Abstract
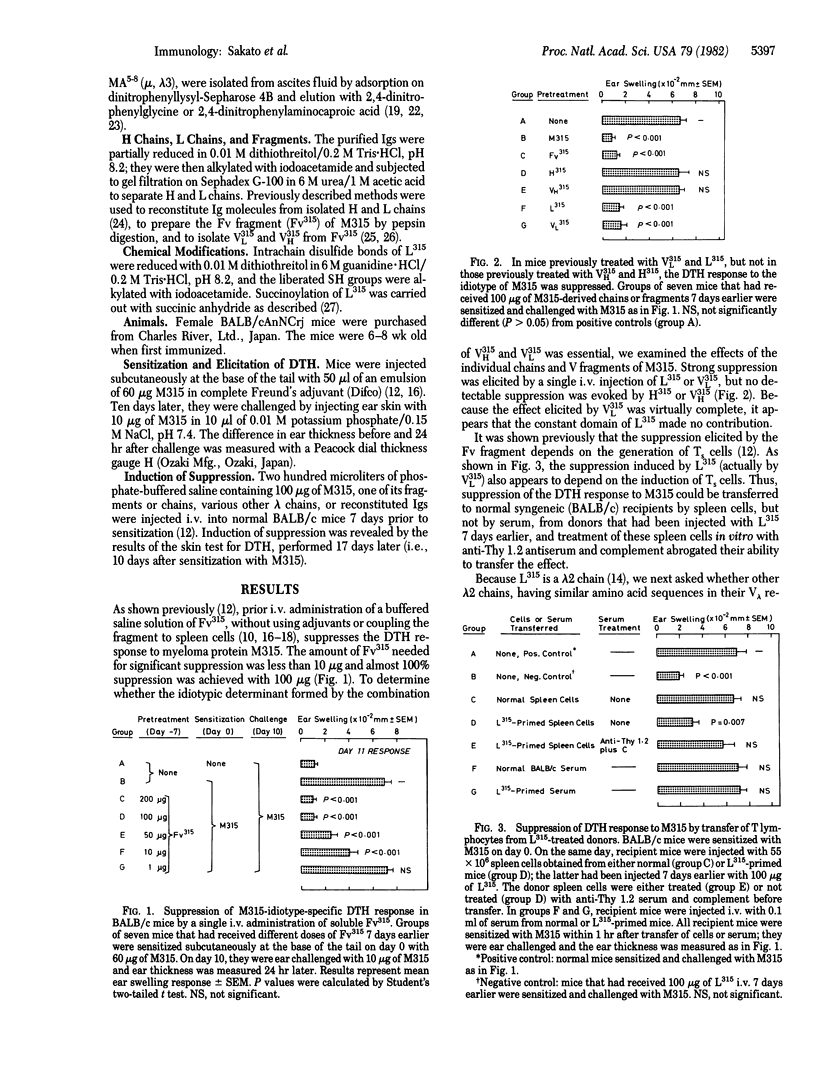
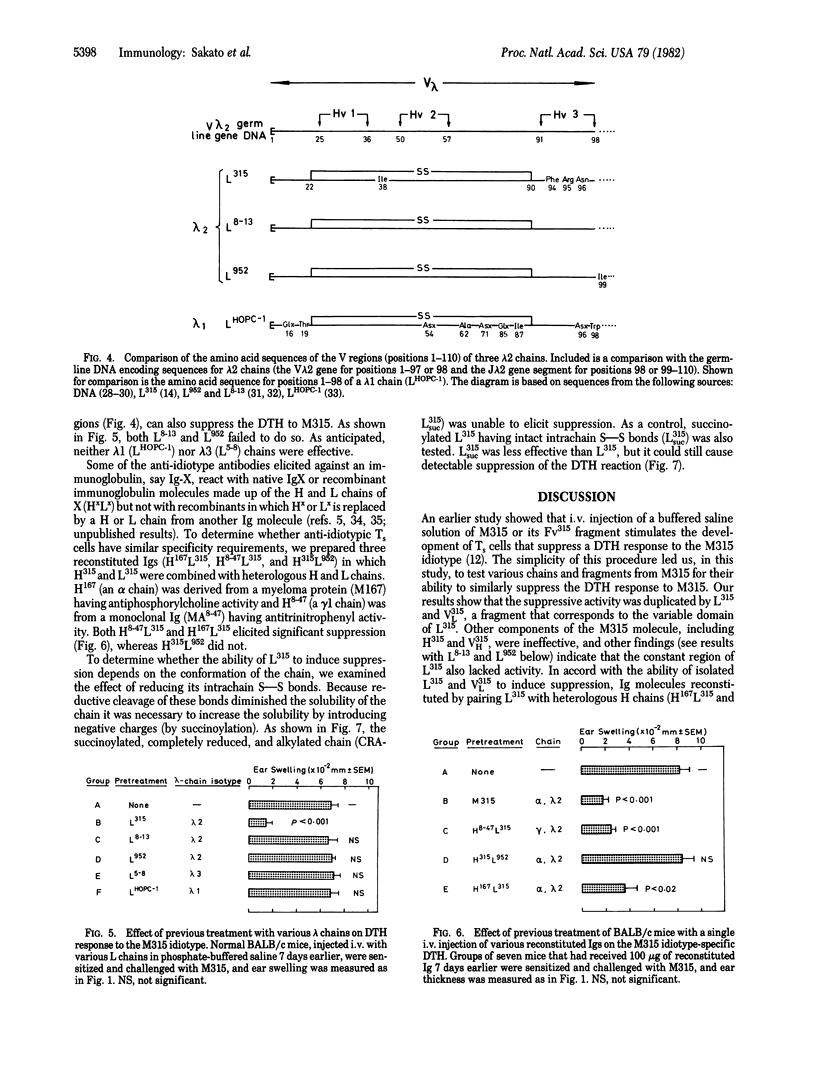
The induction in BALB/c mice of suppressor T cells that block a delayed-type hypersensitivity (DTH) response to the idiotype of M315, a myeloma protein of BALB/c origin, was examined with a variety of immunoglobulin chains and fragments whose amino acid sequences are known. Normal BALB/c mice receiving either the light chain of M315 (L315, lambda 2 isotype) or the variable (V) domain of this chain prior to sensitization with M315 showed marked suppression of DTH to the M315 idiotype. In contrast, neither the heavy chain nor the variable domain of the heavy chain of M315 affected the DTH response. Two other lambda 2 chains were tested and they also failed to suppress DTH to M315. Comparison of amino acid sequences in the three lambda 2 chains indicates that in L315 at most four V region amino acid substitutions (each resulting from a somatic mutation in the V lambda 2 germ-line gene) determine the specificity of the T-cell suppressor pathway. One of the four is in the framework and probably of negligible importance; the other three, however, are all clustered in the third hypervariable loop of the L315 V domain. The tertiary structure of L315 may also be essential, because disruption of intrachain disulfide bonds abolished the ability of the chain to induce suppression.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbas A. K., Perry L. L., Bach B. A., Greene M. I. Idiotype-specific T cell immunity. I. Generation of effector and suppressor T lymphocytes reactive with myeloma idiotypic determinants. J Immunol. 1980 Mar;124(3):1160–1166. [PubMed] [Google Scholar]
- Amzel L. M., Poljak R. J., Saul F., Varga J. M., Richards F. F. The three dimensional structure of a combining region-ligand complex of immunoglobulin NEW at 3.5-A resolution. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1427–1430. doi: 10.1073/pnas.71.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma T., Steiner L. A., Eisen H. N. Identification of a third type of lambda light chain in mouse immunoglobulins. Proc Natl Acad Sci U S A. 1981 Jan;78(1):569–573. doi: 10.1073/pnas.78.1.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomberg B., Tonegawa S. DNA sequences of the joining regions of mouse lambda light chain immunoglobulin genes. Proc Natl Acad Sci U S A. 1982 Jan;79(2):530–533. doi: 10.1073/pnas.79.2.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges S. H., Little J. R. Recovery of binding activity in reconstituted mouse myeloma proteins. Biochemistry. 1971 Jun 22;10(13):2525–2530. doi: 10.1021/bi00789a016. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Metzger H. Affinity labeling of a phosphorylcholine binding mouse myeloma protein. Biochemistry. 1972 Feb 29;11(5):766–771. doi: 10.1021/bi00755a014. [DOI] [PubMed] [Google Scholar]
- Cotner T., Blaser K., Kriedberg J., Potter M., Eisen H. N. Mouse myeloma proteins with lambda 2 and lambda 3 light chains. J Immunol. 1981 Nov;127(5):2150–2155. [PubMed] [Google Scholar]
- Dohi Y., Nisonoff A. Suppression of idiotype and generation of suppressor T cells with idiotype-conjugated thymocytes. J Exp Med. 1979 Oct 1;150(4):909–918. doi: 10.1084/jem.150.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugan E. S., Bradshaw R. A., Simms E. S., Eisen H. N. Amino acid sequence of the light chain of a mouse myeloma protein (MOPC-315). Biochemistry. 1973 Dec 18;12(26):5400–5416. [PubMed] [Google Scholar]
- Eichmann K. Expression and function of idiotypes of lymphocytes. Adv Immunol. 1978;26:195–254. doi: 10.1016/s0065-2776(08)60231-x. [DOI] [PubMed] [Google Scholar]
- Eichmann K., Falk I., Rajewsky K. Recognition of idiotypes in lymphocyte interactions. II. Antigen-independent cooperation between T and B lymphocytes that possess similar and complementary idiotypes. Eur J Immunol. 1978 Dec;8(12):853–857. doi: 10.1002/eji.1830081206. [DOI] [PubMed] [Google Scholar]
- Endres R. O., Grey H. M. Antigen recognition by T cells. I. Suppressor T cells fail to recognize cross-reactivity between native and denatured ovalbumin. J Immunol. 1980 Oct;125(4):1515–1520. [PubMed] [Google Scholar]
- Francis S. H., Leslie R. G., Hood L., Eisen H. N. Amino-acid sequence of the variable region of the heavy (alpha) chain of a mouse myeloma protein with anti-hapten activity. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1123–1127. doi: 10.1073/pnas.71.4.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl E. J., Metzger H. Affinity labeling of a mouse myeloma protein which binds nitrophenyl ligands. Kinetics of labeling and isolation of a labeled peptide. Biochemistry. 1970 Mar 3;9(5):1267–1278. doi: 10.1021/bi00807a031. [DOI] [PubMed] [Google Scholar]
- Habeeb A. F. Quantitation of conformational changes on chemical modification of proteins: use of succinylated proteins as a model. Arch Biochem Biophys. 1967 Sep;121(3):652–664. doi: 10.1016/0003-9861(67)90050-1. [DOI] [PubMed] [Google Scholar]
- Hochman J., Inbar D., Givol D. An active antibody fragment (Fv) composed of the variable portions of heavy and light chains. Biochemistry. 1973 Mar 13;12(6):1130–1135. doi: 10.1021/bi00730a018. [DOI] [PubMed] [Google Scholar]
- Imanishi-Kari T., Rajnavölgyi E., Takemori T., Jack R. S., Rajewsky K. The effect of light chain gene expression on the inheritance of an idiotype associated with primary anti-(4-hydroxy-3-nitrophenyl)acetyl(NP) antibodies. Eur J Immunol. 1979 Apr;9(4):324–331. doi: 10.1002/eji.1830090414. [DOI] [PubMed] [Google Scholar]
- Inbar D., Rotman M., Givol D. Crystallization with hapten of the Fab' fragment from a mouse IgA myeloma protein with antidinitrophenyl activity. J Biol Chem. 1971 Oct 25;246(20):6272–6275. [PubMed] [Google Scholar]
- Janeway C. A., Jr, Sakato N., Eisen H. N. Recognition of immunoglobulin idiotypes by thymus-derived lymphocytes. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2357–2360. doi: 10.1073/pnas.72.6.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerne N. K. Towards a network theory of the immune system. Ann Immunol (Paris) 1974 Jan;125C(1-2):373–389. [PubMed] [Google Scholar]
- Jørgensen T., Gaudernack G., Hannestad K. Immunization with the light chain and the VL domain of the isologous myeloma protein 315 inhibits growth of mouse plasmacytoma MOPC315. Scand J Immunol. 1980;11(1):29–35. doi: 10.1111/j.1365-3083.1980.tb00205.x. [DOI] [PubMed] [Google Scholar]
- Jørgensen T., Hannestad K. T helper lymphocytes recognize the VL domain of the isologous mouse myeloma protein 315. Scand J Immunol. 1979;10(4):317–323. doi: 10.1111/j.1365-3083.1979.tb01357.x. [DOI] [PubMed] [Google Scholar]
- Lynch R. G., Graff R. J., Sirisinha S., Simms E. S., Eisen H. N. Myeloma proteins as tumor-specific transplantation antigens. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1540–1544. doi: 10.1073/pnas.69.6.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjula B. N., Glaudemans C. P., Mushinski E. B., Potter M. Subunit interactions in mouse myeloma proteins with anti-galactan activity. Proc Natl Acad Sci U S A. 1976 Mar;73(3):932–936. doi: 10.1073/pnas.73.3.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J., Selsing E., Storb U. Structural alterations in J regions of mouse immunoglobulin lambda genes are associated with differential gene expression. Nature. 1982 Feb 4;295(5848):428–430. doi: 10.1038/295428a0. [DOI] [PubMed] [Google Scholar]
- Miller S. D., Wetzig R. P., Claman H. N. The induction of cell-mediated immunity and tolerance with protein antigens coupled to syngeneic lymphoid cells. J Exp Med. 1979 Mar 1;149(3):758–773. doi: 10.1084/jem.149.3.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odermatt B. F., Perlmutter R., Lynch R. G. Molecular heterogeneity and fine specificity of BALB/c antibodies elicited by isologous myeloma protein. Eur J Immunol. 1978 Dec;8(12):858–865. doi: 10.1002/eji.1830081207. [DOI] [PubMed] [Google Scholar]
- Oudin J., Cazenave P. A. Similar idiotypic specificities in immunoglobulin fractions with different antibody functions or even without detectable antibody function. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2616–2620. doi: 10.1073/pnas.68.10.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen F. L., Ju S. T., Nisonoff A. Presence on idiotype-specific suppressor T cells of receptors that interact with molecules bearing the idiotype. J Exp Med. 1977 Jun 1;145(6):1559–1566. doi: 10.1084/jem.145.6.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakato N., Eisen H. N. Antibodies to idiotypes of isologous immunoglobulins. J Exp Med. 1975 Jun 1;141(6):1411–1426. doi: 10.1084/jem.141.6.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakato N., Janeway C. A., Jr, Eisen H. N. Immune responses of BALB/c mice to the idiotype of T15 and of other myeloma proteins of BALB/c origin: implications for an immune network and antibody multispecificity. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 2):719–724. doi: 10.1101/sqb.1977.041.01.082. [DOI] [PubMed] [Google Scholar]
- Segal D. M., Padlan E. A., Cohen G. H., Rudikoff S., Potter M., Davies D. R. The three-dimensional structure of a phosphorylcholine-binding mouse immunoglobulin Fab and the nature of the antigen binding site. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4298–4302. doi: 10.1073/pnas.71.11.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirisinha S., Eisen H. N. Autoimmune-like antibodies to the ligand-binding sites of myeloma proteins. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3130–3135. doi: 10.1073/pnas.68.12.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sy M. S., Bach B. A., Brown A., Nisonoff A., Benacerraf B., Greene M. I. Antigen- and receptor-driven regulatory mechanisms. II. Induction of suppressor T cells with idiotype-coupled syngeneic spleen cells. J Exp Med. 1979 Nov 1;150(5):1229–1240. doi: 10.1084/jem.150.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T., Okumura K. The role of antigen-specific T cell factors in the immune response. Adv Immunol. 1979;28:1–87. doi: 10.1016/s0065-2776(08)60799-3. [DOI] [PubMed] [Google Scholar]
- Tonegawa S., Maxam A. M., Tizard R., Bernard O., Gilbert W. Sequence of a mouse germ-line gene for a variable region of an immunoglobulin light chain. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1485–1489. doi: 10.1073/pnas.75.3.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]


