Abstract
Background:
Staphylococcus aureus has been recognized as a major human pathogen and is the major cause of nosocomial infections. Gamma-toxin, leukocidin and other bi-component toxins are a family of proteins encoded by the hlg and luk-PV, respectively. Panton-Valentine leukocidin (PVL) is an example of these toxins and causes leukocyte destruction and tissue necrosis. The aim of this study was to determine the prevalence of bi-component leukocidin in Methicillin Resistant Staphylococcus aureus (MRSA) isolates in staphylococcal infections.
Methods:
Collectively, 143 isolates of S. aureus were obtained from Tehran University of Medical Sciences hospitals and confirmed with biochemical tests. Then polymerase chain reaction was used to detect luk-PV loci and luk-E/D. Coagulase gene was used as internal control. The antibiotic susceptibility patterns of isolates were determined using disk diffusion method.
Results:
Out of 149 S. aureus isolates 24.2% were luk-PV positive and 73.8% were luk-E/D positive.
Conclusion:
There was PVL positive MRSA isolates with high prevalence in evaluated hospitals. The diseases from these bacteria are with extensive necrosis, leucopenia and even death. We desire that, prevent from progress and death by diagnosis and right treatment.
Keywords: Bi-component leukocidin, Methicillin-resistant Staphylococcus aureus, Staphylococcal infections, Iran
Introduction
Methicillin-Resistant Staphylococcus aureus (MRSA) is the most disease generating strain of staphylococci and is the most prevalent pathogen isolated from hospitalized patients (1); it is also resistant to a number of antibiotics classes because mecA induces methicilin resistance in the bacteria (2, 3). It causes a wide range of diseases from mild infections of the skin and soft tissue to life threatening pneumonia and poisonings like toxic shock syndrome (4).
S. aureus has several virulent factors and produces many toxins that are associated with pathogenicity, as well. Some of the exotoxins have the capacity to phagocyte destruction, particularly, polymorphonuclear (PMN) and monocytes. These exotoxins belong to the family of bi-component leukotoxins including S and F proteins (5).These proteins are transcribed by two adjacent and contra scribed genes, encoded and carried on a temperate bacteriophage (6). The mentioned proteins function synergically to create pores on the membrane of the phagocytes (5). These dimeric molecules which are called synergohymenotropic toxins assemble on the neutrophil membrane to create an octameric structure and open calcium channels by providing pores. The secretion of cytolytic enzymes and the production of super oxidize ions can also lead to tissue necrosis (7). The family of the staphylococcus leukotoxins includes Panton-Valentine leukocidin (PVL), γ Hemolysin (HlgA+HlgB+HlgC), LUKM (LUKM-PV+LUKF-PV) and LUKE/D (LUKE+LUKD) (8–11).
Currently, S. aureus carrying Panton-Valentine leukocidin has turned into a serious global problem. Although cutaneous infections and relapsing abscess are usually originated by the S. aureus, it is now ten years that PVL positive strains has caused the increase in pneumonia prevalence among the previously healthy youth; this is accompanied by a high mortality and morbidity rate (12). The prevalence of acute cutaneous infections has been observed among the American school children due to these isolates (13). Similarly, PVL-related cutaneous infections have also been reported among Dutch homosexuals (14), Switzerland schoolchildren (15) and the Scottish hospital staff (16). Today, the increasing number of reports on the PVL positive strains related to necrotizing pneumonia acquired from the community has caused a lot of concerns (5).
Lina et al. (1999) described the relationship between the presence of PVL- positive strains, pneumonia and cutaneous infections (17). Moreover, it was proved in other studies that PVL can be found in both MRSA and MSSA strains and that the possibility of PVL production by CA-MRSA is higher than HA-MRSA (18). Studies on the methicilin-resistant S. aureus (MRSA) isolates carrying PVL genes have also been carried out in hospitals of Florida (19), Germany (20), France (21), Minnesota (22), Latvia (23), Austria (24), Belgium (25), the Netherlands (26) and Middle Tennessee (27). In the present study, using PCR assay, the presence of Panton-Valentine leukocidin (PVL) and leukocidin (LUKE/D) genes in various infections caused by S. aureus is evaluated and the MRSA or MSSA nature of the isolates was determined.
The aim of this study was to determine the frequency of bi-component leukocidins in MRSA and MSSA isolates for epidemiological purposes and determine the relation of these genes with S. aureus infections.
Materials and Methods
Bacterial isolates
Collectively 143 isolates of S. aureus were obtained from Tehran University of Medical Sciences hospitals (Children s Medical Center, Shariati, Sina, Loghman, Imam Khomeini). These isolates were transferred to microbiology laboratory of School of Public Health and subcultured on blood agar. Then all of these isolates were reconfirmed with biochemical tests (Coagulase, Manitol fermentation and DNase tests).
Genomic DNA extraction
DNA was isolated by using genomic DNA extraction kit (Bioneer Inc, Korea) as recommended by the manufacturer, with the modification that 1.5 λ lysostaphin was added to bacterial suspension. Finally the genomic DNA extraction was used as the template for PCR.
PCR analysis
A collection of 149 isolates were screened for the presence of pvl and luke/d genes and coa gene as an amplification internal control by PCR, using previously described primers (17). Primers used in this study were as follow 5′CGAGACCAAGATTCAATAAC 3′ as forward and 5′ AAAGAAAACCACTCACA TC ACA 3′ as reverse. These primers sequences correspond to 900bp of coa gene. Amplification of the pvl gene was performed as a single PCR with a forward 5′ATCATTAGGTAAAA TGT CTGCACATGATCCA3′, and reverse 5′GCA TCAASTGTATTGGATAGCCAAAAGC3′ and of luke/d gene with a forward 5′ ATT CCATAGCATAAGCACTGC 3′ ,and reverse 5 ′ TGAAAAACCTTCAAAGTTGAT ACC AG 3′ primers as described before(17). These primers sequences correspond to 433bp of pvl gene and 269bp of luke/d gene respectively. In this study Staphylococcus aureus strain, NCTC 13300, was used as positive control and distilled water as a negative control for PCR. DNA amplification was performed on an Ependorf cycler in a final volume of 50 μl containing 10 μl of 10X PCR Buffer, 3.5 μl of Mgcl2(10mM), 0.2 mM dNTPmix, 20 ρM of each primer,1U of Taq polymerase and 4 μl of template DNA. Amplification was carried out with first denaturation at 97 °C for 6min (First denaturation) followed by 35 cycles according to the following program: denaturation at 92 °C for 30 S, annealing at 55 °C for 30S,and extension at 72 °C for 45S, plus a final extension at 72 °C for 10 min to complete partial polymerization.
Detection of PCR products
The PCR products were resolved by electrophoresis through a 1/5% agarose gel containing Ethidium bromide.
Antimicrobial susceptibility testing
Susceptibility to oxacillin was determined by agar disc diffusion method using, Muller Hinton agar medium containing 2% NaCl and the plates were incubated at 35°C overnight.
Results
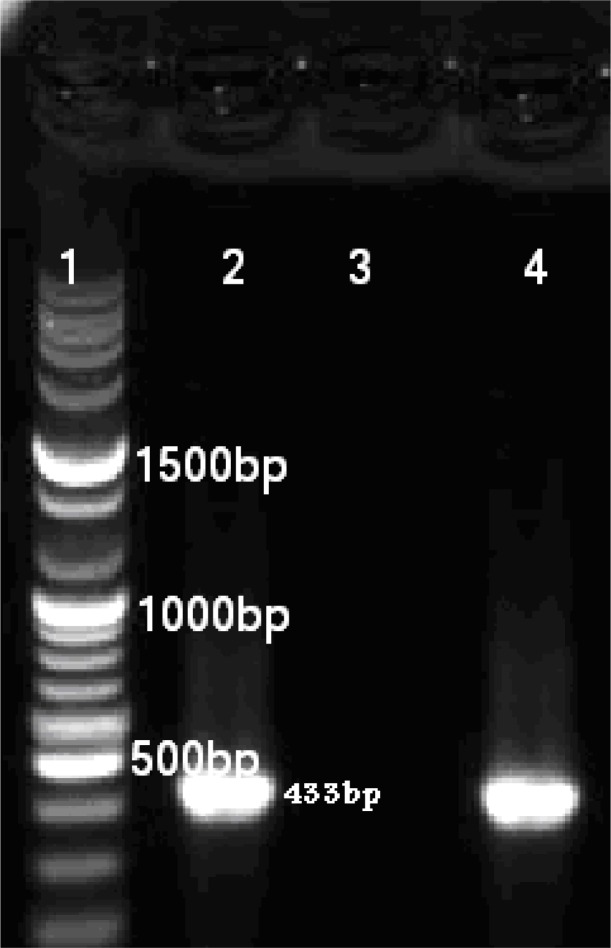
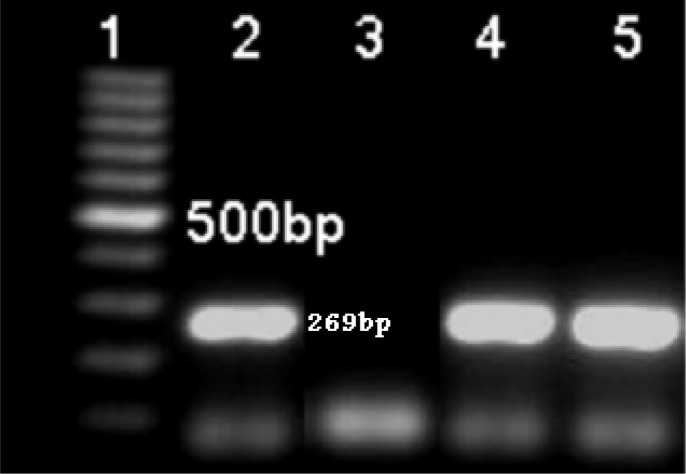
Coa gene was screened and detected in all 149 isolates of S. aureus. The standard S. aureus NCTC13300 produced amplified PCR products of 269bp and 433bp for (PVL) LUKS/FPR and LUKE/D, respectively; in this test, specialized primers were used for each gene (Fig 1 & 2). Among the 149 S. aureus isolates, there were 36(24/16%) PVL positive and 110 (73/82%) LUKE/D positive isolates (Table 1 & 2). Among the PVL positive S. aureus isolates, there were 61.8% MRSA, HA MRSA type.
Fig. 1:
Six μl PCR- product of luk s/f-pv (agarose 1%); Lane 1- molecular size marker (fermentase), Lane 2-Positive control for luk s/f-pv gene (433bp), Lane 3 –Negative control, Lane 4-Positive isolate from patient for luk s/f-pv gene
Fig. 2:
Six μl PCR- product of luk s/f-pv (agarose 1%).Lane 1: molecular size marker (fermentase), Lane 2: Positive control for luk e/d gene (269bp), Lane 3: Negative control, Lane 4& 5: Positive isolates from patients for luk e/d gene
Table 1:
Presence of pvl gene in S.aureus isolates of different origin
| Origin | Total number of isolates | Number (%) of positive isolates | P value |
|---|---|---|---|
| Cutaneous | 52 | 21(40.38) | 0.002 |
| Tracheal | 34 | 9(26.47) | 0.65 |
| Blood | 28 | 4(14.28) | 0.2 |
| Urine | 25 | 1(4) | 0.01 |
| Other | 11 | 1(9.09) | 0.40 |
The P values were assessed by x2 test. Boldface value indicates statistical significance (P≤0.05).
Table 2:
LUK E/D- positive isolates of Staphylococcus aureus in different staphylococcal infections
| Origin | Total number of isolates | Number (%) of positive isolates | P value |
|---|---|---|---|
| Cutaneous | 52 | 42(86.53) | 0.01 |
| Tracheal | 34 | 29(85.29) | 0.08 |
| Blood | 28 | 18(64.28) | 0.34 |
| Urine | 25 | 14(56) | 0.02 |
| Other | 11 | 5(45.45) | 0.009 |
The P values were assessed by x2 test. Boldface value indicates statistical significance (P≤0.05).
Discussion
We collected 149 isolates of S. aureus from five hospitals across Tehran and PCR assays showed that 24.16% of the isolates were LUKS/ F-PV (PVL)-positive and 73.82% were LUK E/D positive. The prevalence for pvl genes is higher than the European countries. For instance, in England and Wales in 2005, 1.6% of the isolates were PVL positive (5). In fact, in all studies, the prevalence of PVL positive S. aureus isolates was reported at 2–35% (3, 28). However, it is noteworthy in Argentina, the prevalence of the isolates was 56% (2). These differences may be due to various geographical areas or the type of assay used for detecting the gene. In the present study, 40.38%, 26.47%, 14.28%, 4% and 9.09% of the isolates were related to cutaneous, tracheal, blood, urine and other isolates, respectively. In fact, 94.4% of the cases were PVL positive isolates related to cutaneous, tracheal and blood while the rest was related to the other samples. In other studies also, PVL-positive S. aureus isolates were more prevalent in cutaneous and pulmonary isolates (5, 18) and our findings was the same. An interesting finding of our study was the presence of PVL genes in S. aureus isolates separated from urine isolates, because in the previous studies, such isolates were not found (18). Moreover, the prevalence of PVL positive S. aureus was high in blood isolates (18). 64.3% of the studied isolates belonged to men and 35.66% belonged to women; among these, 41.67% and 58.33% of the PVL positive S. aureus strains go to women and men, correspondingly. There was no significant difference and the rates were compatible with the previous findings (27). Lina et al. showed an association between pvl genes and cutaneous infections (17), confirming earlier findings by other workers (5). In our study, also there was a significant relation between cutaneous infections and the presence of pvl genes (Table 2). Studies have shown that pvl genes were first represented among the strains of CAMRSA (29–33); pvl genes are rarely reported in HA-MRSA (12). However, the analysis of MRSA isolates has shown that PVL-containing MRSA did not merely exist in the community and they may be found in hospitals, as well (27). In the present study, 61.8% of the PVL positive isolates were MRSA, type HA-MRSA. Conspicuously, methicillin-sensitive (MSSA) PVL containing S. aureus isolates were found in our study; however, such isolates were previously reported, as well (20). In an assay with microarray, it was demonstrated that MRSA isolates harboring genes for several bi-component toxins such as LUKE/D (23). We detected LUKE/D genes in nearly 73.8% of the S. aureus isolates separated from all staphylococcal infection types; harboring the genes was not associated with any blood and tracheal isolates and was compatible with the previous findings (34). But interestingly there was a significant relation between presence of the gene and coetaneous and urine isolates (Table 2)
In succinct, PVL-positive S. aureus exist in several infections, especially cutaneous ones. These strains are not only prevalent among CA-MRSA isolates but are abundantly found in HA-MRSA; carriers of the PVL positive isolates are also found (35) and this highlights the importance of these isolates in hospitals. Since PVL is a very remarkable virulence factor, it is recommended that early treatment is applied following an on-time diagnosis and performing an antibiogram. The reason is that such isolates are very dangerous and life threatening. We can prevent person-to-person transfer and fatal prevalence by making early diagnosis of the diseases in patients and the carriers with a simple PCR assay.
Acknowledgments
This research has been supported by Tehran University of Medical Sciences grant no: 86-03-27-5912. We are grateful to Dr Mohammad Iman Eini for the gift of control positive isolate. The authors declare that they have no conflicts of interest.
References
- 1.Boyle-vavra S, Daum R. Community acquired methicillin- resistant Staphylococcus aureus: the role of Panton- Valentine leukocidin. Lab Invest. 2007;87:3–9. doi: 10.1038/labinvest.3700501. [DOI] [PubMed] [Google Scholar]
- 2.Sola C, Saka A, Vindel A, Bocco J. High Frequency of Panton -Valentine Leukocidin genes in invasive methicillin- susceptible Staphylococcus aureus strains and the relationship with methicillin- resistant Staphylococcus aureus in Cordoba, Argentina. Eur J Clin Infect Dis. 2007;26:281–86. doi: 10.1007/s10096-007-0278-4. [DOI] [PubMed] [Google Scholar]
- 3.Melles D, Gorkink R, Boelens H, Snijders S, et al. Natural Population dynamics and expansion of pathogenic clones of Staphylococcus aureus. J Clin Invest. 2004;114:1732–1740. doi: 10.1172/JCI23083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lowy F. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 5.Holmes A, Ganner M, Guane S, Pitt T, et al. Staphylococcus aureus isolates carrying Panton- Valentine Leukocidin genes in England and Wales: frequency, characterization, and association with clinical disease. J Clin Microbiol. 2005;43:2384–90. doi: 10.1128/JCM.43.5.2384-2390.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rainard P, Corrates J, Bee M, Cochard T, et al. Leukotoxic activities of Staphylococcus aureus strains isolated from cows, ewes and goats with mastitis: importance of LOKM/LUKF’- PV leukotoxin. Clin Diagnos Lab Immun. 2003;10:272–77. doi: 10.1128/CDLI.10.2.272-277.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zetola N, Frances J, Neuremberger E, Bishai N. Community acquired methicillin- resistant Staphylococcus aureus: an emerging threat. Lancet. 2005;5:275–86. doi: 10.1016/S1473-3099(05)70112-2. [DOI] [PubMed] [Google Scholar]
- 8.Choorit W, Kanceco J, Muramoto K, Kamio Y. Existence of a new protein component with the same function as the LUKF component of leukocidin or gamma-Hemolysin and it’s gen in Staphylococcus aureus P63. FEBS left. 1995;357:260–64. doi: 10.1016/0014-5793(94)01372-8. [DOI] [PubMed] [Google Scholar]
- 9.Gravet A, Colin D, Keller D, Giradot R, et al. Characterization of a novel structural member, LUKF-LUKD of the bi-component staphylococcal leukotoxins family. FEBS left. 2002;436:202–208. doi: 10.1016/s0014-5793(98)01130-2. [DOI] [PubMed] [Google Scholar]
- 10.Kaneko J, Muramoto K, Kamio Y. Gene of LUKF-PV-Like component of Panton-Valentine leukocidin in Staphylococcus aureus P83 is linked with LUKM. Biosci Biotechnol Biochem. 1997;61:541–44. doi: 10.1271/bbb.61.541. [DOI] [PubMed] [Google Scholar]
- 11.Woodin A. Purification of the tow components of leukocidin from Staphylococcus aureus (1960) Biochem J. 1960;75:158–65. doi: 10.1042/bj0750158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgan M. Diagnosis and treatment of Panton-Valentine leukocidin (PVL)-associated staphylococcal pneumonia. J Antimicrob Agent. 2007;30:289–96. doi: 10.1016/j.ijantimicag.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 13.Centers for disease control and prevention Outbreaks of community-acquired methicillin resistant Staphylococcus aureus skin infections. Morb Mortal Wkly Rep. 2003;52:88. [PubMed] [Google Scholar]
- 14.Wannet W. Virulent MRSA strains containing the Panton-Valentine leukocidin gen in Netherlands. Eurosurveillance Wkly. 2003;7:1. [Google Scholar]
- 15.Boubaker K, Diebld P, Blanc S, Vandenesch F, et al. Panton-Valentine leukocidin and staphylococcal skin infections in school children. Emerg Infect Dis. 2004;10:121–24. doi: 10.3201/eid1001.030144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scottish center for infection and environmental health Community MRSA and Panton-Valentine leukocidin. SWCIEH Wkly. 2002;36:298. [Google Scholar]
- 17.Lina G, Piemont Y, Godail-Gamot F, Bes M, et al. Involvement of Panton-Valentine leukocidin- producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis. 1999;29:1128–32. doi: 10.1086/313461. [DOI] [PubMed] [Google Scholar]
- 18.Reichert B, Birrel G. Severe nonpneumonic necrotizing infections in children caused by Panton-Valentine Leukocidin producing Staphylococcus aureus Strains. J Infect. 2005;50:438–42. doi: 10.1016/j.jinf.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Shannon M, Heller L, Arbuckle J, Talavera M, et al. Staphylococcal cassette chromosome mec and Panton-Valentine leukocidin characterization of methicillin-resistant Staphylococcus aureus clones. J Chlin Microbiol. 2007;45:1019–21. doi: 10.1128/JCM.01706-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Notte O, Haay H, Zimmerman A, Geiss M. Staphylococcus aureus positive for by Panton-Valentine Leukocidin genes but susceptible to methicillin in patients with furuncles. Eur J Clin Microbiol Infect Dis. 2005;24:477–479. doi: 10.1007/s10096-005-1354-2. [DOI] [PubMed] [Google Scholar]
- 21.Boussaud V, Parrot A, Mayaud C, Wislez M, et al. Life- threatening hemoptysis in adults with community-acquired pneumonia due to Panton-Valentine leukocidin-Secreting Staphylococcus aureus. Intensive Care Med. 2003;29:1840–43. doi: 10.1007/s00134-003-1918-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naimi S, Kathleen H, Boxrud J, Groom V, et al. Epidemiology and cllonality of community acquired methicillin-resistant Staphylococcus aureus in Minnesota, 1996–1998. Clin Infect Dis. 2001;33:990–96. doi: 10.1086/322693. [DOI] [PubMed] [Google Scholar]
- 23.Miklaevis E, Hoeggman S, Balode A, Sanchez B, et al. Report on the first PVL-Positive Community acquired MRSA strain in Latvia. Eurosurveillance. 2004;9:10–12. [PubMed] [Google Scholar]
- 24.Krziwanek K, Luger C, Sammer B, Stumvoll S, et al. PVL-Positive MRSA in Austria. Eur J Clin Microbiol Infect Dis. 2007;26:931–35. doi: 10.1007/s10096-007-0391-4. [DOI] [PubMed] [Google Scholar]
- 25.Nashev D, Bizeva L, Toshkova K. First cases of infections caused by Panton-Valentine leukocidin positive community-acquired methicillin-resistant Staphylococcus aureus in Bulgaria. Eurosurveill. 2007;12:3225. doi: 10.2807/esw.12.26.03225-en. [DOI] [PubMed] [Google Scholar]
- 26.Wannet W, Heck M, Pluister G, Spalbary A, et al. Panton-Valentine leukocidin positive MRSA in 2003: the Dutch situation. Eurosurveill. 2004;9:484. [PubMed] [Google Scholar]
- 27.Abdullah K, Haijing L, Charles W. Antimicrobial susceptibility patterns and staphylococcal cassette chromosome mec types of, as well as by Panton-Valentine leukocidin occurrence among methicillin-resistant Staphylococcus aureus isolates from children and adults in Middle Tennessee. J Clin Microbiol. 2006;44:4436–440. doi: 10.1128/JCM.01546-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aires-ed-sousa M, Conceicao T, Lencastre H. Unusually high prevalence of nosocomial Panton-Valentine leukocidin positive Staphylococcus aureus isolates in Cape Verde Islands. J Clin Microbiol. 2006;44:3790–93. doi: 10.1128/JCM.01192-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dufour P, Gillet Y, Bes M, Lina G, et al. Community-acquired methicillin-resistant Staphylococcus aureus infections in France: emergence of a single clone that produces Panton-Valentine leukocidin. Clin Infect Dis. 2002;35:813–24. doi: 10.1086/342576. [DOI] [PubMed] [Google Scholar]
- 30.Naimi S, Ledell H, Como-Sabetti K, Borchardl M, et al. Comparison of community and healthcare-associated methicillin-resistant Staphylococcus aureus infection. JAMA. 2003;290:2976–84. doi: 10.1001/jama.290.22.2976. [DOI] [PubMed] [Google Scholar]
- 31.Tenover C, McDougal K, Goering V, Kiligore G, et al. Characterization of a strain of community- associated methicillin-resistant Staphylococcus aureus widely disseminated in the United States. J Clin Microbiol. 2006;44:108–18. doi: 10.1128/JCM.44.1.108-118.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vandenesch F, Naimi S, Enright C, Lina G, et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: World emergence. Emerg Infect Dis. 2003;9:978–84. doi: 10.3201/eid0908.030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monecke S, Slickers P, Holzel H, Richter-Huhn G, et al. Microarray-based characterization of a Panton-Valentine leukocidin community acquired strain of methicillin-resistant Staphylococcus aureus. Clin Microbiol Infect. 2006;12:718–28. doi: 10.1111/j.1469-0691.2006.01420.x. [DOI] [PubMed] [Google Scholar]
- 34.Bronner S, Stoessel P, Gravet A, Monteil H, et al. Variable expressions of Staphylococcus aureus bi-component leukotoxins semi quantified by competitive reverse transcription- PCR. Environ Microbiol. 2006;66:3431–38. doi: 10.1128/aem.66.9.3931-3938.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baggett H, Hennessy T, Rudolph K. Community- onset methicillin- resistant Staphylococcus aureus associated with antibiotic use and the cytotoxin Panton-Valentine leukocidin during a forunclosis outbreak in rural Alaska. J Infect Dis. 2004;189:1565–73. doi: 10.1086/383247. [DOI] [PubMed] [Google Scholar]