Abstract
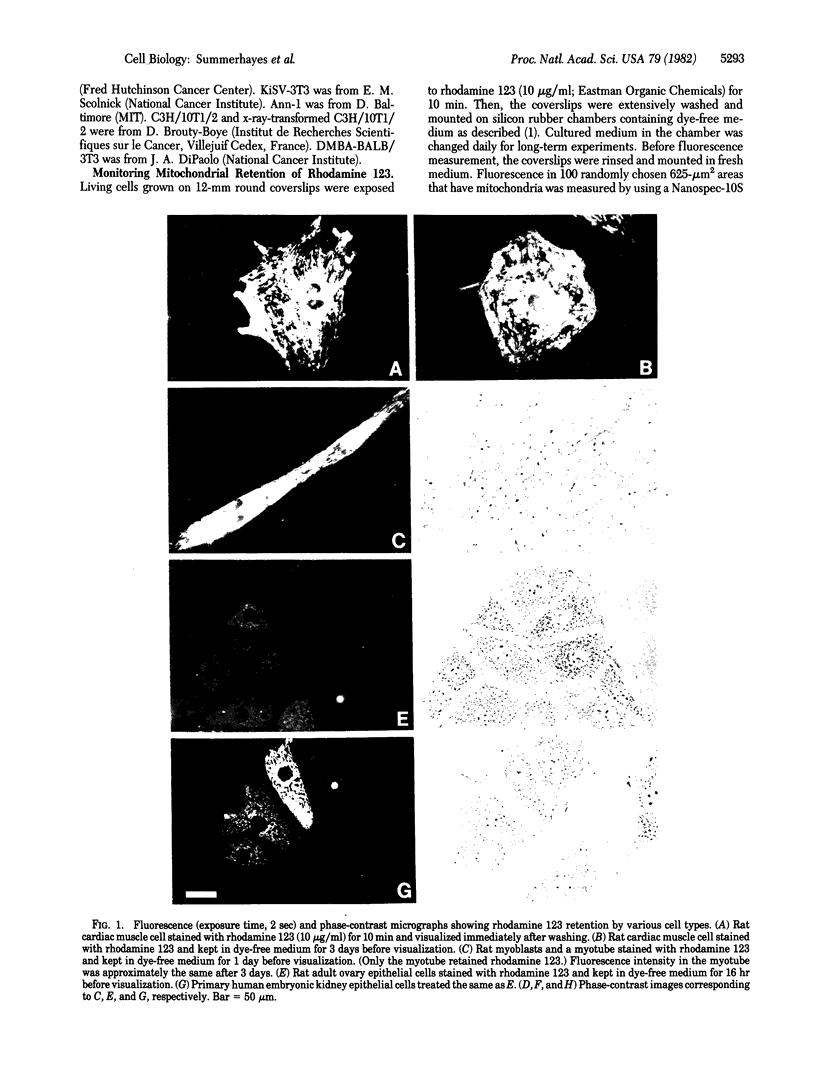
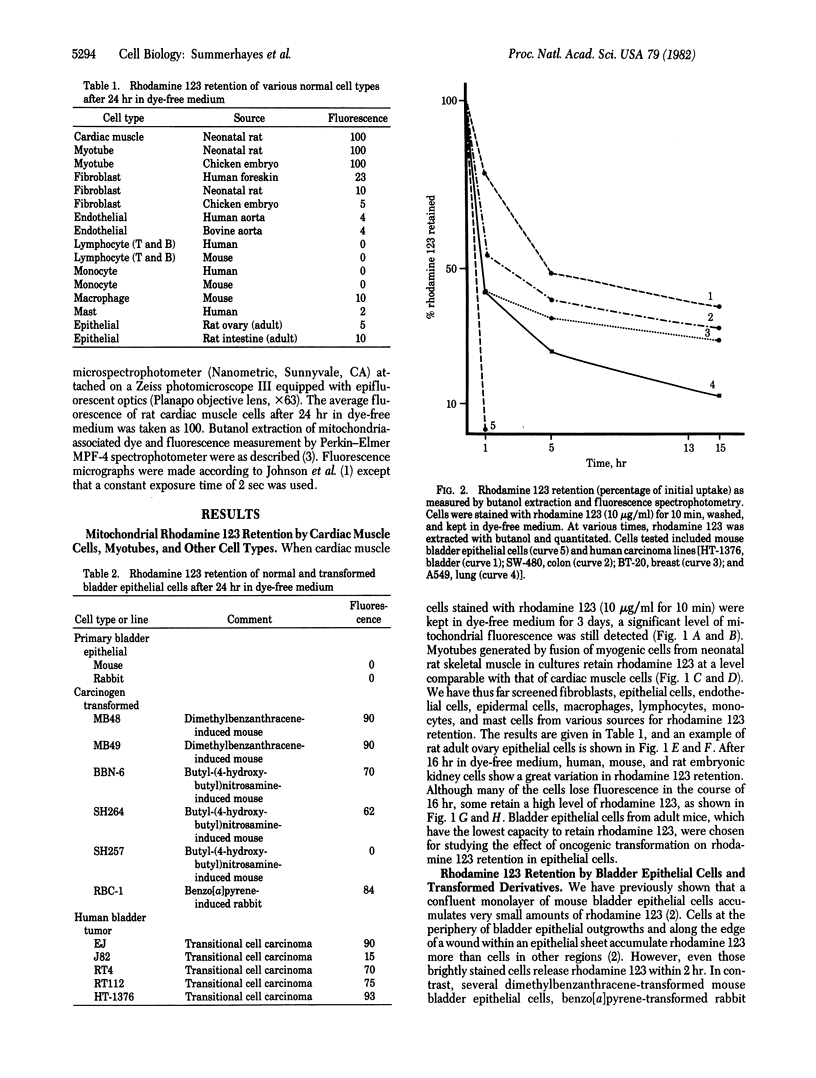
Mitochondria in cardiac muscle cells and myoblast-fused myotubes display unusually long (3-5 days) retention times of rhodamine 123, a mitochondria-specific fluorescent probe, in living cells. Among 50 keratin-positive carcinoma or transformed epithelial cell lines tested, mitochondria with prolonged rhodamine 123 retention are detected in most of the transitional cell carcinoma, adenocarcinoma, and chemical carcinogen-transformed epithelial cell lines and in some squamous cell carcinoma lines but not in any oat cell carcinoma lines. The presence of mitochondria having unusual dye retention may be useful for diagnosis and exploitable for chemotherapy of certain human carcinomas.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen L. B., Summerhayes I. C., Johnson L. V., Walsh M. L., Bernal S. D., Lampidis T. J. Probing mitochondria in living cells with rhodamine 123. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):141–155. doi: 10.1101/sqb.1982.046.01.018. [DOI] [PubMed] [Google Scholar]
- Cohen R. L., Muirhead K. A., Gill J. E., Waggoner A. S., Horan P. K. A cyanine dye distinguishes between cycling and non-cycling fibroblasts. Nature. 1981 Apr 16;290(5807):593–595. doi: 10.1038/290593a0. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Staiano-Coico L., Melamed M. R. Increased mitochondrial uptake of rhodamine 123 during lymphocyte stimulation. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2383–2387. doi: 10.1073/pnas.78.4.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Traganos F., Staiano-Coico L., Kapuscinski J., Melamed M. R. Interaction of rhodamine 123 with living cells studied by flow cytometry. Cancer Res. 1982 Mar;42(3):799–806. [PubMed] [Google Scholar]
- Goldstein S., Korczack L. B. Status of mitochondria in living human fibroblasts during growth and senescence in vitro: use of the laser dye rhodamine 123. J Cell Biol. 1981 Nov;91(2 Pt 1):392–398. doi: 10.1083/jcb.91.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hightower M. J., Fairfield F. R., Lucas J. J. A staining procedure for identifying viable cell hybrids constructed by somatic cell fusion, cybridization, or nuclear transplantation. Somatic Cell Genet. 1981 May;7(3):321–329. doi: 10.1007/BF01538857. [DOI] [PubMed] [Google Scholar]
- James T. W., Bohman R. Proliferation of mitochondria during the cell cycle of the human cell line (HL-60). J Cell Biol. 1981 May;89(2):256–260. doi: 10.1083/jcb.89.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. V., Summerhayes I. C., Chen L. B. Decreased uptake and retention of rhodamine 123 by mitochondria in feline sarcoma virus-transformed mink cells. Cell. 1982 Jan;28(1):7–14. doi: 10.1016/0092-8674(82)90369-5. [DOI] [PubMed] [Google Scholar]
- Johnson L. V., Walsh M. L., Bockus B. J., Chen L. B. Monitoring of relative mitochondrial membrane potential in living cells by fluorescence microscopy. J Cell Biol. 1981 Mar;88(3):526–535. doi: 10.1083/jcb.88.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. V., Walsh M. L., Chen L. B. Localization of mitochondria in living cells with rhodamine 123. Proc Natl Acad Sci U S A. 1980 Feb;77(2):990–994. doi: 10.1073/pnas.77.2.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KONIGSBERG I. R. Clonal analysis of myogenesis. Science. 1963 Jun 21;140(3573):1273–1284. doi: 10.1126/science.140.3573.1273. [DOI] [PubMed] [Google Scholar]
- Lampidis T. J., Schaiberger G. E. Age-related loss of DNA repair synthesis in isolated rat myocardial cells. Exp Cell Res. 1975 Dec;96(2):412–416. doi: 10.1016/0014-4827(75)90276-1. [DOI] [PubMed] [Google Scholar]
- Levenson R., Macara I. G., Smith R. L., Cantley L., Housman D. Role of mitochondrial membrane potential in the regulation of murine erythroleukemia cell differentiation. Cell. 1982 Apr;28(4):855–863. doi: 10.1016/0092-8674(82)90064-2. [DOI] [PubMed] [Google Scholar]
- Rein A., Rubin H. Effects of local cell concentrations upon the growth of chick embryo cells in tissue culture. Exp Cell Res. 1968 Mar;49(3):666–678. doi: 10.1016/0014-4827(68)90213-9. [DOI] [PubMed] [Google Scholar]
- Rheinwald J. G., Beckett M. A. Tumorigenic keratinocyte lines requiring anchorage and fibroblast support cultured from human squamous cell carcinomas. Cancer Res. 1981 May;41(5):1657–1663. [PubMed] [Google Scholar]
- Rheinwald J. G., Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975 Nov;6(3):331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- Siemens A., Walter R., Liaw L. H., Berns M. W. Laser-stimulated fluorescence of submicrometer regions within single mitochondria of rhodamine-treated myocardial cells in culture. Proc Natl Acad Sci U S A. 1982 Jan;79(2):466–470. doi: 10.1073/pnas.79.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerhayes I. C., Cheng Y. S., Sun T. T., Chen L. B. Expression of keratin and vimentin intermediate filaments in rabbit bladder epithelial cells at different stages of benzo[a]pyrene-induced neoplastic progression. J Cell Biol. 1981 Jul;90(1):63–69. doi: 10.1083/jcb.90.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerhayes I. C., Franks L. M. Effects of donor age on neoplastic transformation of adult mouse bladder epithelium in vitro. J Natl Cancer Inst. 1979 Apr;62(4):1017–1023. [PubMed] [Google Scholar]
- Yaffe D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci U S A. 1968 Oct;61(2):477–483. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler M. L., Davidson R. L. Elimination of mitochondrial elements and improved viability in hybrid cells. Somatic Cell Genet. 1981 Jan;7(1):73–88. doi: 10.1007/BF01544749. [DOI] [PubMed] [Google Scholar]