Abstract
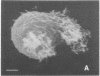
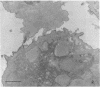
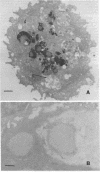
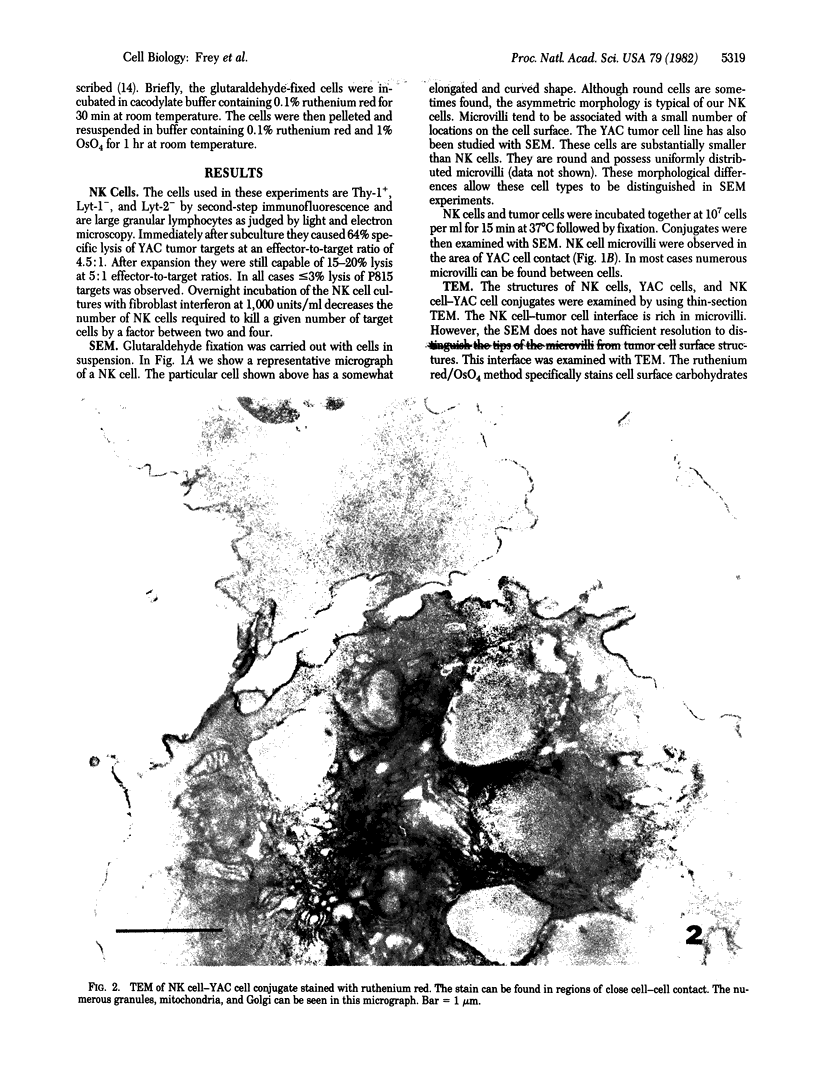
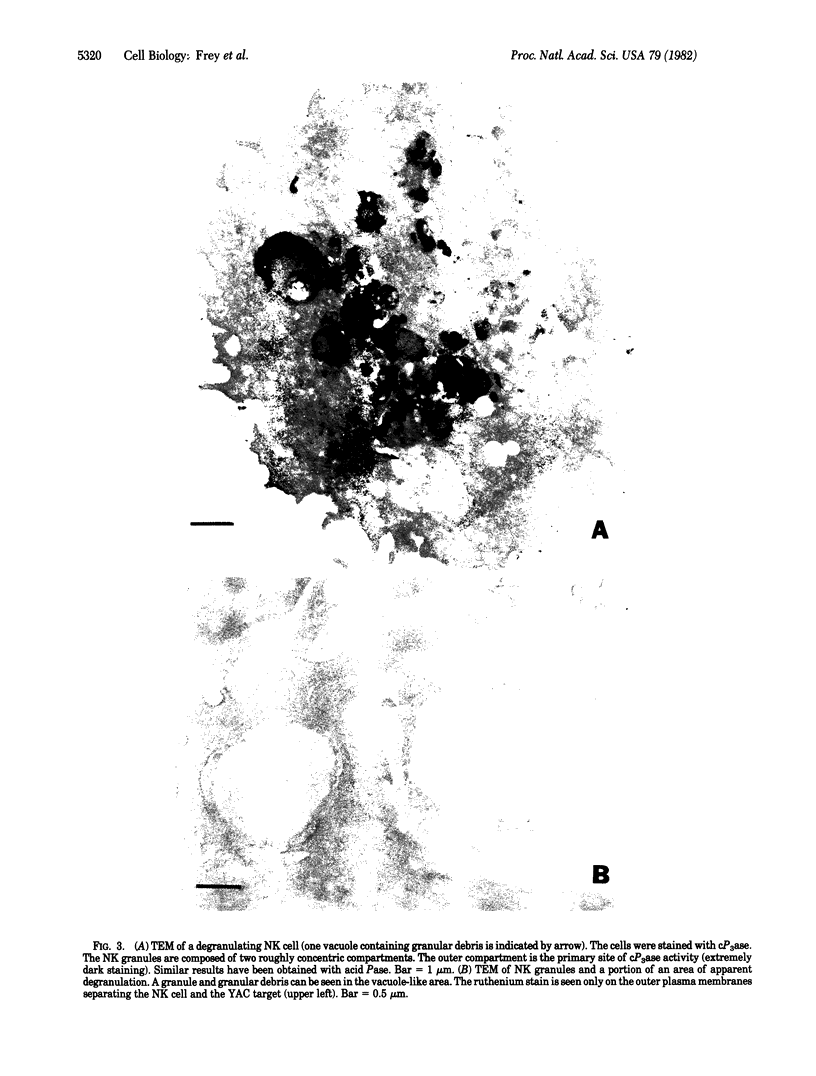
Natural killer (NK) cells were obtained from C3H mouse spleens according to a modified version of the method of Kuribayashi et al. [Kuribayashi, K., Gillis, S., Dern, D. E. & Henney, C. S. (1981) J. Immunol. 126, 2321-2327]. These cells retain in vitro cytotoxicity against certain model tumor cell targets and appear homogeneous by morphological criteria. NK cells, YAC (tumor) cells, and NK cell-YAC cell conjugates have been examined with scanning (SEM) and transmission (TEM) electron microscopy. SEM experiments have shown that: (i) NK cells are large and possess various shapes in contrast to the YAC target cells which are smaller and round, (ii) YAC cells have uniformly distributed microvilli whereas the NK cell microvilli are most prominent in the area of effector-to-target contact, and (iii) in the absence of target cells, NK cell microvilli are found in a small number (usually 1-3) of cell surface locations. The region of NK cell-tumor cell contact has also been examined with TEM. The cells were stained with ruthenium red/OsO4. The electron-dense ruthenium red/OsO4 reaction product was consistently found in regions of close cell-cell contact, suggesting that carbohydrates were not completely cleared from areas of contact and that target and effector membranes do not fuse extensively. TEM observations indicate that NK cells have structurally unique granules. The granules are composed of at least two distinct compartments. The outer compartment contains the lysosome-associated enzymes acid phosphatase and inorganic trimetaphosphatase. No enzymatic activities have been found associated with the inner compartment. NK cells appear to degranulate when incubated with YAC cells. Under those circumstances, limited areas of the NK cytoplasm contain vacuole-like areas possessing granules and apparent granular debris. Degranulation appears to be involved in the cytotoxic function of NK cells.
Keywords: cytotoxic mechanism, degranulation, lysosomal enzymes, immune surveillance
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bainton D. F., Farquhar M. G. Segregation and packaging of granule enzymes in eosinophilic leukocytes. J Cell Biol. 1970 Apr;45(1):54–73. doi: 10.1083/jcb.45.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton D. R., Golde D. W. Differentiation of macrophages from normal human bone marrow in liquid culture. Electron microscopy and cytochemistry. J Clin Invest. 1978 Jun;61(6):1555–1569. doi: 10.1172/JCI109076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks C. G., Kuribayashi K., Sale G. E., Henney C. S. Characterization of five cloned murine cell lines showing high cytolytic activity against YAC-1 cells. J Immunol. 1982 May;128(5):2326–2335. [PubMed] [Google Scholar]
- Carpén O., Virtanen I., Saksela E. The cytotoxic activity of human natural killer cells requires an intact secretory apparatus. Cell Immunol. 1981 Feb;58(1):97–106. doi: 10.1016/0008-8749(81)90152-0. [DOI] [PubMed] [Google Scholar]
- Dennert G. Cloned lines of natural killer cells. Nature. 1980 Sep 4;287(5777):47–49. doi: 10.1038/287047a0. [DOI] [PubMed] [Google Scholar]
- Doty S. B., Smith C. E., Hand A. R., Oliver C. Inorganic trimetaphosphatase as a histochemical marker for lysosomes in light and electron microscopy. J Histochem Cytochem. 1977 Dec;25(12):1381–1384. doi: 10.1177/25.12.200672. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Holden H. T. Natural cell-mediated immunity. Adv Cancer Res. 1978;27:305–377. doi: 10.1016/s0065-230x(08)60936-7. [DOI] [PubMed] [Google Scholar]
- Kiessling R., Haller O. Natural killer cells in the mouse: an alternative immune surveillance mechanism? Contemp Top Immunobiol. 1978;8:171–201. doi: 10.1007/978-1-4684-0922-2_6. [DOI] [PubMed] [Google Scholar]
- Kuribayashi K., Gillis S., Kern D. E., Henney C. S. Murine NK cell cultures: effects of interleukin-2 and interferon on cell growth and cytotoxic reactivity. J Immunol. 1981 Jun;126(6):2321–2327. [PubMed] [Google Scholar]
- Lagunoff D. Vital staining of mast cells with ruthenium red. J Histochem Cytochem. 1972 Nov;20(11):938–944. doi: 10.1177/20.11.938. [DOI] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec. 1971 Nov;171(3):347–368. doi: 10.1002/ar.1091710302. [DOI] [PubMed] [Google Scholar]
- Luini W., Boraschi D., Alberti S., Aleotti A., Tagliabue A. Morphological characterization of a cell population responsible for natural killer activity. Immunology. 1981 Aug;43(4):663–668. [PMC free article] [PubMed] [Google Scholar]
- Neighbour P. A., Huberman H. S. Sr++-induced inhibition of human natural killer (NK) cell-mediated cytotoxicity. J Immunol. 1982 Mar;128(3):1236–1240. [PubMed] [Google Scholar]
- Petty H. R. Response of the resident macrophage to concanavalin A. Alterations of surface morphology and anionic site distribution. Exp Cell Res. 1980 Aug;128(2):439–454. doi: 10.1016/0014-4827(80)90079-8. [DOI] [PubMed] [Google Scholar]
- Quan P. C., Ishizaka T., Bloom B. R. Studies on the mechanism of NK cell lysis. J Immunol. 1982 Apr;128(4):1786–1791. [PubMed] [Google Scholar]
- Seeman P. M., Palade G. E. Acid phosphatase localization in rabbit eosinophils. J Cell Biol. 1967 Sep;34(3):745–756. doi: 10.1083/jcb.34.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura K., Habu S., Fukui H., Akatsuka A., Okumura K., Tamaoki N. Morphology and function of ganglio-N-tetraosylceramide-positive lymphocyte mediators of natural killer activity. J Natl Cancer Inst. 1982 Mar;68(3):449–455. [PubMed] [Google Scholar]
- Spiess P. J., Rosenberg S. A. A simplified method for the production of murine T-cell growth factor free of lectin. J Immunol Methods. 1981;42(2):213–222. doi: 10.1016/0022-1759(81)90151-4. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Timonen T., Saksela E., Ranki A., Häyry P. Fractionation, morphological and functional characterization of effector cells responsible for human natural killer activity against cell-line targets. Cell Immunol. 1979 Nov;48(1):133–148. doi: 10.1016/0008-8749(79)90106-0. [DOI] [PubMed] [Google Scholar]