Abstract
Background:
The effect of curcumin as a natural safe compound with different biological activities was examined on fungal growth and aflatoxin production in Aspergillus parasiticus NRRL 2999.
Methods:
The fungus was cultured in presence of serial two-fold concentrations of curcumin (125–2000 μg/ml) in yeast extract sucrose broth for 3 days at 28°C. Mycelia dry weight was determined as an index of fungal growth, while aflatoxin production was assessed by high performance liquid chromatography (HPLC). The expression of ver-1, nor-1, pksA, omtA and aflR genes in aflatoxin biosynthetic pathway was evaluated by real time PCR.
Results:
Curcumin strongly inhibited aflatoxin B1 production in the range of 26.6 to 94.9% by serial two-fold concentrations from 125 to 2000 μg/ml. Fungal growth was also inhibited by the compound in the range of 34.0 to 60.8%. Analysis of the expression of aflatoxin pathway genes by real time PCR showed that curcumin inhibited the expression of ver-1, nor-1, pksA, omtA and aflR genes at concentrations of 250 and 1000 μg/ml. In concentration of 1000 μg/ml, gene expression was reduced by 31.3%, 44.6%, 57.1% 110.9% and 286.7% accordingly. Reduction in the expression of aflatoxin biosynthesis genes was significant only for aflR. In ferric reducing ability of plasma (FRAP) assay, curcumin showed strong antioxidant activity at all concentrations tested.
Conclusion:
Curcumin may be employed successfully as a good candidate in controlling of toxigenic fungal growth on food and feed and subsequent contamination with aflatoxins in practice.
Keywords: Aflatoxin, Aspergillus parasiticus, Curcumin, Gene expression, FRAP, Real-Time PCR
Introduction
Aflatoxins (AFs) are toxic, carcinogenic, mutagenic, and polyketide-derived secondary metabolites produced mainly by certain strains of Aspergillus flavus and A. parasiticus and in a less extent by several strains of A. nomius, A. pseudotamarii, A. bombycis, and A. ochraceoroseus (1). Among the at least 16 structurally related aflatoxins characterized, there are only four major aflatoxins, B1, B2, G1, and G2 which they are important natural contaminants of crops and agricultural commodities. Among naturally occurring AFs, aflatoxin B1 (AFB1) is considered as the most potent hepatotoxic and hepatocarcinogenic chemical. AFs have been detected in numerous agricultural commodities, such as corn, peanuts, cereal grains, whole wheat, rye breads, oil seeds, cottonseed, tree nuts, and contaminated environments (2, 3). Consumption of AF-contaminated foods and feeds is a serious problem from the viewpoint of not only public health, but also economic losses.
The biosynthesis of AFB1 is a multistep process and more than 23 enzymatic reactions are required for this pathway, which is initiated by hexanoate synthesis from acetate (4). It has been shown that 25 genes clustered within a 75-kb DNA region in the chromosomes of A. parasiticus and A. flavus are involved in aflatoxin biosynthesis. The ver-1 encodes a ketoreductase required for the conversion of VERA to demethylsterigmatocystin (DMST) (5, 6). Norsolonic acid is an intermediate in the aflatoxin biosynthetic pathway in A. parasiticus that is converted to AVN by a reductase/dehydrogenase enzyme, and this reaction is reversible depending on NADPH or NADH (7). The aflR gene encodes a sequence-specific DNA-binding binuclear zinc cluster (Zn (II)2Cys6) protein, required for transcriptional activation of most, if not all, of the structural genes (8–10). The involvement of omtA in the later step of aflatoxin production has been established. This gene encodes O-methyltransferase A or O-methyltransferase II required for the conversion of ST to O-methylsterigmatocystin (OMST) and DHST to dihydro-O-methylsterigmatocystin (DHOMST) (11–13). It has been demonstrated that pksA is important for aflatoxin biosynthesis, because of involvement in the synthesis of a polyketide from the primary metabolite, acetate (14).
With respect to the rapid expanding the list of food-borne pathogens, searching of novel strategies to prevent food contamination with these hazardous organisms is an urgent need. Many researchers have now been involved to find out the safer and more effective compounds from natural sources with lower toxicity for eukaryotic systems (15–17). Natural inhibitors of either fungal growth or AF production from plants and microorganisms are possible candidates for reducing AF contamination without incurring the problems arise from synthetic chemicals (18). Among the thousands of naturally occurring compounds so far identified in plants, there is none that poses, or reasonably might be expected to pose a significant risk to public health at the levels of intake when used as additives.
Certain organic acids and some herbal compounds are known to suppress growth of Aspergillus and reduce aflatoxin production (19). Curcumin (1, 7-bis (4- hydroxyl – 3 - methoxyphenyl) - 1, 6 - heptadiene- 3,5- dione/diferuloyl methane), is a phenolic compound chemically characterized in 1910. As a generally regarded as safe (GRAS) compound found in various natural sources such as different medicinal plants, it is known for a diverse range of beneficial biological properties including antimicrobial, antioxidant and free radical scavenging activities (20–23). To our knowledge, very little has been documented about its effect of aflatoxigenic fungi regard to aflatoxin production at genomic level.
In the present study, antifungal and antioxidant activities of curcumin was studied against an aflatoxin-producing A. parasiticus with special attention to the expression of important key genes of aflatoxin biosynthesis pathway catalyzed various enzymatic reactions in different steps of toxin biosynthesis.
Materials and Methods
Strain and culture conditions
Aspergillus parasiticus NRRL 2999 was cultured on Sabouraud Dextrose Agar (E. Merck, Germany) slant for 7 days at 28°C. The spores were harvested by adding sterile water containing 0.01% Tween 80. The tip culture method was used for monitoring the effect of curcumin on AFB1 production. In this method, a 1-ml Pipetman tip was used as a culture vessel for liquid stationary culture (24). Curcumin (Sigma–Aldrich, St. Louis, MO, USA) was prepared in final concentrations of 2000, 1000, 500, 250, 125 μg/ml in dimethylsulfoxide (DMSO; a final concentration of 1%). A 10 μl spore suspension of the A. parasiticus NRRL 2999 (200 cell/μl) was inoculated into 500 μl of YES broth (2% yeast extract and 20% sucrose) exposed to different concentrations of the compound in separate tips and incubated for 3 days at 28°C. The control cultures were treated in the same manner with the test cultures except that they were not contained curcumin. Two separate experiments were carried out in two triplicate sets each.
Fungal dry weight and growth inhibitory determination
After separating the culture medium from the tips using centrifugation and incubation at 80°C for 3 h, the mycelia weight was calculated by reducing the weight of mycelia-containing tips from those of pre-weighted empty tips. The growth inhibitory effect expressed as percent inhibition of growth by the following formula:
Inhibition of growth (%) = Dc- Ds / Dc × 100; Where Dc is the dry weight of colony in control sample, Ds is the dry weight of colony in treated sample.
Analysis of aflatoxin production by TLC and HPLC
For detection of AFB1, chloroformic extract of the culture broth and the standard of AFB1 (Sigma-Aldrich) was spotted on TLC silica gel 60F254 plates and developed by chloroform- ethyl acetate- formic acid 90% (6:3:1, v/v/v). AFB1 were visualized under UV light (365 nm) and photographed with a TLC scanner CAMAG Reprostar 3 (CAMAG, Switzerland). To quantitative measurement the amounts of AFB1, fifty ml of each chloroformic extract was injected into the HPLC (KNAUER D-14163 UV–VIS system, Berlin, Germany) column (Cosmosil 5-ph-AR-300 Waters; 4.6×150 mm) and eluted at a flow rate of 1 ml/min. by water/acetonitrile/methanol (60:25:15, v/v/v) as mobile phase. The amounts of AFB1 were measured at wavelength of 365 nm. The elution time of the samples was compared with pure AFB1 standard and quantified based on the ratio of the peak area of samples to those of the standards (25).
Measurement of antioxidant activity
The antioxidant capacity of curcumin was determined by ferric reducing ability of plasma (FRAP) assay according to Benzie and Strain (26). The method is based on the reduction of a ferric 2, 4, 6-tripyridyl-s-triazine complex (Fe3+-TPTZ) to the ferrous form (Fe2+-TPTZ). The working FRAP reagent was prepared by mixing 10 volumes of 300 mmol/l acetate buffer, pH 3.6, with 1 volume of 10 mmol/l TPTZ (2, 4, 6-tripyridyl-s-triazine) in 40 mmol/l hydrochloric acid and with 1 volume of 20 mmol/l ferric chloride. FeSO4. 7H2O in range of 100–1000 mmol/l was used for calibration. 50 μl of each concentration of curcumin (500, 250, 125, 62.5 and 31.25) was added to 1.5 ml of FRAP reagent and the absorbance of reaction mixture at 593 nm was measured after incubation at 37°C for 10 min. The FRAP values for the samples were then determined using this standard curve. The experiment was carried out in triplicate.
RNA preparation and Real-Time PCR
A. parasiticus NRRL 2999 was cultured on YES broth (as control) and YES broth contained 250 and 1000 μg/ml of curcumin in 50 ml Erlenmeyer flasks. After 3 days incubation of cultures at 28°C, the harvested mycelia mass was flash-frozen in liquid nitrogen and grounded to a fine powder in a porcelain mortar. Total RNA was purified from the homogenized fungal mycelia using GITC reagent. The total RNA was treated with RNase-free DNase (Fermentase), First-strand cDNA was prepared with the Fermentase Kit, using random hexamer primers, according to the protocol. Real-Time quantitative RT-PCR was carried out using the SYBR Green Master Mix (GENET BIO), in a final volume of 25 μl for each reaction, and an ABI PRISM 7500 thermal cycler (Applied Biosystems). Two-step PCR conditions were as follows: after an initial incubation at 95°C for 10 min, 40 cycles of 95°C for 15s and 60°C for 1 min were performed. The primer sets were aflR, 5′-GGCTGGTCAGGAGCAAAGC-3′ and 5′-CCCCGAATTCCGAATCG-3′; ver-1, 5′-CCAATGCGGCCGTTGT-3′ and 5′-TGAGAAAAACGACGCAATGAA-3′; nor-1, 5′-GTCCAAGCAACAGGCCAAGT-3′ and 5′-TCG TGCATGTTGGTGATGGT-3′; pksA, 5′-TGCATGGCGATGTGGTAGTT-3′ and 5′ GTAAGGCCGCGGAAGAAAG-3′; omtA, 5′-GGCCATATCCGCGAGCTT-3′ and 5′-CGCATGACCACATCCCAAT-3′;β-actin,5′TGCTCTCGTTATCGACAATGGT-3′ and 5′-CATCGTCACCGGCGAAA-3′(27, 28).
Mathematical method for relative quantification in Real-Time PCR
The mathematical method was used to determine the relative quantification of a target gene in comparison a reference gene. Real-Time PCR efficiencies were calculated from the given slopes in LightCycler software. The corresponding Real-Time PCR efficiency (E) of one cycle in the exponential phase was calculated according to the equation: E = 10[–1/slope].
The relative expression ratio (R) of a target gene is calculated based on E and the crossing points (CP) deviation of an unknown sample versus a control, and expressed in comparison to a reference gene. Equation 1 shows a mathematical model of relative expression ratio in Real-Time PCR.
| (1) |
The ratio of a target gene is expressed in a sample versus a control in comparison to a reference gene. E target is the real-time PCR efficiency of target gene transcript; E ref is the Real-Time PCR efficiency of a reference gene transcript; ΔCPtarget is the CP deviation of control – sample of the target gene transcript; ΔCPref= CP deviation of control – sample of reference gene transcript. The reference gene could be a stable and secure unregulated transcript, e.g. a house- keeping gene transcript. For the calculation of R, the individual Real-Time PCR efficiencies and the CD deviation (ΔCP) of the investigated transcripts must be known. CP deviations of control cDNA minus sample of the target gene and reference genes were calculated according to the derived CP values (29).
Statistical analysis
The data of fungal growth, AFB1 contents and gene expression were subjected to the Analysis of Variance (One-way ANOVA) in Tukey range. The differences with P<0.05 were considered significant.
Results
Effects of curcumin on A. parasiticus growth and AFB1 production
For assessing the antifungal effect of curcumin, the fungus was exposed to different concentrations of the compound in YES broth at 28 °C for 3 days. Fungal growth was inhibited in the range of 34.0 to 60.8% in the fungus exposed to serial two-fold concentrations of 125 to 2000 μg/ml an IC50 value around 900 μg/ml (Table 1). Growth inhibition was found to be significant at concentrations of ≥250 μg/ml compared with non-treated controls (P<0.01). The qualitative TLC results of curcumin treated cultures showed that curcumin was able to inhibit strongly AFB1 production in a dose dependent manner. HPLC analysis was shown that curcumin strongly inhibited AFB1 production by in the range of 26.6 to 94.9%, with an IC50 value around 400 μg/ml at serial two-fold concentrations of 125 to 2000 μg/ml after comparing with non-treated controls (Table 1).
Table 1:
Effect of curcumin on the growth and AFB1 production by Aspergillus parasiticus NRRL 2999
| Curcumin (μg/ml) | Fungal dry weight (mg) | Growth inhibition (%) | Total AFB1 (ng) | AFB1 (ng/mg dry weight) | AFB1 inhibition (%) |
|---|---|---|---|---|---|
| Control (0.0) | 9.7 ± 0.59 | 0.00 | 7361.4 ± 448.6 | 758.9 ± 50.1 | 0.00 |
| 125 | 6.4 ± 0.85 | 34.0 | 3566.0 ± 509.9 | 557.2 ± 67.3 | 26.6 |
| 250 | 5.2 ± 0.31 | 46.4 | 2300.2 ± 343.6 | 442.3 ± 53.6 | 41.7 |
| 500 | 5.2 ± 0.47 | 46.4 | 1877.7 ± 286.7 | 361.1 ± 40.9 | 52.4 |
| 1000 | 4.8 ± 0.59 | 50.5 | 708.4 ± 170.3 | 147.6 ± 17.5 | 80.5 |
| 2000 | 3.8 ± 0.59 | 60.8 | 147.8 ± 15.0 | 38.9 ± 3.8 | 94.9 |
Results are the means ± SD of two separate experiments in triplicate sets.
Antioxidant activity of curcumin by FRAP
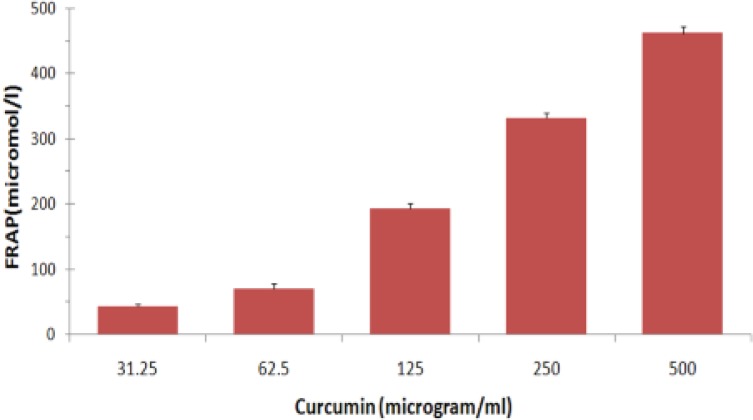
Based on the FRAP analysis, it was shown that curcumin is a potent antioxidant compound and the antioxidant values was obtained in range between 43.3 to 461.6 μmol/l in serial two-fold concentrations of the compound from 31.25 to 500 μg/ml (Fig. 1).
Fig. 1:
Mean FRAP values (μM/l) and standard errors of the mean are given for different concentrations of curcumin (n=3) (P< 0.05)
Effects of curcumin on the expression of aflatoxin biosynthetic pathway genes
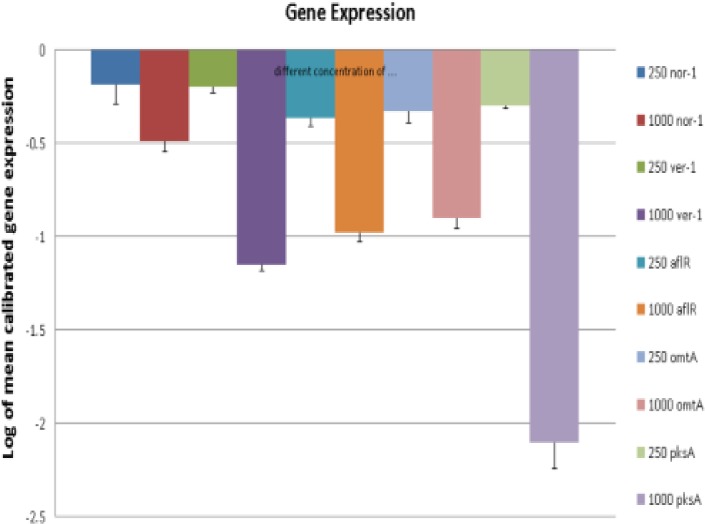
For evaluating the effects of curcumin on expression of genes encoding proteins involved in aflatoxin biosynthesis, A. parasiticus NRRL 2999 was cultured on YES broth in presence of curcumin (250 and 1000 μg/ml) for 3 days at 28°C. The fungal mycelia were separated by filtration, total RNA was extracted, and the expression of nor-1, ver-1, omtA, pksA and aflR genes was evaluated by Real-Time PCR. The result showed that curcumin decreased mRNA levels of all tested genes in both concentrations dose-dependently (Fig. 2). In 250 μg/ml concentration, the expression of ver-1, nor-1, pksA, omtA and aflR was affected by 7.5%, 11.6%, 15.7%, 20.8% and 106.3%, respectively. In concentration of 1000 μg/ml, gene expression was reduced by 31.3%, 44.6%, 57.1% 110.9% and 286.7% accordingly. Based on the statistical analyses, reduction in the expression of aflatoxin biosynthesis genes examined in this study was significant only for aflR (P<0.05).
Fig. 2:
Effect of curcumin on the expression of nor-1, pksA, ver-1, omtA and aflR at concentrations of 250 and 1000 μg/ml. Data are means ± SD (n=6) of two separate experiments in triplicate sets each (P< 0.05)
Discussion
In the present study, antifungal and antioxidant activities of curcumin was established against an aflatoxin-producing A. parasiticus in relation to the reduction in the expression of important key genes of aflatoxin biosynthesis pathway i.e. ver-1, nor-1, pksA, omtA and aflR. To date, different studies achieved to find out bioactive compounds from plants and microorganisms with inhibitory effect on toxigenic fungal growth and aflatoxin production (16–19, 30). Beyond laboratory-based studies, some researchers showed the practical potential of plant formulations in control of aflatoxin contamination of crops. From the study of Bluma et al. (31), it was shown that thyme and poleo and clove essential oils were effective to control aflatoxigenic fungi in stored maize. In clove and poleo EOs, AFB1 production was completely inhibited at 0.982 aw, whereas mountain thyme and eucalyptus EOs inhibited AFB1 production at highest percentage (85–90%) in all concentrations used. Razzaghi-Abyaneh et al. (32) demonstrated that carvacrol and thymol isolated from Satureja hortensis L. strongly inhibited aflatoxin production by A. parasiticus, with IC50 values of 0.79 and 0.86 mM, respectively. Yoshinari et al. (27) showed that dioctatin A, a natural compound isolated from Streptomyces strongly inhibited aflatoxin production by A. parasiticus, with an IC50 value of 4.0 mM. Using RT-PCR, they showed that dioctatin A inhibited the transcription of pksA, ver-1 and omtA and significantly repressed the pathway regulatory gene, aflR. In a similar study, Yoshinari et al. (33) reported that (E)- and (Z)-spiroethers isolated from the essential oil of German chamomile inhibited AFG1 production by Aspergillus parasiticus with IC50 values of 2.8 and 20.8 mM, respectively. The authors suggested that a possible inhibitory mechanism of spiroethers on AFG1 production was on the activity of a cytochrome P450-dependent enzyme.
In the present study, we showed a potent inhibition of AFB1 production in A. parasiticus exposed to curcumin could be due to a meaningful reduction in the expression of some important aflatoxin pathway genes i.e. ver-1, nor-1, pksA, omtA and aflR. Previous studies showed that curcumin is effective on the growth of some microorganisms including fungi (21–23). Despite the known antibacterial and antifungal activities of curcumin, very little has been demonstrated about its effect on aflatoxin biosynthesis in gene expression level. It has also been reported that curcumin affects the cell cycle of Candida albicans in different ranges (23). De et al. (22) showed that curcumin inhibited the growth of Helicobacter pylori with MIC ranges from 5 to 50 μg/ml.
Here we showed that curcumin strongly inhibited AFB1 production in A. parasiticus in a dose dependent manner by a possible mechanism involved a meaningful reduction in the expression of some genes in aflatoxin biosynthetic pathway including; ver-1, nor-1, pksA, omtA and aflR. The aflR was the most affected gene under the test condition, which showed significant reduction at mRNA level compared with the control. Since aflR is a regulatory gene in aflatoxin biosynthetic pathway, we purpose that a significant reduction in the expression of this gene in curcumin-exposed A. parasiticus is responsible in part not only for AFB1 inhibition by the fungus, but also for down regulating other genes studied in the present work. Another explanation is the direct inhibition of all genes tested by curcumin via affecting corresponding mRNAs expression. We reported for the first time that curcumin reduces expression of nor-1, ver-1, omtA, pksA and aflR in A. parasiticus in relation to a remarkable inhibition in AFB1 production by the fungus. All together, these results indicate that curcumin may be employed successfully as a good candidate in controlling of toxigenic fungal growth on food and feed and subsequent contamination with aflatoxins in practice.
Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc) have been completely observed by the authors.
Acknowledgments
This work was supported financially by Tarbiat Modares University. We would like to thank Dr. Asghar Sepahvand, Mrs. Maryam Razeghi and Mr. Kayhan Karvandian for their helpful assistance. The authors declare that there is no conflict of interests.
References
- 1.Jamali M, Ebrahimi MA, Karimipour M, Shams-Ghahfarokhi M, Dinparast-Djadid N, Kalantari S, Pilehvar-Soltanahmadi Y, Amani A, Razzaghi-Abyaneh M. An insight into the distribution, genetic diversity and mycotoxin production of Aspergillus section Flavi in soils of pistachio orchards. Folia Microbiol. 2012;57:27–36. doi: 10.1007/s12223-011-0090-5. [DOI] [PubMed] [Google Scholar]
- 2.Razzaghi-Abyaneh M, Shams-Ghahfarokhi M, Allameh A, Kazeroon-Shiri M, Ranjbar-Bahadori S, Mirzahoseini H, Rezaee MB. A survey on distribution of Aspergillus section Flavi in corn field soils in Iran: population patterns based on aflatoxins, cyclopiazonic acid and sclerotia production. Mycopathologia. 2006;161:183–192. doi: 10.1007/s11046-005-0242-8. [DOI] [PubMed] [Google Scholar]
- 3.Sepahvand A, Shams-Ghahfarokhi M, Jahanshiri Z, Jamali M, Allameh A, Razzaghi-Abyaneh M. A survey on distribution and toxigenicity of Aspergillus flavus from indoor and outdoor hospital environments. Folia Microbiol. 2011;56:527–534. doi: 10.1007/s12223-011-0078-1. [DOI] [PubMed] [Google Scholar]
- 4.Yu J, Chang PK, Ehrlich KC, Cary JW, Bhatnagar D, Cleveland TE, Payne GA, Linz JE, Woloshuk CP, Bennett JW. The clustered pathway genes in aflatoxin biosynthesis. Appl Environ Microbiol. 2004;70:1253–1262. doi: 10.1128/AEM.70.3.1253-1262.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skory CD, Chang P-K, Cary J, Linz JE. Isolation and characterization of a gene from Aspergillus parasiticus associated with the conversion of versicolorin A to sterigmatocystin in aflatoxin biosynthesis. Appl Environ Microbiol. 1992;58:3527–3537. doi: 10.1128/aem.58.11.3527-3537.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang S-H, Wu TS, Lee R, Chu FS, Linz JE. Analysis of mechanisms regulating expression of the ver-1 gene, involved in aflatoxin biosynthesis. Appl Environ Microbiol. 1997;63:1058–1065. doi: 10.1128/aem.63.3.1058-1065.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett JW, Chang P-K, Bhatnagar D. One gene to whole pathway: the role of norsolorinic acid in aflatoxin research. Adv Appl Microbiol. 1997;45:1–15. doi: 10.1016/s0065-2164(08)70260-0. [DOI] [PubMed] [Google Scholar]
- 8.Ehrlich KC, Montalbano BG, Bhatnagar D, Cleveland TE. Alteration of different domains in aflR affects aflatoxin pathway metabolism in Aspergillus parasiticus transformants. Fungal Genet Biol. 1998;23:279–287. doi: 10.1006/fgbi.1998.1045. [DOI] [PubMed] [Google Scholar]
- 9.Chang P-K, Yu J, Bhatnagar D, Cleveland TE. Repressor-AflR interaction modulates aflatoxin biosynthesis in Aspergillus parasiticus. Mycopathologia. 1999;147:105–112. doi: 10.1023/a:1007157309168. [DOI] [PubMed] [Google Scholar]
- 10.Price MS, Yu J, Nierman WC, Kim HS, Pritchard B, et al. The aflatoxin pathway regulator aflR induces gene transcription inside and outside of the aflatoxin biosynthetic cluster. FEMS Microbiol Lett. 2006;255:275–279. doi: 10.1111/j.1574-6968.2005.00084.x. [DOI] [PubMed] [Google Scholar]
- 11.Bhatnagar D, Ullah AHJ, Cleveland TE. Purification and characterization of a methyltransferase from Aspergillus parasiticus SRRC 163 involved in aflatoxin biosynthetic pathway. Prep Biochem. 1988;18:321–349. doi: 10.1080/00327488808062532. [DOI] [PubMed] [Google Scholar]
- 12.Yabe K, Ando Y, Hashimoto J, Hamasaki T. Two distinct O-methyltransferases in aflatoxin biosynthesis. Appl Environ Microbiol. 1989;55:2172–2177. doi: 10.1128/aem.55.9.2172-2177.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keller NP, Dischinger JHC, Bhatnagar D, Cleveland TE, Ullah AHJ. Purification of a 40-kilodalton methyltransferase active in the aflatoxin biosynthetic pathway. Appl Environ Microbiol. 1993;59:479–484. doi: 10.1128/aem.59.2.479-484.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown DW, Adams TH, Keller NP. Aspergillus has distinct fatty acid synthases for primary and secondary metabolism. Proc Natl Acad Sci USA. 1996;19:14873–14877. doi: 10.1073/pnas.93.25.14873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Razzaghi-Abyaneh M, Shams-Ghahfarokhi M, Chang PK. Aflatoxins: Mechanisms of Inhibition by Antagonistic Plants and Microorganisms. In: Guevara-Gonzalez Ramon G., editor. Aflatoxins: Biochemistry and Molecular Biology. INTECH Open Access Publisher; 2011. pp. 285–304. [Google Scholar]
- 16.Alinezhad S, Kamalzadeh A, Shams-Ghahfarokhi M, Rezaee MB, Jaimand K, Kawachi M, Zamani Z, Tolouei R, Razzaghi-Abyaneh M. Search for novel antifungals from 49 indigenous medicinal plants: Foeniculum vulgare and Platycladus orientalis as strong inhibitors of aflatoxin production by Aspergillus parasiticus. Ann Microbiol. 2011;61:673–681. [Google Scholar]
- 17.Hua S-ST, Grosjean O-K, Baker JL. Inhibition of aflatoxin biosynthesis by phenolic compounds. Lett Appl Microbiol. 1999;29:289–291. doi: 10.1046/j.1472-765x.1999.00635.x. [DOI] [PubMed] [Google Scholar]
- 18.Razzaghi-Abyaneh M, Shams-Ghahfarokhi M, Rezaee MB, Sakuda S. Natural Aflatoxin Inhibitors from Medicinal Plants. In: Rai M, Varma A, editors. Mycotoxins in Food, Feed and Bioweapons. Springer-Verlag Publication; 2010. pp. 329–354. [Google Scholar]
- 19.Gowda NKS, Malathi V, Suganthi RU. Effect of some chemical and herbal compounds on growth of Aspergillus parasiticus and aflatoxin production. Animal Feed Sci Technol. 2004;116:281–291. [Google Scholar]
- 20.Shukla PK, Khanna VK, Khan M, Srimal RC. Protective effect of curcumin against lead neurotoxicity in rat. Hum Exp Toxicol. 2003;22:653–658. doi: 10.1191/0960327103ht411oa. [DOI] [PubMed] [Google Scholar]
- 21.Moghaddam KM, Iranshahi M, Yazdi MC, Shahverdi AR. The combination effect of curcumin with different antibiotics against Staphylococcus aureus. IJGP. 2009;3:141–143. [Google Scholar]
- 22.De R, Kundu P, Swarnakar S, Ramamurthy T, Chowdhury A, Nair GB, Mukhopadhyay AK. Antimicrobial activity of curcumin against Helicobacter pylori isolates from India and during infections in mice. Antimicrob Ag Chemother. 2009;53:1592–1597. doi: 10.1128/AAC.01242-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jianhua W, Hai W. Antifungal susceptibility analysis of berberine, baicalin, eugenol and curcumin on Candida albicans. J Med Col PLA. 2009;24:142–147. [Google Scholar]
- 24.Yabe K, Nakamura H, Ando Y, Terakado N, Nakajima H, Hamasaki T. Isolation and characterization of Aspergillus parasiticus mutants with impaired aflatoxin production by a novel tip culture method. Appl Environ Microbial. 1988;54:2096–2100. doi: 10.1128/aem.54.8.2096-2100.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Razzaghi-Abyaneh M, Yoshinari T, Shams-Ghahfarokhi M, Rezaee M-B, Nagasawa H, Sakuda S. Dillapiol and apiol as specific inhibitors for the biosynthesis of aflatoxin G1 in Aspergillus parasiticus. Biosci Biotechnol Biochem. 2007;71:2329–2332. doi: 10.1271/bbb.70264. [DOI] [PubMed] [Google Scholar]
- 26.Benzie IFF, Strain JJ. The Ferric Reducing Ability of Plasma (FRAP) as a measure of “Antioxidant Power”: The FRAP Assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 27.Yoshinari T, Akiyama T, Nakamura K, Kondo T, Takahashi Y, Muraoka Y, Nonomura Y, Nagasawa H, Sakuda S. Dioctatin A is a strong inhibitor of aflatoxin production by Aspergillus parasiticus. Microbiology. 2007;153:2774–2780. doi: 10.1099/mic.0.2006/005629-0. [DOI] [PubMed] [Google Scholar]
- 28.Mayer Z, Bagnara A, Färber P, Geisen R. Quantification of the copy number of nor-1, a gene of the aflatoxin biosynthetic pathway by real-time PCR, and its correlation to the cfu of Aspergillus flavus in foods. Int J Food Microbiol. 2003;82:143–151. doi: 10.1016/s0168-1605(02)00250-7. [DOI] [PubMed] [Google Scholar]
- 29.Pfaffl MW. A new mathematical model for relative quantification in real time RT-PCR. Nucleic Acids Res. 2001;29(9):2002–2007. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils-A review. Food Chem Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 31.Bluma R, Amaiden MR, Etcheverry M. Screening of argentine plant extracts: Impact on growth parameters and aflatoxin B1 accumulation by Aspergillus section Flavi. Int J Food Microbiol. 2008;122:114–125. doi: 10.1016/j.ijfoodmicro.2007.11.050. [DOI] [PubMed] [Google Scholar]
- 32.Razzaghi-Abyaneh M, Shams-Ghahfarokhi M, Yoshinari T, Rezaee M-B, Jaimand K, Nagasawa H, Sakuda S. Inhibitory effects of Satureja hortensis L. essential oil on growth and aflatoxin production by Aspergillus parasiticus. Int J Food Microbiol. 2008;123:228–233. doi: 10.1016/j.ijfoodmicro.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 33.Yoshinari T, Yaguchi A, Takahashi-Ando N, Kimura M, Takahashi H, Nakajima T, Sugita-Konishi Y, Nagasawa H, Sakuda S. Spiroethers of German chamomile inhibit production of afatoxin G1 and trichothecene mycotoxin by inhibiting cytochrome P-450 monooxygenases involved in their biosynthesis. FEMS Microbiol Lett. 2008;284:184–190. doi: 10.1111/j.1574-6968.2008.01195.x. [DOI] [PubMed] [Google Scholar]