Summary
Retrocyclin-101 (RC101) and Protegrin-1 (PG1) are two important antimicrobial peptides that can be used as therapeutic agents against bacterial and/or viral infections, especially those caused by the HIV-1 or sexually transmitted bacteria. Because of their antimicrobial activity and complex secondary structures, they have not yet been produced in microbial systems and their chemical synthesis is prohibitively expensive. Therefore, we created chloroplast transformation vectors with the RC101 or PG1 coding sequence, fused with GFP to confer stability, furin or Factor Xa cleavage site to liberate the mature peptide from their fusion proteins and a His-tag to aid in their purification. Stable integration of RC101 into the tobacco chloroplast genome and homoplasmy were confirmed by Southern blots. RC101 and PG1 accumulated up to 32%–38% and 17%~26% of the total soluble protein. Both RC101 and PG1 were cleaved from GFP by corresponding proteases in vitro, and Factor Xa–like protease activity was observed within chloroplasts. Confocal microscopy studies showed location of GFP fluorescence within chloroplasts. Organic extraction resulted in 10.6-fold higher yield of RC101 than purification by affinity chromatography using His-tag. In planta bioassays with Erwinia carotovora confirmed the antibacterial activity of RC101 and PG1 expressed in chloroplasts. RC101 transplastomic plants were resistant to tobacco mosaic virus infections, confirming antiviral activity. Because RC101 and PG1 have not yet been produced in other cell culture or microbial systems, chloroplasts can be used as bioreactors for producing these proteins. Adequate yield of purified antimicrobial peptides from transplastomic plants should facilitate further preclinical studies.
Keywords: antimicrobial peptide, chloroplast genetic engineering, molecular farming, plant-made biopharmaceuticals
Introduction
Antimicrobial peptides are evolutionarily conserved components of the innate immune response and are found in different organisms, including bacteria, vertebrates, invertebrates and plants (Boman, 1995; Nicolas and Mor, 1995; Broekaert et al., 1997; Hancock and Chapple, 1999). Antimicrobial peptides are also called peptide antibiotics. When compared with conventional antibiotics, development of resistance is less likely with antimicrobial peptides. Many bacteria species remain sensitive to antimicrobial peptides after a long time of evolution (Yeaman and Yount, 2003; Nizet, 2006). Adaptive immune systems can remember the pathogen and elicit a much faster and stronger immune response against that pathogen at subsequent encounters (Boman, 1995). Without such specificity and memory, antimicrobial peptides evolved a different mechanism against pathogen infections. Most antimicrobial peptides are efficient against a broad spectrum of pathogens rather than specific against one pathogen, which makes them especially suitable for use against local and systematic infections (Bals, 2000; Schaller-Bals et al., 2002). Other than the antimicrobial activities, some antimicrobial peptides are shown to have immunomodulatory activities. Some studies show that antimicrobial peptides such as defensins are likely to play a role in recruiting effector T cells to inflammatory sites, thereby contributing to the effector phase of adaptive immunity (Yang et al., 2001). These intriguing characteristics of antimicrobial peptides facilitate development of novel antibiotics. However, the high cost of production of antimicrobial peptides and lack of suitable expression systems could be potential barriers for their development and clinical studies.
The chloroplast, as a bioreactor, is able to express foreign proteins at high levels because of their high copy numbers. When a transgene is integrated into the inverted repeat region of the chloroplast genome, up to 20 000 copies of the transgene per cell could be expressed. Several therapeutic proteins have been expressed in chloroplasts, including human blood proteins somatotropin (Staub et al., 2000), insulin-like growth factor (Daniell et al., 2009), proinsulin (Ruhlman et al., 2007), IFN-α2b (Arlen et al., 2007), serum albumin (Fernandez-San et al., 2003), IFN-γ (Leelavathi and Reddy, 2003), cardiotrophin-1 (Farran et al., 2008), alpha1-anti-trypsin (Nadai et al., 2009) and glutamic acid decarboxylase (Wang et al., 2008). In addition, several vaccine antigens have been expressed in chloroplasts against several bacterial pathogens including cholera toxin B subunit (Daniell et al., 2001), tetanus toxin (Tregoning et al., 2003), anthrax protective antigen (Watson et al., 2004; Koya et al., 2005), plague F1-V fusion antigen (Arlen et al., 2008), and outer surface lipoprotein A (OspA) for Lyme disease (Glenz et al., 2006), and their functionality has been evaluated in cell culture systems or animal models after pathogen or toxin challenges. Antigens produced against protozoan pathogens were immunogenic against amoeba (Chebolu and Daniell, 2007) or effective against the malarial parasite (Davoodi-Semiromi et al., 2009). Although several viral antigens have been expressed in chloroplasts, neutralizing antibodies were shown only against human papillomavirus (Fernandez-San et al., 2008) and canine parvovirus 2L21 peptide (Molina et al., 2004). Other proteins expressed in chloroplasts include bovine mammary-associated serum amyloid (Manuell et al., 2007), aprotinin (Tissot et al., 2008) and monoclonal large single-chain (lsc) antibody against glycoprotein D of the herpes simplex virus (Mayfield et al., 2003). The expression levels of these proteins are mostly 2%~20% of total soluble protein (TSP) but could be even higher than RuBisCo (Oey et al., 2009; Ruhlman et al., 2010). Other advantages of chloroplast transformation include multi-gene engineering, transgene containment, lack of position effect, gene silencing and maternal inheritance (Daniell et al., 2005; 2009).
Retrocyclin is a cyclic octadecapeptide, which is artificially synthesized based on a human pseudogene that is homologous to rhesus monkey circular minidefensins. Retrocyclin contains six cysteines and has large β-sheet structure that is stabilized by three intramolecular disulphide bonds. Structure–function studies indicate that the cyclic backbone, intramolecular tri-disulphide ladder, and arginine residues of retrocyclin contributed substantially to its protective effects (Trabi et al., 2001; Jenssen et al., 2006). Retrocyclin peptides are small antimicrobial agents with potent activity against bacteria and viruses, especially against HIV retrovirus or sexually transmitted bacteria. Previous studies have shown that Retrocyclin-101 (RC101) and other retrocyclins can protect human CD4+ cells from infection by T- and M-tropic strains of HIV-1 in vitro (Cole et al., 2002) and prevent HIV-1 infection in an organ-like construct of human cervicovaginal tissue (Cole et al., 2007). The ability of RC101 to prevent HIV-1 infection and retain full activity in the presence of vaginal fluid makes it a good candidate for topical microbicide to prevent sexual transmission of HIV-1.
Protegrin-1 (PG1) belongs to the protegrin family, which is discovered in porcine leucocytes (Kokryakov et al., 1993). PG1 is a cysteine-rich, 18-residue β-sheet peptide. It has a high content of arginine, an amidated C-terminus, and four conserved cysteines at positions 6, 8, 13 and 15, which would form two disulphide bonds. The antimicrobial activity of PG1 is strongly related to the stability of β-hairpin conformation, and the β-hairpin conformation of PG1 is stabilized by the two disulphide bonds. Removal of both disulphide bonds would result in substantial reduction of PG1's activity (Harwig et al., 1996; Chen et al., 2000). Therefore, the disulphide bridges are very important to the activity of PG1. It was shown that PG1 had potent antimicrobial activity against a broad spectrum of microorganisms, including bacteria, fungi and yeasts (Kokryakov et al., 1993; Steinberg et al., 1997). Chlamydia trachomatis and Neisseria gonorrhoeae are two kinds of pathogenic bacteria that can cause sexually transmitted diseases (STDs) in humans. Two previous studies that compare the efficiency of PG1 with human neutrophil defensins demonstrated that PG1 is more potent than human neutrophil defensins in inactivating C. trachomatis and N. gonorrhoeae (Qu et al., 1996; Yasin et al., 1996). Therefore, a combination of RC101 and PG1 should be able to inactivate most bacterial and viral pathogens, and they will be especially effective against bacteria and viruses that cause STDs.
In a previous study, our laboratory expressed the antimicrobial peptide MSI-99, an analogue of magainin 2, via the chloroplast genome to obtain high levels of protection against bacterial and fungal pathogens (DeGray et al., 2001). Recently, a proteinaceous antibiotic, PlyGBS lysin, was also expressed in the chloroplast, and it was shown that the protein synthesis capacity of the chloroplast was exhausted by the massive production of the foreign protein (Oey et al., 2009). However, antimicrobial peptides containing multiple intramolecular disulphide bonds have not yet been expressed in chloroplasts. In this study, we investigated expression of functional disulphide-bonded antimicrobial peptides in chloroplasts. The rationale is that chloroplasts have already been shown in previous studies to be fully functional in expressing biologically active, disulphide-bonded therapeutic proteins, such as human somatotropin (Staub et al., 2000), cholera toxin B (Daniell et al., 2001), human interferon-α2b (Arlen et al., 2007) and alkaline phosphatases (Bally et al., 2008). Because of the high cost associated with chemical synthesis and inability of cell culture or microbial systems to produce these proteins, expression of RC101 or PG1 antimicrobial peptides in chloroplasts would be an ideal solution for their large-scale economic production.
Results
Construction of chloroplast transformation vectors
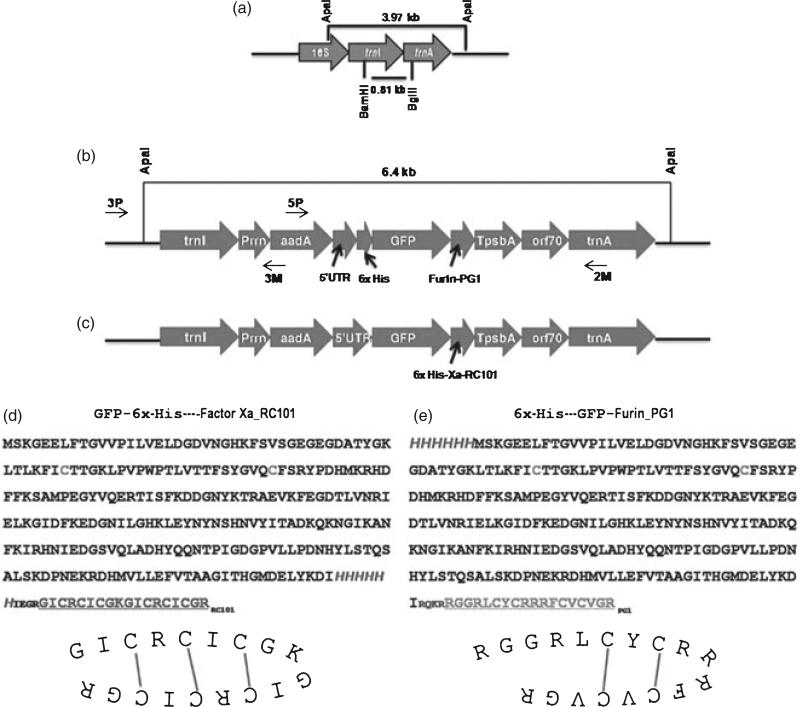
Two chloroplast transformation vectors were designed for expressing RC101 and PG1 in chloroplasts. They were constructed using the basic pLD vector, which was developed in our laboratory for chloroplast transformation (Daniell et al., 1998; Verma et al., 2008). Both PG1 and RC101 were fused with GFP gene because of their small sizes (18 amino acids). Besides, GFP was used as a reporter and to aid in quantification of the fusion proteins. A 6-histidine tag was also engineered upstream of RC101/PG1 to facilitate purification of these fusion proteins. A furin protease cleavage site was engineered between PG1 and GFP, while a Factor Xa protease cleavage site was engineered between RC101 and the 6-histidine tag to facilitate release of PG1/RC101 from these fusion proteins. The promoter and 5′-untranslated region (UTR) of the tobacco psbA gene was placed upstream of the His6-GFP-Furin-PG1/GFP-His6-Xa-RC101 transgene cassette to enhance expression of these fusion proteins. The aadA gene, which conferred resistance to spectinomycin, was driven by the constitutive Prrn promoter. The flanking sequences of trnI and trnA facilitated recombination with the native chloroplast genome (Figure 1b,c). The transgene fragment sequences and the disulphide bonds of RC101 and PG1 are shown in Figure 1d,e.
Figure 1.
Schematic representation of chloroplast vectors. (a) The native chloroplast genome showing both homologous recombination sites (trnI and trnA) and the restriction enzyme sites used for Southern blot analysis. (b) The pLD-His6-GFP-Furin-PG1 vector map with the primer annealing sites. (c) The pLD-GFP-His6-Factor Xa-retrocyclin-101 (RC101) vector map; primer annealing sites are the same as shown on the PG1 vector map. (d) The nucleotide sequence of GFP-6xHis-Factor Xa-RC101 and the schematic representation of disulphide bonds in RC101. (e) The nucleotide sequence of 6xHis-GFP-Furin-PG1 and the schematic representation of disulphide bonds in PG1.
Confirmation of transgene cassette integration and homoplasmy
Several primary shoots appeared from the RC101- and PG1-bombarded tobacco leaves, and they were developed through three rounds of selection. To confirm integration of transgene cassettes into the chloroplast genome, the putative transformed shoots were screened by PCR. Two pairs of primers were used for screening. The 3P and 3M primers were used to check site-specific integration of the selectable marker gene (aadA) into the chloroplast genome. The 5P and 2M primers were used to check integration of the transgene expression cassette (Figure 1b,c). DNA template from the RC101-GFP and PG1-GFP transplastomic shoots yielded PCR products with both primers (Figure 2a,b). The 3P-3M PCR products for both the RC101 and PG1 transformants were 1.65 kbp, and 5P-2M PCR products were 2.6 kbp. Because the sizes of the RC101 and PG1 transgene expression cassette (including GFP) were similar, PCR product sizes were also similar. These PCR products could be generated only from transformed chloroplasts and not nuclear transformants or spontaneous mutants.
Figure 2.
PCR and Southern blot analysis to investigate transgene integration and homoplasmy. (a) PCR analysis of the untransformed and transplastomic lines using the primer pair 3P/3M. Lanes 1–3: retrocyclin-101 (RC101) transplastomic lines; 4–6: PG1 transplastomic lines. (b) PCR analysis of the untransformed and transplastomic lines using the primer pair 5P/2M. Lanes 1–3: RC101 transplastomic lines; 4–6: PG1 transplastomic lines. (c) Southern blot hybridized with the flanking sequence trnI-trnA probe to investigate the homoplasmy of RC101 and PG1 transplastomic lines. Lanes 1–2, DNA samples from RC101 transplastomic plants; lanes 3–4, PG1 transplastomic plants. M, 1-kbp DNA plus ladder; WT, untransformed tobacco.
Because there are thousands of copies of chloroplast genomes in each plant cell, some of them may not be transformed. Therefore, Southern blot was performed to investigate whether RC101 and PG1 transplastomic plants achieved homoplasmy. The probe used was made by digesting the flanking sequences trnI and trnA with BamHI and BglII (Figure 1a). Flanking sequence probe identified a single 4.0-kbp fragment in the untransformed tobacco, as expected. In the RC101 and PG1 transplastomic lines, only one 6.4-kbp fragment was observed (Figure 2c). Absence of the 4.0-kbp fragment confirmed that all the chloroplast genomes were transformed (to the detection limit of Southern blots), and therefore, they are considered to be homoplasmic.
Evaluation of RC101 or PG1 expression in transgenic chloroplasts
To evaluate expression of foreign genes in chloroplasts of RC101-GFP and PG1-GFP transplastomic lines, immunoblots using GFP antibodies were performed. Based on the TSP concentration, same amount of protein extracts from RC101 and PG1 transplastomic lines (before and after pro-tease digestion) were resolved on 12% SDS-PAGE gels. The size of RC101 is 1.9 kDa, while the size of PG1 is 2.1 kDa. Therefore, the sizes of RC101-GFP and PG1-GFP are both ~29 kDa. After cleavage of RC101 and PG1 from GFP, we should observe only the 27 kDa GFP polypeptide. The immunoblot result is shown in Figure 3a. Clearly, the fusion proteins were cleaved after protease digestion.
Figure 3.
Protease cleavage of the fusion proteins by immunoblot and quantification of expression by densitometric analysis. (a) Immunoblot analysis of retrocyclin-101 (RC101)-GFP and PG1-GFP expression and cleavage. 1: untransformed protein extract, 10 μg; 2: precision plus protein marker, 5 μg; 3: RC101-GFP transplastomic line protein extract, 3 μg; 4: RC101-GFP protein extract digested by Factor Xa protease, 3 μg; 5: PG1-GFP protein extract, 6 μg; 6: PG1-GFP protein extract digested by furin protease, 6 μg; 7: GFP standard, 100 ng. (b) Native polyacrylamide gel electrophoresis of RC101-GFP and PG1-GFP protein extracts. Lanes 1–3, GFP standard (150, 300, 600 ng); lane 4, untransformed plant extract, 10 μg; lanes 5–6, RC101 transplastomic extracts (6, 8 μg); lanes 7–8, PG1 transplastomic extracts (6, 8 μg). (c) GFP standard curve based on the integrated density values of 150, 300 and 600 ng of GFP standard. (d) Estimation of RC101-GFP and PG1-GFP expression levels in transplastomic plants.
An alternative approach to confirm the expression of RC101-GFP and PG1-GFP proteins is to observe the green fluorescence emitted by GFP. After crude protein extracts were resolved on the native polyacrylamide gel, the green fluorescence emitted by GFP fusion proteins was observed under the UV light. The green peptides shown correspond to the GFP fusion proteins. The strong green fluorescence observed indicated that GFP fusion proteins were expressed at high levels (Figure 3b). The expression of RC101 and PG1 transplastomic plants was quantified using the GFP fluorescence by densitometric analysis. The integrated density values (IDVs) of GFP fluorescence were measured by spot densitometry. The linear GFP standard curve was established using 150–600 ng of GFP standard protein (Figure 3c). Based on this GFP standard curve, the expression levels of RC101 and PG1 transplastomic plants were estimated to be approximately 35% and 25% of TSP (Figure 3d). To confirm the expression levels of the transplastomic plants, ELISA was also performed to determine the quantities of RC101-GFP and PG1-GFP fusion proteins in transplastomic tobacco plants. Because the antimicrobial peptides RC101 and PG1 were fused with GFP proteins, ELISA was performed using the GFP antibodies to quantify the RC101-GFP and PG1-GFP fusion proteins. RC101-GFP accumulated to 32%~38% of TSP, and PG1-GFP accumulated to 17%~26% of TSP. This variation of expression levels could be attributable to leaf samples harvested from plants under different periods of illumination.
Dot blot analysis was also performed to evaluate the expression of RC101 in transgenic chloroplasts. Factor Xa-cleaved samples and Factor Xa-uncleaved samples from RC101 transplastomic plants were tested by dot blots. It is shown that both uncut and cut samples of RC101-GFP appeared positive (Figure 4a). As shown in previous experiments (Figure 3a), RC101-GFP fusion proteins were already partially cleaved by Factor Xa within chloroplasts. Because PG1 was not immunogenic, dot blot analysis could not be performed with PG1 transplastomic plants. Instead, PG1 protein expression was examined by silver staining. By comparison of cut and uncut samples from PG1 transplastomic plants, it is clear that there is a 2 kDa polypeptide present in the furin-digested sample but absent in the uncut sample and untransformed tobacco protein extract (Figure 4b). The size of PG1 is 2.16 kDa, and therefore, this polypeptide should correspond to the PG1 protein.
Figure 4.
Dot blot analysis and silver staining to investigate expression of retrocyclin-101 (RC101) and PG1. (a) Dot blot analysis of RC101 before and after cleavage. Indicated amount of RC101 was used as standards. Uncut, RC101-GFP without Factor Xa cleavage; Cut, RC101-GFP after Factor Xa cleavage. (b) Silver-stained gel of plant extracts before or after furin cleavage of PG1-GFP protein. 1: Marker 12 (invitrogen); 2: Untransformed plant protein extract, 40 μg; 3: PG1-GFP protein extract without furin digestion, 40 μg; 4: PG1-GFP protein extract digested by furin protease, 40 μg.
RC101 and PG1 were expressed and contained within chloroplasts
To investigate whether the chloroplasts remained intact when RC101 or PG1 antimicrobial peptides were highly expressed in chloroplasts, fresh leaves were examined under the confocal microscope. Strong green fluorescence was emitted from the RC101 and PG1 transplastomic lines (Figure 5a,b). We observed that chloroplasts emitting green fluorescence formed circles around each cell. There was no GFP fluorescence outside chloroplasts. This observation confirmed that chloroplasts remained intact because GFP-fused antimicrobial proteins were not released into the cytoplasm in any detectable quantity.
Figure 5.
Confocal microscopy of retrocyclin-101 (RC101)-GFP and PG1-GFP transplastomic plants. The left panels show chloroplasts from RC101-GFP (a) or PG1-GFP (b) transplastomic lines (bars = 20 μm). The right panels show four times higher magnification of the boxed regions (bars = 5 μm).
Purification of RC101-GFP and PG1-GFP fusion proteins
We then tried to purify the RC101-GFP and PG1-GFP fusion proteins. The engineered His-tag and GFP protein facilitated purification of RC101-GFP and PG1-GFP fusion proteins. We tried to purify the fusion proteins by affinity chromatography using His-tag or organic extraction through GFP. Results of purification using both methods are shown in Figure 6. Approximately 8 μg of purified PG1-GFP and 5 μg of purified RC101-GFP were obtained from one gram of fresh tobacco leaf using the affinity chromatography method. In contrast, purification of RC101-GFP using the organic extraction method resulted in a yield of 53 μg of purified RC101-GFP per gram of fresh tobacco leaf. The organic extraction method resulted in much higher yield than the affinity chromatography method. It is evident that monomers, dimers and multimers of the RC101-GFP were recovered by organic extraction method, resulting in 10.6-fold higher yield, whereas only the monomer was recovered using the affinity chromatography. The highly enriched fraction was the RC101-GFP monomer, ~29 kDa in size. The upper bands should be dimers and multimers formed by RC101-GFP proteins. This same pattern was observed in the native gel electrophoresis of RC101-GFP transplastomic plant protein extracts (Figure 3b). PG1-GFP protein was purified only by affinity chromatography, and we could observe a single band, which should be the monomer form of PG1-GFP.
Figure 6.
Purified retrocyclin-101 (RC101)-GFP and PG1-GFP fusion proteins were separated on native PAGE and observed by Coomassie staining or fluorescence under UV light. PG1-GFP was purified by affinity chromatography, and RC101-GFP was purified by both affinity chromatography and organic extraction method. Samples were loaded in duplicate. M, Precision Plus protein marker, 5 μg; St, GFP standard, 500 ng. The same gel was observed under UV light (bottom) or stained by Coomassie staining (top). The yield of RC101-GFP was 5 μg/g leaf by affinity chromatography and 53 μg/g leaf by organic extraction; PG1-GFP yield was 8 μg/g leaf by affinity chromatography purification.
RC101 and PG1 retained their antimicrobial activity when expressed in chloroplasts
Retrocyclin-101, as a member of the θx-defensin family, possesses antibacterial activity as well as antiviral activity (Tang et al., 1999). To investigate the functionality of RC101 and PG1 expressed in the tobacco chloroplasts, we performed both antibacterial and antivirus assays using plant pathogens because use of HIV and other human bacterial pathogens requires higher levels of containment than our current facilities. The antibacterial activity of RC101 and PG-1 was studied by investigating enhanced resistance to Erwinia soft rot using either the syringe or sand paper method. One day after inoculation with Erwinia, the first signs of damage were observed on leaves of untransformed plants in the regions of inoculation. On the third day, virtually all inoculated untransformed leaf surfaces underwent necrosis, whereas in leaves of RC101 or PG1 transplastomic plants, no or minimally damaged zones were observed depending on the number of bacteria inoculated. Inoculation of potted plants with Erwinia carotovora using a syringe method resulted in areas of necrosis surrounding the point of inoculation in untransformed control for all cell densities (Figure 7b,f), whereas transplastomic RC101 and PG-1 mature leaves showed no areas of necrosis (Figure 7a,e). Even inoculation of 108 cells resulted in no or minimal necrosis in mature transplastomic leaves. In contrast, untransformed plants inoculated with 102 cells displayed obvious necrosis. Similar results were obtained with E. carotovora inoculated by the sand paper method. Transplastomic mature leaves inoculated with E. carotovora showed no necrosis (Figure 7c) or a mild discoloration at the site of inoculation of 108 cells (Figure 7g), and untransformed plants inoculated with 102 cells or higher density displayed obvious necrosis (Figure 7d,h).
Figure 7.
In planta antimicrobial bioassays to investigate functionality of retrocyclin-101 (RC101) and PG1 expressed in chloroplasts. Twenty microliter of the 108, 106, 104 and 102 cells from an overnight culture of Erwinia carotovora was injected into leaves of (a) RC101, (e) PG-1 transplastomic and (b, f) untransformed (UT) plants using a syringe with a precision glide needle. Five- to 7-mm areas of (c) RC101, (g) PG-1 and (d, h) untransformed leaves were scraped with fine-grain sandpaper. Twenty microliter of the 108, 106, 104 and 102 cells of Erwinia was inoculated to each prepared area. Photographs were taken 5 days after inoculation.
The bacterial count in inoculated plants was also estimated. Bacterial suspensions (1.0 × 105 cfu/mL) of E. carotovora were inoculated into transplastomic and untransformed leaves by a syringe. Following inoculation, the density of E. carotovora in untransformed, RC101 and PG-1 transplastomic leaves was less than 1 × 105 cfu/cm2 at 0 day postinoculation. Three days after inoculation, the population of E. carotovora in untransformed tobacco leaves reached 2.0 × 108 cfu/cm2 (Figure 8a,b). In comparison, the density of E. carotovora was less than 1 × 104 cfu/cm2 in both RC101 (Figure 8a) and PG1 (Figure 8b) transplastomic leaves 3 days after inoculation, a 10 000-fold reduction in bacterial burden. In addition, no apparent symptoms of necrosis were observed in any of the RC101 or PG1 plants. These results demonstrated that the RC101 and PG1 transplastomic plants are resistant to E. carotovora. Therefore, RC101 and PG1 maintained their antibacterial activity when expressed in chloroplasts.
Figure 8.
Bacterial density in the PG1, retrocyclin-101 (RC101) and untransformed (UT) plants inoculated with Erwinia carotovora. (a) Bacterial density in RC101 and untransformed leaves. (b) Bacterial density in PG1 and untransformed leaves. The bacterial density in plants on 0, 1 and 3 days after inoculation. All values represent means of six replications with standard deviations shown as error bars.
To determine the antiviral activity of PG1 and RC101 when expressed in tobacco chloroplasts, transplastomic and untransformed control plants were tested for tobacco mosaic virus (TMV) infection for 20 days. In susceptible untransformed control and PG1 plants, TMV multiplied and spread throughout the plants, causing typical mosaic, necrosis and wrinkle symptoms within 20 days after inoculation (Figure 9a,b). However, the RC101 transplastomic plants did not show obvious symptoms of TMV infection, and the plants grew well (Figure 9c). These results confirmed the antiviral activity of RC101 by conferring resistance to TMV when expressed in chloroplasts.
Figure 9.
Response of untransformed and retrocyclin-101 (RC101)/PG1 transplastomic plants to tobacco mosaic virus (TMV). (a) TMV-inoculated leaf from untransformed plant; (b) TMV-inoculated leaf from transplastomic PG1 plant. (c) TMV-inoculated leaf from transplastomic RC101 plant. Pictures were taken on 20 days after inoculation.
Discussion
RC101 and PG1 are antimicrobial peptides that have potent antimicrobial activities against a broad spectrum of microorganisms. Both RC101 and PG1 are disulphide-bonded proteins. RC101 contains three and PG1 contains two intramolecular disulphide bonds that are important for their antimicrobial activities (Harwig et al., 1996; Chen et al., 2000; Trabi et al., 2001; Jenssen et al., 2006). Because RC101 and PG1 are microbicidal and contain multiple disulphide bonds, they have not yet been produced in microbial or cell culture systems. The goal of our study is to produce low-cost and functional RC101 and PG1 antimicrobial peptides in transgenic tobacco chloroplasts.
Our laboratory has previously expressed antimicrobial peptide MSI-99 in transgenic tobacco chloroplasts without harmful effects to transplastomic plants. MSI-99 is an analogue of a naturally occurring peptide (magainin 2) found in the skin of the African frog (Jacob and Zasloff, 1994). In another study, a proteinaceous antibiotic called PlyGBS lysine was expressed in tobacco chloroplasts to high levels (>70% TSP, Oey et al., 2009). The PlyGBS transplastomic plants showed delayed growth and a slightly pale-green phenotype when compared to the untransformed plants. The authors suggested that it was attributable to the exhaustion of protein synthesis capacity of transgenic chloroplasts by the massive over-expression of PlyGBS although expression of >70% TSP of CTB-proinsulin yielded healthy transplastomic plants (Ruhlman et al., 2010). Previously expressed antimicrobial peptides did not contain disulphide bonds, whereas the RC101 and PG1 antimicrobial peptides have β-sheet structures and contain multiple intramolecular disulphide bonds. Therefore, efforts to express RC101 and PG1 in transgenic chloroplasts should further expand the applications of the chloroplast transformation system.
To facilitate expression of small antimicrobial peptides RC101 and PG1 in tobacco chloroplasts, each peptide was translationally fused with the GFP. This also facilitated detection and quantification of RC101-GFP and PG1-GFP in chloroplasts. The expression of GFP fusion proteins was visualized by examination under UV light or in immunoblots using the anti-GFP antibody. ELISA was also performed using anti-GFP antibody to quantify the expression of fusion proteins. Factor Xa protease cleavage site was inserted between RC101 and GFP and the furin cleavage site was inserted between PG1 and GFP, so that they could be cleaved from their fusion proteins by appropriate proteases. It is interesting to note that RC101-GFP protein was already partially cleaved within chloroplasts, suggesting the presence of Factor Xa–like protease activity within chloroplasts.
The smaller green fluorescent peptides observed in RC101 and PG1 lanes in Figure 3b should be the monomer form of RC101-GFP or PG1-GFP. The monomers ran faster than the GFP standard, probably because GFP, when fused with RC101 or PG1, has higher electrophoretic mobility in native gels. Different sizes correspond to the multimers formed by the GFP fusion proteins. GFP protein did not form multimers. Therefore, the formation of multimers by RC101-GFP or PG1-GFP fusion proteins is probably because of folded antimicrobial peptides RC101 or PG1, which are disulphide-bonded proteins. Similar folding pattern has also been observed before when proteins containing multiple disulphide bonds were expressed in chloroplasts, including CTB-proinsulin (Ruhlman et al., 2007) and interferon-α2b (Arlen et al., 2007).
The toxicity of antimicrobial peptides is specific against microbial membranes and therefore can be safely applied to mammals, including human beings. The composition of the membranes is likely to be the determining factor for their selectivity. Biomembranes of prokaryotic or eukaryotic cells differ significantly. Mammalian cytoplasmic membranes are mainly composed of phosphatidylcholine, phosphatidylethanolamine, sphingomyelin and cholesterol, which are all generally neutrally charged. In contrast, in many bacterial pathogens, the membranes are composed predominantly of phosphatidylglycerol (PG), cardiolipin and phosphatidylserine, which are highly electronegative (Yeaman and Yount, 2003). Most antimicrobial peptides, including RC101 and PG1, are positively charged under physiological pH because they are rich in Arginine. Therefore, the net negative charge of the biomembranes makes them the preferred target sites of antimicrobial peptides. The chloroplast envelope and thylakoid membranes predominantly possess three glycolipids: monogalactosyl diacylglycerol (MGDG), digalactosyl diacylglycerol (DGDG) and sulfoquinovosyl diacylglycerol (SQDG), and a sole phospholipid: PG. SQDG and PG, distinct from the noncharged MGDG and DGDG, are negatively charged. However, MGDG makes up 50% of chloroplast membrane lipid and DGDG makes up 30%, suggesting that the major components of chloroplast membranes are neutral. In this study, we examined fresh leaves of RC101 and PG1 transplastomic plants under confocal microscope. Confocal images showed that GFP fusion proteins were contained within chloroplasts and were not released into the cytoplasm. Cationic antimicrobial peptides including RC101 and PG1 kill bacteria by disrupting their membranes. Although the chloroplast membrane structure cannot be resolved from the confocal images shown in Figure 5, no GFP fluorescence was detected outside the chloroplasts, suggesting that chloroplasts are not disrupted.
RC101-GFP and PG1-GFP accumulated up to 32%~38% and 17%~26% of TSP, and they were purified by affinity chromatography or organic extraction method. The results showed that organic extraction resulted in nearly 10-fold higher yield than the affinity chromatography method (53 vs 5 μg/g fresh leaf). PG1 was only purified by affinity chromatography, and the yield was 8 μg/g of fresh leaf. We did not observe dimers or multimers in RC101-GFP or PG1-GFP samples purified by affinity chromatography, which indicated that they were lost during the purification process. The His-tag was not accessible in the dimer or multimer forms of RC101-GFP and PG1-GFP. Therefore, most of the fusion proteins were not bound to the affinity column and lost during purification.
Previous study reported that the minimum inhibitory concentrations of PG-1 against gram-positive or gram-negative bacteria ranged from 0.12 to 2 μg/mL (Steinberg et al., 1997). Retrocyclin (10–20 μg/mL) can inhibit proviral DNA formation and protect human CD4+ lymphocytes from in vitro infection by both T-tropic and M-tropic strains of HIV-1 (Cole et al., 2002). RC101, as low as 2 μg, can prevent HIV-1 infection in an organ-like construct of human cervicovaginal tissue (Cole et al., 2007). In another study, it was reported that Retrocyclin-1, an analogue of RC101, can kill vegetative Bacillus anthracis cells with an minimum effective concentration <1 μg/mL (Wang et al., 2006). As can be seen from these published data, antimicrobial peptides are highly potent and their effective dosage is only few microgram per millilitre. Although our purification yield is relatively low, tobacco can be scaled up to yield up to 40 metric tons of biomass/acre/year. One acre of RC101 transplastomic tobacco plants could potentially yield up to 2 kg of purified RC101 by organic extraction. Therefore, adequate quantities of RC101 or PG1 could be purified from transplastomic plants for preclinical or clinical studies.
RC101 and PG1 are shown to be functional when expressed in chloroplasts. Both RC101 and PG1 protected the transgenic tobacco plants from bacterial infection caused by E. carotovora. In the antiviral assays, RC101 transgenic plants were resistant to TMV infection, but PG1 transgenic plants showed the symptoms of mosaic, necrosis and wrinkle as untransformed plants. Although PG1 has a broad-spectrum antimicrobial activity against bacteria, virus and fungus, it is most effective against bacterial infections, especially antibiotic-resistant bacteria (Kokryakov et al., 1993; Qu et al., 1996; Yasin et al., 1996; Steinberg et al., 1997). In our study, PG1 is not effective in protecting plants from TMV infection. RC101 is an analogue of retrocyclin, and it is especially effective in protecting against viral infections. Several previous studies have shown that RC101 can be used to prevent HIV-1 infection (Cole et al., 2002, 2007). Our study shows that RC101 is active against the retrovirus TMV when expressed in chloroplasts. The antimicrobial activities of RC101 and PG1 can protect plants from phytopathogen infections, which make them good candidates to engineer disease-resistant plants. Because the use of HIV and other human bacterial or viral pathogens requires higher levels of containment than our current facilities, these studies were not performed. Future studies will include testing RC101 and PG1 in suitable animal models against bacterial or viral pathogens.
Experimental procedures
Construction of chloroplast transformation vectors
The 6xHis-Factor Xa-RC101 sequence was synthesized by Klenow fragment, and it was flanked by EcoRV and NotI restriction sites. The oligomers used were the following: C2Fwd (5′-GATATCCATCATCATCATCATCATATCGAAGGCCGCGGTATTTGTAGATGTATTTGTGGTAAAGGTATTT-3′) and C2Rev (3′-CGGCGCCATAAACATCTACATAAAC ACCATTTCCATAAACATCTACATAAACACCATCTATTCGCCGGCG-5′ or 5′- GCGGCCGCTTATCTACCACAAATACATCTACAAATACCTTTACCACAAATACATCTACAAATACCGCGGC-3′). Soluble modified GFP (sm-GFP) protein was cloned into the pGEM-T vector. The 6xHis-Factor Xa-RC101 sequence was cleaved by EcoRV and NotI and subcloned into the pGEM-GFP vector. Then GFP-6xHis-Factor Xa-RC101 was digested by NdeI (partial) and NotI and subcloned into the pLD vector (Daniell et al., 1998, 2001).
The EcoRV-Furin-PG1-NotI sequence was synthesized by Klenow fragment. The oligomers used were the following: EcoRV-Start Codon-Furin-PG1 (5′-GTC-GATATC-ATG-GGCCAAAAACGAAGGGGAGGTCGCCTGTGCTATTGTAGGCGTAGGTTCTGCGTCTGT) and NotI-stop codon-reverse PG1 (5′-GCA-GCGGCCGC-TCA-TCCTCGTCCGACACAGACGCAGAACCTACGCCTACAATAGCACAGGCGACCTCCCCT-3′). A 6xHis tag was introduced by PCR to the 5′ end of smGFP sequence and the PCR products were then cloned into the pGEM-T vector. The synthesized Furin-PG1 gene sequence was digested by EcoRV and NotI and then inserted into the 3′ end of 6xHis-smGFP sequence in the pGEM-T vector. The 6xHis-smGFP-Furin-PG1 sequence was digested by the NdeI (partial) and NotI enzymes and subcloned into the pLD vector.
Bombardment and selection of transplastomic plants
Sterile tobacco leaves were bombarded using the Bio-rad PDS 1000/He biolistic device as described previously (Verma et al., 2008). Bombarded leaves were then subjected to three rounds of selection. First two rounds of selection were performed on the regeneration medium of plants, and the third round of selection was on Murashige and Skoog medium without hormone medium. All these were supplemented with 500 mg/L of spectinomycin. After selection, RC101 and PG1 transplastomic shoots were transferred to pots in the greenhouse.
PCR analysis to confirm transplastomic plants
Total plant DNA was isolated from transplastomic tobacco leaves using the DNeasy Plant Mini Kit from Qiagen (Valencia, CA, USA). PCR was set up with two pairs of primers, 3P-3M and 5P-2M (Verma et al., 2008), to confirm the successful transformation of tobacco chloroplasts. The 3P primer (AAAACCCGTCCTCAGTTCGGATTGC) anneals with the native chloroplast genome, and 3M primer (CCGCGTTGTTTCATCAAGCCTTACG) anneals with the aadA gene. Therefore, this pair of primers was used to check site-specific integration of selectable marker genes into the chloroplast genome. The 5P primer (CTGTAGAAGTCACCATTGTTGTGC) anneals with the aadA gene, and 2M primer (TGACTGCCCACCTGAGAGCGGACA) anneals with the trnA gene, which were used to check integration of the transgene expression cassette.
Southern blot to confirm homoplasmy
Total plant DNA was digested with ApaI enzyme and then separated on a 0.8% agarose gel. After electrophoresis, the gel was soaked in 0.25N HCl depurination solution for 15 min and then rinsed twice in water, 5 min each. After that, the gel was soaked in transfer buffer (0.4 N NaOH, 1 m NaCl) for 20 min, and then the dry transfer was set up. After transfer, the membrane was rinsed with 2 × SSC twice for 5 min each. After the membrane was dry, it was cross-linked using GS GeneLinker UV Chamber at C3 setting. The 0.81-kbp flanking sequence probe was prepared by digesting pUC-CT vector with BamHI and BglII (Figure 1a). After the probe was labelled with 32P, hybridization of the membrane was performed using Stratagene QUICK-HYB hybridization solution and protocol (Stratagene, La Jolla, CA, USA).
Factor Xa and furin cleavage assays
RC101 tobacco transplastomic leaves (100 mg) were ground in liquid nitrogen and homogenized in 200 μL of plant extraction buffer (0.1 N NaOH, 1 m Tris–HCl, pH 4.5) using a mechanical mixer. The homogenized plant extract was then centrifuged for 5 min at 16 000 g at 4 °C. The extract (10 μg) was then incubated with 1 μ of Factor Xa protease in 20 mm Tris–HCl (pH 8.0 @ 25 °C) with 100 mm NaCl and 2 mm CaCl2 overnight at 23 °C. The cleaved products were loaded with uncleaved RC101 protein extracts on the same gel to investigate cleavage of RC101-GFP fusion protein. Western blot analysis was performed as described.
Total protein from the PG1-GFP transplastomic tobacco leaves was extracted the same way as RC101-GFP described earlier. The extract (10 μg) from PG1-GFP transplastomic tobacco leaves was incubated with 1 unit of furin in a total reaction volume of 25 μL containing 100 mm Hepes (pH 7.5, 25 °C), 0.5% Triton X-100, 1 mm CaCl2 and 1 mm 2-mercaptoethanol at 25 °C.
Native polyacrylamide gel electrophoresis and densitometric analysis
Total protein from the RC101-GFP and PG1-GFP transplastomic plants was extracted as described earlier. The TSP concentration was determined by the Bradford assay, and then different amount of TSP was loaded with native gel loading buffer (60 mm pH 6.8 Tris–HCl, 25% glycerol and 0.01% Bromophenol blue) into the 12% native polyacrylamide gel. After electrophoresis, the gel was scanned and analysed for the presence of GFP fusion proteins using AlphaImager® and AlphaEase® FC software (Alpha Innotech, San Leandro, CA, USA). The IDVs of the GFP standards and samples were recorded and analysed further.
Western blot analysis
Frozen leaf materials (100 mg) were ground in liquid nitrogen and then resuspended in 200 μL of plant extraction buffer. The supernatant was collected after centrifuging the sample for 5 min at 14 000 rpm. The plant extract was mixed with 2× sample loading buffer and then boiled for 5 min before loading. The transformed and untransformed plant extracts and recombinant GFP standard (Vector Labs, Burlingame, CA, USA) were loaded onto the 12% SDS-PAGE gel. The proteins in the gel were then transferred to the nitrocellulose membrane at 100 V for 1 h. After transfer, the membrane was first blocked in PTM (1X PBS, 0.1% Tween-20, 3% milk) for 2 h at room temperature and then incubated with chick anti-GFP primary antibody (Chemicon, Billerica, MA, USA) at 1 : 3000 dilution in PTM for 2 h at room temperature. After the membrane was washed 3 times with PBS-T (1X PBS, 0.1% Tween-20), 5 min each time, rabbit anti-chick secondary antibody conjugated with HRP was added at 1 : 3000 dilution in PTM and then incubated for 1 h at room temperature.
Dot blot assay
The Immobilon-P (PVDF) membrane was prewet in methanol for 1–2 min, rinsed twice with TBS (500 mm NaCl, 20 mm Tris–HCl pH 7.5) and soaked in TBS until use. Protein extracts from RC101 transplastomic line and standards (0.25–8 ng of RC101 peptides) were resuspended in 0.1% acetic acid and then dotted onto an PVDF membrane. Once the last dot was soaked in, the membrane was placed in fixation buffer (0.05% glutaraldehyde in 1X TBS) and rocked on the orbital shaker at room temperature for 20 min. The membrane was blocked for 30 min at 37 °C using Superblock (Pierce, Rockford, IL, USA) and then incubated overnight with anti-RC101 polyclonal antisera (Invitrogen custom antibody service, Carlsbad, CA, USA) diluted 1 : 2000 in antibody buffer (Superblock diluted 1 : 3 in TBS containing 0.05% Tween-20 and 0.01% thimerosal). After washing twice and blocked again for 15 min, the membrane was incubated with peroxidaseconjugated anti-rabbit immunoglobulin G for 1 h. After washing, the membrane was developed with Immun-Star HRP (Bio-Rad, Hercules, CA, USA). Images were captured and analysed using the Bio-Rad ChemiDoc system.
PG-1 furin cleavage assay and silver staining
After furin digestion, PG1 was cleaved off from GFP. Because of nonavailability of PG1 antibody, we used silver staining to investigate the presence of the 2.1 kDa PG1 protein after furin cleavage. The cleaved products of PG1-GFP fusion protein were separated in a 16.8% tris–tricine gel to get the maximum resolution in the ≤10 kDa range. Untransformed plant extracts, Marker 12 unstained standard (Invitrogen), PG1-GFP plant protein extracts before and after furin digestion were mixed with sample loading buffer and loaded on the 16.8% gel. After electrophoresis, the gel was stained by silver staining.
Confocal microscopy
Untransformed, RC101-GFP and PG1-GFP transplastomic tobacco leaves were harvested fresh before microscopic analysis. They were cut into 5 × 5 mm small pieces and fixed on slides. Confocal microscope (Olympus FluoView, Center Valley, PA, USA) with adjustable bandwidths of the detected fluorescence wavelength was used. The filter used was 505–525 nm. GFP fluorescence from the samples was detected and saved as digital format files.
ELISA quantification of RC101-GFP and PG1-GFP fusion proteins
All untransformed, transplastomic plant protein extracts (all the extracts used here were the same as used in Bradford assay) and recombinant GFP standard (Vector Laboratories, Burlingame, CA, USA, MB-0752) were diluted using the ELISA coating buffer (15 mm Na2CO3, 35 mm NaHCO3, pH 9.6). The recombinant GFP standard was serially diluted from 100 to 3.125 ng/mL. Different dilutions of test samples were prepared ranging from 1 : 1000 to 1 : 9000. The wells of a 96-well microtiter EIA plate were coated with 100 μL of diluted test samples and standards. The plate was covered with an adhesive plastic and incubated for 2 h at room temperature. After incubation, the coating solution was removed and the plate was washed twice by filling the wells with 200 μL PBS and once by water. The coated wells were blocked by adding 200 μL of blocking buffer (3% dry milk in PBS). Then, the plate was covered and incubated for 2 h at room temperature. After removing the blocking buffer, the plate was washed again as described before. Mouse anti-GFP IgG monoclonal antibody (Chemicon, MAB3836) at 1 : 2000 dilution was added and incubated for 2 h at room temperature. After washing twice with PBS and once with water, HRP-conjugated goat anti-mouse IgG antibody (American Qualex, San Clemente, CA, USA) at 1 : 2000 dilution was added and incubated for 2 h at room temperature. After washing, the plate was developed with TMB (3, 3′, 5, 5′—Tetramethylbenzidine). The absorbance of each well was read with a microplate reader (Bio-rad, model 680).
Purification of RC101-GFP and PG1-GFP fusion proteins by affinity chromatography or organic extraction
Fresh leaves (10 g) were ground in liquid nitrogen. Lysis buffer (10 mm Imidazole (pH 8.0), 50 mm Na/K Phosphate buffer, 20 mm Tris–HCl, 300 mm NaCl) (75–80 mL) and one tablet of pro-tease inhibitor cocktail (Roche-Complete, EDTA-free) were added to the ground leaf powder. The sonicated sample was centrifuged at 75 000 g for 1 h at 10 °C. The supernatant was filtered using a Mira cloth to remove debris and loaded onto the column.
The sample lines of the AKTA-3D FPLC were primed and purged before loading the samples at the rate of 3 mL/min. The fraction size was 2.5 mL. The samples were subjected to affinity chromatography, and the elution was performed at 100% gradient, which was 250 mm imidazole. The peak at the right wavelength (498 nm) was noted, and all the fractions comprising that peak were taken. The purified proteins were then separated on the native PAGE gel. The gel was stained by coomassie staining and viewed directly.
The organic extraction protocol described by Skosyrev et al. (2003) was used. Saturated ammonium sulphate (pH 7.8) was added to a final saturation of 70% to the plant protein extract. The entire suspension was extracted twice with a one-fourth and a 1/16th volume of ethanol by vigorous shaking for 1 min. After centrifugation, both ethanol phases were collected carefully to avoid disturbance of the interphase. A one-fourth volume of n-butanol was added to the combined ethanol extract. After vigorous shaking and centrifugation, the lower phase containing fusion protein was carefully collected. Lower phase was adjusted to 20% saturation of ammonium sulphate and loaded directly to a column with Butyl-Toyopearl equilibrated with 20% ammonium sulphate in 10 mm Tris–HCl, pH 7.8. After washing with the equilibration buffer, protein was eluted with salt-free 10 mm Tris–HCl, pH 7.8.
In planta assay for resistance to Erwinia soft rot
To investigate bacterial resistance of RC101 and PG-1 transplastomic line, untransformed control and transplastomic leaves were inoculated with bacterial suspension culture. E. carotovora strain was obtained from Dr. Jerry Bartz's laboratory (University of Florida, Gainesville) and grown for 24 h at 25 °C in 5 mL of nutrient broth (NB) medium (Difco, Lawrence, KS, USA). Different dilutions of bacteria were prepared. Five- to 7-mm areas of greenhouse-grown untransformed, RC101 and PG-1 transplastomic tobacco leaves were scraped with fine-grain sandpaper, and 20 μL of 108, 106, 104 and 102 of Erwinia cells was inoculated to each prepared area. In a parallel study, 20 μL of 108, 106, 104 and 102 of Erwinia cells was injected into leaves of untransformed, RC101 and PG-1 transplastomic tobacco using a syringe with a precision glide needle. Photographs were taken 5 days after inoculation.
Erwinia carotovora inoculation and analysis
The leaves of untransformed and transplastomic tobacco plants were inoculated with 20 μL of bacterial suspension (1.0 × 105 cfu/mL) through a syringe. Each leaf disc (0.8 cm diameter) was punched off from the inoculated area of an individual plant after 0, 1 or 3 days of incubation. The bacterial population inside the leaf was calculated as follows. Leaf tissue was ground in 100 μL sterilized water in a microcentrifuge tube. The suspension was serially diluted with sterilized water and was then plated on nutrient broth agar plates (Difco). Colonies were counted after 1 day of incubation at 25 °C.
Tobacco Mosaic Virus (TMV) inoculation and analysis
Full-length infectious TMV RNA transcripts were generated by in vitro transcription of KpnI-linearized Klenow-filled pTMV004 vector (Obtained from Prof. William Dawson, University of Florida Citrus Research and Education Center, Lake Alfred) using T7 RNA polymerase (Promega, Madison, WI, USA), as described before (Dinesh-Kumar and Baker, 2000). In vitro-generated TMV transcripts were rub-inoculated onto tobacco plants, and infected leaves were harvested 14 days after inoculation and re-inoculated onto tobacco plants for virus multiplication. The inoculum for plant infection was prepared by grinding infected TMV-sensitive tobacco leaf tissues in 10 mm sodium phosphate buffer, pH 7.0. The leaf sap with virus was then injected into the main veins of 4 to 5-week-old PG1, RC101 transplastomic and untransformed tobacco plant leaves using a syringe. Plants were evaluated for the development of symptoms to TMV infection for 20 days after inoculation.
Acknowledgements
Investigations reported here were supported by NIH R01 GM 63879 and USDA 3611-21000-021-02S grants to Henry Daniell. Authors are thankful to Dr. Alexander Cole and his laboratory colleagues for providing data on Figure 4a and helpful discussions, Dr. Jerry Bartz (University of Florida, Gainesville) for providing Erwinia carotovora culture and Dr. William Dawson (Citrus Research and Education Center, UF, Lake Alfred) for providing the cDNA clone of TMV.
References
- Arlen PA, Falconer R, Cherukumilli S, Cole A, Cole AM, Oishi KK, Daniell H. Field production and functional evaluation of chloroplast-derived interferon-alpha2b. Plant Biotechnol. J. 2007;5:511–525. doi: 10.1111/j.1467-7652.2007.00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlen PA, Singleton M, Adamovicz JJ, Ding Y, voodi-Semiromi A, Daniell H. Effective plague vaccination via oral delivery of plant cells expressing F1-V antigens in chloroplasts. Infect. Immun. 2008;76:3640–3650. doi: 10.1128/IAI.00050-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bally J, Paget E, Droux M, Job C, Job D, Dubald M. Both the stroma and thylakoid lumen of tobacco chloroplasts are competent for the formation of disulphide bonds in recombinant proteins. Plant Biotechnol. J. 2008;6:46–61. doi: 10.1111/j.1467-7652.2007.00298.x. [DOI] [PubMed] [Google Scholar]
- Bals R. Epithelial antimicrobial peptides in host defense against infection. Respir. Res. 2000;1:141–150. doi: 10.1186/rr25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman HG. Peptide antibiotics and their role in innate immunity. Annu. Rev. Immunol. 1995;13:61–92. doi: 10.1146/annurev.iy.13.040195.000425. [DOI] [PubMed] [Google Scholar]
- Broekaert WF, Cammue BPA, De Bolle MFC, Thevissen K, De Samblanx GW, Osborn RW. Antimicrobial peptides from plants. Crit. Rev. Plant Sci. 1997;16:297–323. [Google Scholar]
- Chebolu S, Daniell H. Stable expression of Gal/GalNAc lectin of Entamoeba histolytica in transgenic chloroplasts and immunogenicity in mice towards vaccine development for amoebiasis. Plant Biotechnol. J. 2007;5:230–239. doi: 10.1111/j.1467-7652.2006.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Falla TJ, Liu H, Hurst MA, Fujii CA, Mosca DA, Embree JR, Loury DJ, Radel PA, Cheng CC, Gu L, Fiddes JC. Development of protegrins for the treatment and prevention of oral mucositis: structure-activity relationships of synthetic protegrin analogues. Biopolymers. 2000;55:88–98. doi: 10.1002/1097-0282(2000)55:1<88::AID-BIP80>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Cole AM, Hong T, Boo LM, Nguyen T, Zhao C, Bristol G, Zack JA, Waring AJ, Yang OO, Lehrer RI. Retrocyclin: a primate peptide that protects cells from infection by T- and M-tropic strains of HIV-1. Proc. Nat. Acad. Sci. 2002;99:1813–1818. doi: 10.1073/pnas.052706399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole AL, Herasimtschuk A, Gupta P, Waring AJ, Lehrer RI, Cole AM. The retrocyclin analogue RC-101 prevents human immunodeficiency virus type 1 infection of a model human cervicovaginal tissue construct. Immunology. 2007;121:140–145. doi: 10.1111/j.1365-2567.2006.02553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell H, Datta R, Varma S, Gray S, Lee SB. Containment of herbicide resistance through genetic engineering of the chloroplast genome. Nat. Biotechnol. 1998;16:345–348. doi: 10.1038/nbt0498-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell H, Lee SB, Panchal T, Wiebe PO. Expression of the native cholera toxin B subunit gene and assembly as functional oligomers in transgenic tobacco chloroplasts. J. Mol. Biol. 2001;311:1001–1009. doi: 10.1006/jmbi.2001.4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell H, Chebolu S, Kumar S, Singleton M, Falconer R. Chloroplast-derived vaccine antigens and other therapeutic proteins. Vaccine. 2005;23:1779–1783. doi: 10.1016/j.vaccine.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Daniell H, Ruiz G, Denes B, Sandberg L, Langridge W. Optimization of codon composition and regulatory elements for expression of human insulin like growth factor-1 in transgenic chloroplasts and evaluation of structural identity and function. BMC. Biotechnol. 2009;9:33. doi: 10.1186/1472-6750-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell H, Singh ND, Mason H, Streatfield SJ. Plant-made vaccine antigens and biopharmaceuticals. Trends Plant Sci. 2009;14:669–679. doi: 10.1016/j.tplants.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoodi-Semiromi A, Samson N, Daniell H. The green vaccine: a global strategy to combat infectious and autoimmune diseases. Hum. Vaccin. 2009;5:488–493. doi: 10.4161/hv.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGray G, Rajasekaran K, Smith F, Sanford J, Daniell H. Expression of an antimicrobial peptide via the chloroplast genome to control phytopathogenic bacteria and fungi. Plant Physiol. 2001;127:852–862. [PMC free article] [PubMed] [Google Scholar]
- Dinesh-Kumar SP, Baker BJ. Alternatively spliced N resistance gene transcripts: their possible role in tobacco mosaic virus resistance. Proc. Natl. Acad. Sci. U S A. 2000;97:1908–1913. doi: 10.1073/pnas.020367497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farran I, Rio-Manterola F, Iniguez M, Garate S, Prieto J, Mingo-Castel AM. High-density seedling expression system for the production of bioactive human cardiotrophin-1, a potential therapeutic cytokine, in transgenic tobacco chloroplasts. Plant Biotechnol. J. 2008;6:516–527. doi: 10.1111/j.1467-7652.2008.00334.x. [DOI] [PubMed] [Google Scholar]
- Fernandez-San MA, Mingo-Castel A, Miller M, Daniell H. A chloroplast transgenic approach to hyper-express and purify Human Serum Albumin, a protein highly susceptible to proteolytic degradation. Plant Biotechnol. J. 2003;1:71–79. doi: 10.1046/j.1467-7652.2003.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-San MA, Ortigosa SM, Hervas-Stubbs S, Corral-Martinez P, Segui-Simarro JM, Gaetan J, Coursaget P, Veramendi J. Human papillomavirus L1 protein expressed in tobacco chloroplasts self-assembles into virus-like particles that are highly immunogenic. Plant Biotechnol. J. 2008;6:427–441. doi: 10.1111/j.1467-7652.2008.00338.x. [DOI] [PubMed] [Google Scholar]
- Glenz K, Bouchon B, Stehle T, Wallich R, Simon MM, Warzecha H. Production of a recombinant bacterial lipoprotein in higher plant chloroplasts. Nat. Biotechnol. 2006;24:76–77. doi: 10.1038/nbt1170. [DOI] [PubMed] [Google Scholar]
- Hancock REW, Chapple DS. Peptide antibiotics. Antimicrob. Agents Chemother. 1999;43:1317–1323. doi: 10.1128/aac.43.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwig SS, Waring A, Yang HJ, Cho Y, Tan L, Lehrer RI. Intramolecular disulfide bonds enhance the antimicrobial and lytic activities of protegrins at physiological sodium chloride concentrations. Eur. J. Biochem. 1996;240:352–357. doi: 10.1111/j.1432-1033.1996.0352h.x. [DOI] [PubMed] [Google Scholar]
- Jacob L, Zasloff M. Potential therapeutic applications of magainins and other antimicrobial agents of animal origin. Ciba Found. Symp. 1994;186:197–216. doi: 10.1002/9780470514658.ch12. [DOI] [PubMed] [Google Scholar]
- Jenssen H, Hamill P, Hancock REW. Peptide antimicrobial agents. Clin. Microbiol. Rev. 2006;19:491–511. doi: 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokryakov VN, Harwig SSL, Panyutich EA, Shevchenko AA, Aleshina GM, Shamova OV, Korneva HA, Lehrer RI. Protegrins: leukocyte antimicrobial peptides that combine features of corticostatic defensins and tachyplesins. FEBS Lett. 1993;327:231–236. doi: 10.1016/0014-5793(93)80175-t. [DOI] [PubMed] [Google Scholar]
- Koya V, Moayeri M, Leppla SH, Daniell H. Plant-based vaccine: mice immunized with chloroplast-derived anthrax protective antigen survive anthrax lethal toxin challenge. Infect. Immun. 2005;73:8266–8274. doi: 10.1128/IAI.73.12.8266-8274.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leelavathi S, Reddy VS. Chloroplast expression of His-tagged GUS-fusions: a general strategy to overproduce and purify foreign proteins using transplastomic plants as bioreactors. Mol. Breeding. 2003;11:49–58. [Google Scholar]
- Manuell AL, Beligni MV, Elder JH, Siefker DT, Tran M, Weber A, McDonald TL, Mayfield SP. Robust expression of a bioactive mammalian protein in Chlamydomonas chloroplast. Plant Biotechnol. J. 2007;5:402–412. doi: 10.1111/j.1467-7652.2007.00249.x. [DOI] [PubMed] [Google Scholar]
- Mayfield SP, Franklin SE, Lerner RA. Expression and assembly of a fully active antibody in algae. Proc. Natl. Acad. Sci. U S A. 2003;100:438–442. doi: 10.1073/pnas.0237108100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina A, Hervas-Stubbs S, Daniell H, Mingo-Castel AM, Veramendi J. High-yield expression of a viral peptide animal vaccine in transgenic tobacco chloroplasts. Plant Biotechnol. J. 2004;2:141–153. doi: 10.1046/j.1467-7652.2004.00057.x. [DOI] [PubMed] [Google Scholar]
- Nadai M, Bally J, Vitel M, Job C, Tissot G, Botterman J, Dubald M. High-level expression of active human alpha1-antitrypsin in transgenic tobacco chloroplasts. Transgenic Res. 2009;18:173–183. doi: 10.1007/s11248-008-9209-0. [DOI] [PubMed] [Google Scholar]
- Nicolas P, Mor A. Peptides as weapons against microorganisms in the chemical defense system of vertebrates. Annu. Rev. Microbiol. 1995;49:277–304. doi: 10.1146/annurev.mi.49.100195.001425. [DOI] [PubMed] [Google Scholar]
- Nizet V. Antimicrobial peptide resistance mechanisms of human bacterial pathogens. Curr. Issues Mol. Biol. 2006;8:11–26. [PubMed] [Google Scholar]
- Oey M, Lohse M, Kreikemeyer B, Bock R. Exhaustion of the chloroplast protein synthesis capacity by massive expression of a highly stable protein antibiotic. Plant J. 2009;57:436–445. doi: 10.1111/j.1365-313X.2008.03702.x. [DOI] [PubMed] [Google Scholar]
- Qu XD, Harwig SS, Oren AM, Shafer WM, Lehrer RI. Susceptibility of Neisseria gonorrhoeae to protegrins. Infect. Immun. 1996;64:1240–1245. doi: 10.1128/iai.64.4.1240-1245.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhlman T, Ahangari R, Devine A, Samsam M, Daniell H. Expression of cholera toxin B-proinsulin fusion protein in lettuce and tobacco chloroplasts – oral administration protects against development of insulitis in non-obese diabetic mice. Plant Biotechnol. J. 2007;5:495–510. doi: 10.1111/j.1467-7652.2007.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhlman T, Verma D, Samson N, Daniell H. The role of heterologous chloroplast sequence elements in transgene integration and expression. Plant Physiol. 2010;152:2088–2104. doi: 10.1104/pp.109.152017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller-Bals S, Schulze A, Bals R. Increased levels of antimicrobial peptides in tracheal aspirates of newborn infants during infection. Am. J. Respir. Crit. Care Med. 2002;165:992–995. doi: 10.1164/ajrccm.165.7.200110-020. [DOI] [PubMed] [Google Scholar]
- Skosyrev VS, Rudenko NV, Yakhnin AV, Zagranichny VE, Popova LI, Zakharov MV, Gorokhovatsky AY, Vinokurov LM. EGFP as a fusion partner for the expression and organic extraction of small polypeptides. Protein Expr. Purif. 2003;27:55–62. doi: 10.1016/s1046-5928(02)00595-8. [DOI] [PubMed] [Google Scholar]
- Staub JM, Garcia B, Graves J, Hajdukiewicz PT, Hunter P, Nehra N, Paradkar V, Schlittler M, Carroll JA, Spatola L, Ward D, Ye G, Russell DA. High-yield production of a human therapeutic protein in tobacco chloroplasts. Nat. Biotechnol. 2000;18:333–338. doi: 10.1038/73796. [DOI] [PubMed] [Google Scholar]
- Steinberg DA, Hurst MA, Fujii CA, Kung AH, Ho JF, Cheng FC, Loury DJ, Fiddes JC. Protegrin-1: a broad-spectrum, rapidly microbicidal peptide with in vivo activity. Antimicrob. Agents Chemother. 1997;41:1738–1742. doi: 10.1128/aac.41.8.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YQ, Yuan J, Sapay G, Sapay K, Tran D, Miller CJ, Ouellette AJ, Selsted ME. A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated -defensins. Science. 1999;286:498–502. doi: 10.1126/science.286.5439.498. [DOI] [PubMed] [Google Scholar]
- Tissot G, Canard H, Nadai M, Martone A, Botterman J, Dubald M. Translocation of aprotinin, a therapeutic protease inhibitor, into the thylakoid lumen of genetically engineered tobacco chloroplasts. Plant Biotechnol. J. 2008;6:309–320. doi: 10.1111/j.1467-7652.2008.00321.x. [DOI] [PubMed] [Google Scholar]
- Trabi M, Schirra HJ, Craik DJ. Three-dimensional structure of RTD-1, a cyclic antimicrobial defensin from rhesus macaque leukocytes. Biochemistry. 2001;40:4211–4221. doi: 10.1021/bi002028t. [DOI] [PubMed] [Google Scholar]
- Tregoning JS, Nixon P, Kuroda H, Svab Z, Clare S, Bowe F, Fairweather N, Ytterberg J, van Wijk KJ, Dougan G, Maliga P. Expression of tetanus toxin fragment C in tobacco chloroplasts. Nucleic Acids Res. 2003;31:1174–1179. doi: 10.1093/nar/gkg221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma D, Samson NP, Koya V, Daniell H. A protocol for expression of foreign genes in chloroplasts. Nat. Protoc. 2008;3:739–758. doi: 10.1038/nprot.2007.522. [DOI] [PubMed] [Google Scholar]
- Wang W, Mulakala C, Ward SC, Jung G, Luong H, Pham D, Waring AJ, Kaznessis Y, Lu WY, Bradley KA, Lehrer RI. Retrocyclins kill bacilli and germinating spores of Bacillus anthracis and inactivate anthrax lethal toxin. J. Biol. Chem. 2006;281:32755–32764. doi: 10.1074/jbc.M603614200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Brandsma M, Tremblay R, Maxwell D, Jevnikar AM, Huner N, Ma S. A novel expression platform for the production of diabetes-associated autoantigen human glutamic acid decarboxylase (hGAD65). BMC Biotechnol. 2008;8:87–90. doi: 10.1186/1472-6750-8-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J, Koya V, Leppla SH, Daniell H. Expression of Bacillus anthracis protective antigen in transgenic chloroplasts of tobacco, a non-food/feed crop. Vaccine. 2004;22:4374–4384. doi: 10.1016/j.vaccine.2004.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Chertov O, Oppenheim JJ. The role of mammalian antimicrobial peptides and proteins in awakening of innate host defenses and adaptive immunity. Cell. Mol. Life Sci. 2001;58:978–989. doi: 10.1007/PL00000914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasin B, Harwig SS, Lehrer RI, Wagar EA. Susceptibility of Chlamydia trachomatis to protegrins and defensins. Infect. Immun. 1996;64:709–713. doi: 10.1128/iai.64.3.709-713.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman MR, Yount NY. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 2003;55:27–55. doi: 10.1124/pr.55.1.2. [DOI] [PubMed] [Google Scholar]