Abstract
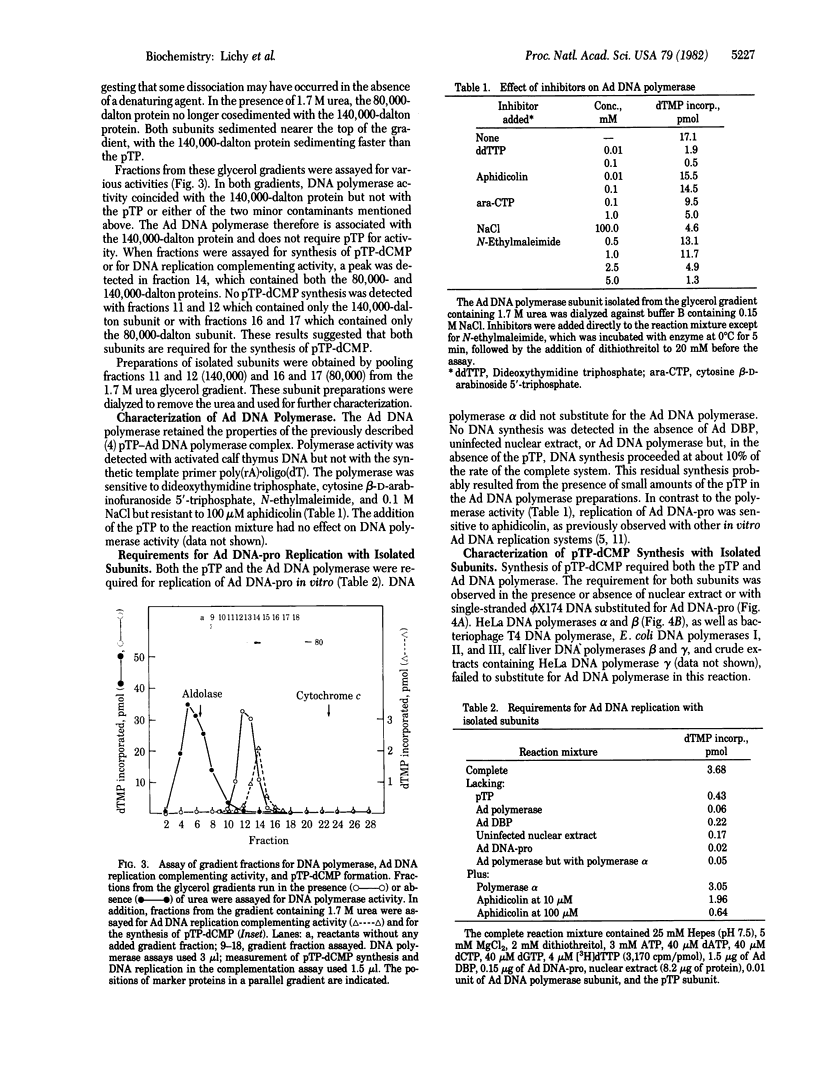
A complex containing the 80,000-dalton precursor to the adenovirus (Ad)-encoded terminal protein (pTP) and a 140,000-dalton protein is required for Ad DNA replication in vitro. This complex has been separated into subunits by glycerol gradient centrifugation in the presence of urea. The isolated 140,000-dalton subunit contains a DNA polymerase activity which can be differentiated from all host DNA polymerases. No enzyme activity was detected with the isolated pTP. The requirements for reactions involved in the initiation of Ad DNA replication were determined by using the isolated subunits. The covalent addition of dCMP, the first nucleotide in the DNA chain, to the pTP, which serves as the primer for replication, required the DNA polymerase subunit as well as the pTP. Synthesis of viral DNA in vitro also required both subunits. The properties of the DNA polymerase suggest that it may be a viral gene product.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Challberg M. D., Desiderio S. V., Kelly T. J., Jr Adenovirus DNA replication in vitro: characterization of a protein covalently linked to nascent DNA strands. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5105–5109. doi: 10.1073/pnas.77.9.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J., Jr Adenovirus DNA replication in vitro: origin and direction of daughter strand synthesis. J Mol Biol. 1979 Dec 25;135(4):999–1012. doi: 10.1016/0022-2836(79)90524-2. [DOI] [PubMed] [Google Scholar]
- Chang L. M., Plevani P., Bollum F. J. Evolutionary conservation of DNA polymerase beta structure. Proc Natl Acad Sci U S A. 1982 Feb;79(3):758–761. doi: 10.1073/pnas.79.3.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conaway R. C., Lehman I. R. A DNA primase activity associated with DNA polymerase alpha from Drosophila melanogaster embryos. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2523–2527. doi: 10.1073/pnas.79.8.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desiderio S. V., Kelly T. J., Jr Structure of the linkage between adenovirus DNA and the 55,000 molecular weight terminal protein. J Mol Biol. 1981 Jan 15;145(2):319–337. doi: 10.1016/0022-2836(81)90208-4. [DOI] [PubMed] [Google Scholar]
- Enomoto T., Lichy J. H., Ikeda J. E., Hurwitz J. Adenovirus DNA replication in vitro: purification of the terminal protein in a functional form. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6779–6783. doi: 10.1073/pnas.78.11.6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galos R. S., Williams J., Binger M. H., Flint S. J. Location of additional early gene sequences in the adenoviral chromosome. Cell. 1979 Aug;17(4):945–956. doi: 10.1016/0092-8674(79)90334-9. [DOI] [PubMed] [Google Scholar]
- Hübscher U., Spanos A., Albert W., Grummt F., Banks G. R. Evidence that a high molecular weight replicative DNA polymerase is conserved during evolution. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6771–6775. doi: 10.1073/pnas.78.11.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda J. E., Enomoto T., Hurwitz J. Adenoviral protein-primed initiation of DNA chains in vitro. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2442–2446. doi: 10.1073/pnas.79.8.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda J. E., Enomoto T., Hurwitz J. Replication of adenovirus DNA-protein complex with purified proteins. Proc Natl Acad Sci U S A. 1981 Feb;78(2):884–888. doi: 10.1073/pnas.78.2.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda J. E., Longiaru M., Horwitz M. S., Hurwitz J. Elongation of primed DNA templates by eukaryotic DNA polymerases. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5827–5831. doi: 10.1073/pnas.77.10.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan L. M., Ariga H., Hurwitz J., Horwitz M. S. Complementation of the temperature-sensitive defect in H5ts125 adenovirus DNA replication in vitro. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5534–5538. doi: 10.1073/pnas.76.11.5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwant M. M., van der Vliet P. C. Differential effect of aphidicolin on adenovirus DNA synthesis and cellular DNA synthesis. Nucleic Acids Res. 1980 Sep 11;8(17):3993–4007. doi: 10.1093/nar/8.17.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichy J. H., Horwitz M. S., Hurwitz J. Formation of a covalent complex between the 80,000-dalton adenovirus terminal protein and 5'-dCMP in vitro. Proc Natl Acad Sci U S A. 1981 May;78(5):2678–2682. doi: 10.1073/pnas.78.5.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longiaru M., Ikeda J. E., Jarkovsky Z., Horwitz S. B., Horwitz M. S. The effect of aphidicolin on adenovirus DNA synthesis. Nucleic Acids Res. 1979 Jul 25;6(10):3369–3386. doi: 10.1093/nar/6.10.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Pincus S., Robertson W., Rekosh D. Characterization of the effect of aphidicolin on adenovirus DNA replication: evidence in support of a protein primer model of initiation. Nucleic Acids Res. 1981 Oct 10;9(19):4919–4938. doi: 10.1093/nar/9.19.4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekosh D. M., Russell W. C., Bellet A. J., Robinson A. J. Identification of a protein linked to the ends of adenovirus DNA. Cell. 1977 Jun;11(2):283–295. doi: 10.1016/0092-8674(77)90045-9. [DOI] [PubMed] [Google Scholar]
- Stillman B. W., Lewis J. B., Chow L. T., Mathews M. B., Smart J. E. Identification of the gene and mRNA for the adenovirus terminal protein precursor. Cell. 1981 Feb;23(2):497–508. doi: 10.1016/0092-8674(81)90145-8. [DOI] [PubMed] [Google Scholar]
- Tamanoi F., Stillman B. W. Function of adenovirus terminal protein in the initiation of DNA replication. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2221–2225. doi: 10.1073/pnas.79.7.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villani G., Sauer B., Lehman I. R. DNA polymerase alpha from Drosophila melanogaster embryos. Subunit structure. J Biol Chem. 1980 Oct 10;255(19):9479–9483. [PubMed] [Google Scholar]
- Wilkie N. M., Ustacelebi S., Williams J. F. Characterization of temperature-sensitive mutants of adenovirus type 5: nucleic acid synthesis. Virology. 1973 Feb;51(2):499–503. doi: 10.1016/0042-6822(73)90450-9. [DOI] [PubMed] [Google Scholar]