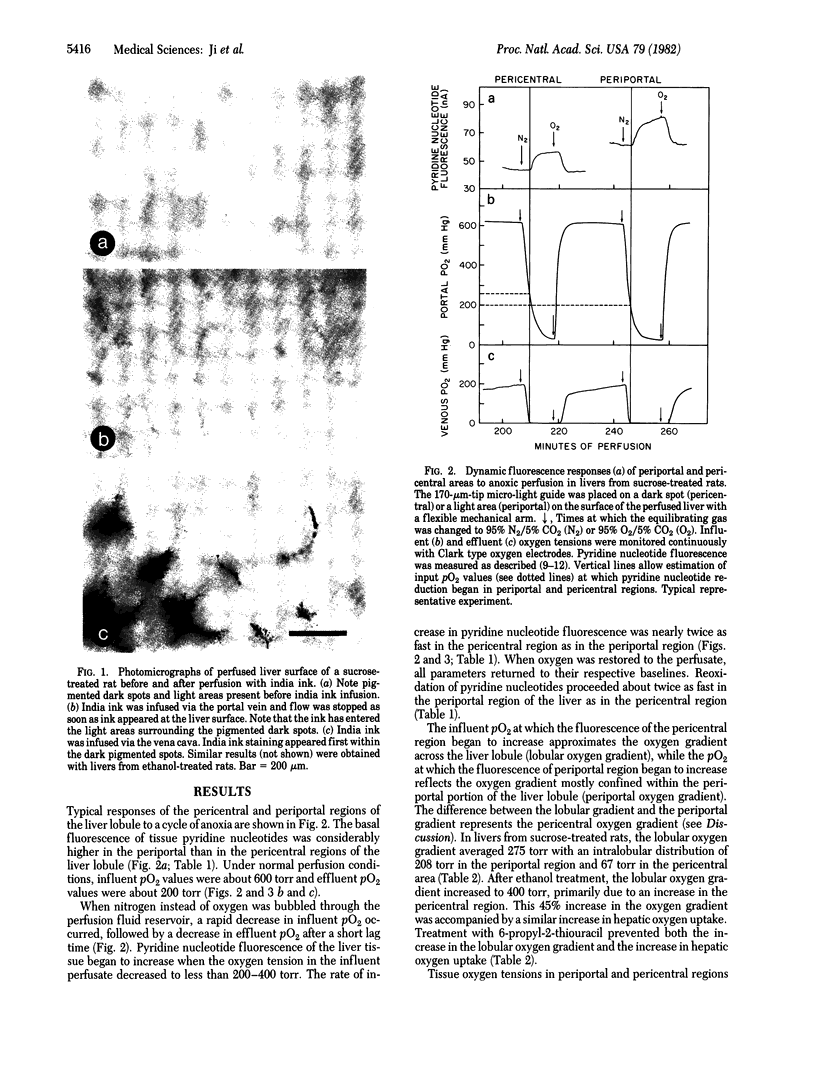
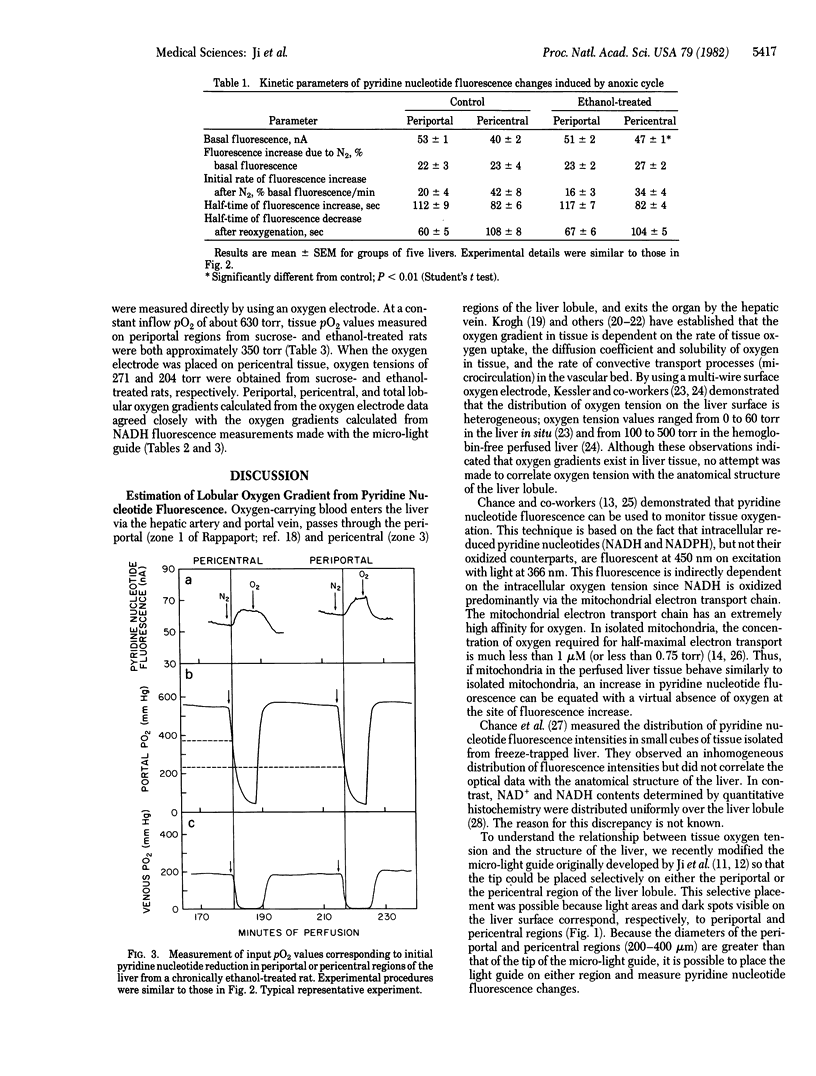
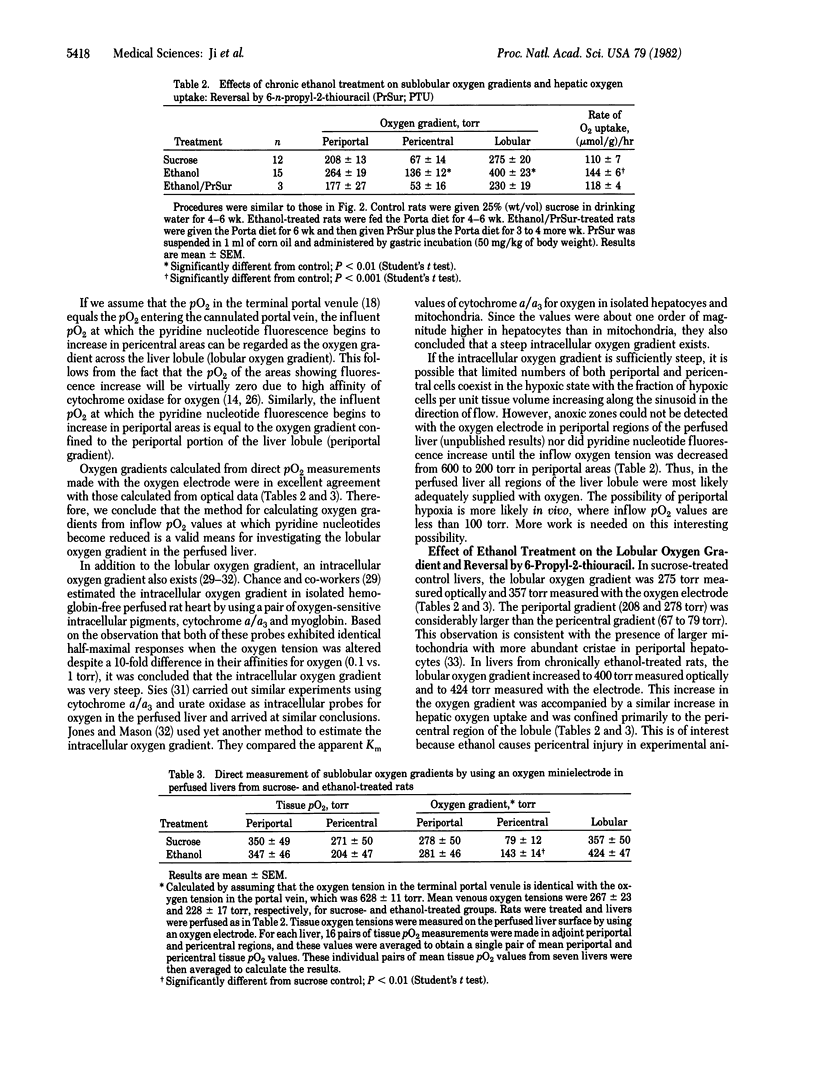
Abstract
Pyridine nucleotide fluorescence made from the surface of the hemoglobin-free perfused rat liver was measured continuously by using a "micro-light guide" placed on selected periportal and pericentral regions of the liver lobule. From the portal oxygen tension at which pyridine nucleotide reduction first occurred in pericentral regions, the oxygen gradient across the liver lobule was estimated in livers from rats treated chronically with ethanol or sucrose. Chronic treatment with ethanol increased the average lobular oxygen gradient from 275 to 400 torr (1 torr = 133 Pa), primarily due to the increase in the oxygen gradient in pericentral regions. Ethanol treatment also increased hepatic oxygen uptake significantly, from 110 to 144 (mumol/g)/hr. Treatment with the antithyroid drug 6-propyl-2-thiouracil reversed the effect of ethanol on O2 uptake and on the lobular oxygen gradient. The oxygen gradients measured with the micro-light guide were confirmed by direct measurement of tissue oxygen tensions in periportal and pericentral areas by using an oxygen electrode. These data are consistent with the hypothesis that chronic treatment with ethanol causes the pericentral region of the liver lobule to become susceptible to hypoxic cellular injury. This may be responsible, at least in part, for the localized hepatotoxic effects of ethanol.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein J., Videla L., Israel Y. Metabolic alterations produced in the liver by chronic ethanol administration. Changes related to energetic parameters of the cell. Biochem J. 1973 Jun;134(2):515–521. doi: 10.1042/bj1340515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Mayevsky A., Goodwin C., Mela L. Factors in oxygen delivery to tissue. Microvasc Res. 1974 Nov;8(3):276–282. doi: 10.1016/s0026-2862(74)80003-8. [DOI] [PubMed] [Google Scholar]
- Chance B. Reaction of oxygen with the respiratory chain in cells and tissues. J Gen Physiol. 1965 Sep;49(1 Suppl):163–195. doi: 10.1085/jgp.49.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman U., Lübbers D. W. The size of the hypoxic zone at the border of an anoxic region within the tissue. Adv Exp Med Biol. 1977 Jul 4;94:655–665. doi: 10.1007/978-1-4684-8890-6_90. [DOI] [PubMed] [Google Scholar]
- Grunewald W. A., Sowa W. Capillary structures and O 2 supply to tissue. An analysis with a digital diffusion model as applied to the skeletal muscle. Rev Physiol Biochem Pharmacol. 1977;77:149–209. [PubMed] [Google Scholar]
- Israel Y., Kalant H., Orrego H., Khanna J. M., Videla L., Phillips J. M. Experimental alcohol-induced hepatic necrosis: suppression by propylthiouracil. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1137–1141. doi: 10.1073/pnas.72.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel Y., Walfish P. G., Orrego H., Blake J., Kalant H. Thyroid hormones in alcoholic liver disease. Effect of treatment with 6-n-propylthiouracil. Gastroenterology. 1979 Jan;76(1):116–122. [PubMed] [Google Scholar]
- Ji S., Chance B., Nishiki K., Smith T., Rich T. Micro-light guides: a new method for measuring tissue fluorescence and reflectance. Am J Physiol. 1979 Mar;236(3):C144–C156. doi: 10.1152/ajpcell.1979.236.3.C144. [DOI] [PubMed] [Google Scholar]
- Ji S., Lemasters J. J., Thurman R. G. A non-invasive method to study metabolic events within sublobular regions of hemoglobin-free perfused liver. FEBS Lett. 1980 Apr 21;113(1):37–42. doi: 10.1016/0014-5793(80)80489-3. [DOI] [PubMed] [Google Scholar]
- Ji S., Lemasters J. J., Thurman R. G. Ethanol-induced changes in the intralobular oxygen gradient of perfused rat liver. Pharmacol Biochem Behav. 1980;13 (Suppl 1):41–45. doi: 10.1016/s0091-3057(80)80007-4. [DOI] [PubMed] [Google Scholar]
- Jones D. P., Mason H. S. Gradients of O2 concentration in hepatocytes. J Biol Chem. 1978 Jul 25;253(14):4874–4880. [PubMed] [Google Scholar]
- Kessler M., Höper J., Krumme B. A. Monitoring of tissue perfusion and cellular function. Anesthesiology. 1976 Aug;45(2):184–197. doi: 10.1097/00000542-197608000-00007. [DOI] [PubMed] [Google Scholar]
- Krogh A. The number and distribution of capillaries in muscles with calculations of the oxygen pressure head necessary for supplying the tissue. J Physiol. 1919 May 20;52(6):409–415. doi: 10.1113/jphysiol.1919.sp001839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemasters J. J., Ji S., Thurman R. G. Centrilobular injury following hypoxia in isolated, perfused rat liver. Science. 1981 Aug 7;213(4508):661–663. doi: 10.1126/science.7256265. [DOI] [PubMed] [Google Scholar]
- Loud A. V. A quantitative stereological description of the ultrastructure of normal rat liver parenchymal cells. J Cell Biol. 1968 Apr;37(1):27–46. doi: 10.1083/jcb.37.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshino N., Jamieson D., Chance B. The properties of hydrogen peroxide production under hyperoxic and hypoxic conditions of perfused rat liver. Biochem J. 1975 Jan;146(1):53–65. doi: 10.1042/bj1460053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshino N., Sugano T., Oshino R., Chance B. Mitochondrial function under hypoxic conditions: the steady states of cytochrome alpha+alpha3 and their relation to mitochondrial energy states. Biochim Biophys Acta. 1974 Dec 19;368(3):298–310. doi: 10.1016/0005-2728(74)90176-5. [DOI] [PubMed] [Google Scholar]
- Porta E. A., Gomez-Dumm C. L. A new experimental approach in the study of chronic alcoholism. I. Effects of high alcohol intake in rats fed a commercial laboratory diet. Lab Invest. 1968 Apr;18(4):352–364. [PubMed] [Google Scholar]
- Rappaport A. M. The microcirculatory acinar concept of normal and pathological hepatic structure. Beitr Pathol. 1976 May;157(3):215–243. doi: 10.1016/s0005-8165(76)80083-2. [DOI] [PubMed] [Google Scholar]
- Schaffer W. T., Denckla W. D., Veech R. L. The effect of chronic ethanol consumption on the rate of whole animal and perfused liver oxygen consumption. Adv Exp Med Biol. 1980;132:587–593. doi: 10.1007/978-1-4757-1419-7_61. [DOI] [PubMed] [Google Scholar]
- Schaffner F., Popper H. Alcoholic hepatitis in the spectrum of ethanol-induced liver injury. Scand J Gastroenterol Suppl. 1970;7:69–78. [PubMed] [Google Scholar]
- Scholz R., Hansen W., Thurman R. G. Interaction of mixed-function oxidation with biosynthetic processes. 1. Inhibition of gluconeogenesis by aminopyrine in perfused rat liver. Eur J Biochem. 1973 Sep 21;38(1):64–72. doi: 10.1111/j.1432-1033.1973.tb03034.x. [DOI] [PubMed] [Google Scholar]
- Sies H. Cytochrome oxidase and urate oxidase as intracellular O2 indicators in studies of O2 gradients during hypoxia in liver. Adv Exp Med Biol. 1977 Jul 4;94:561–566. doi: 10.1007/978-1-4684-8890-6_75. [DOI] [PubMed] [Google Scholar]
- Tamura M., Oshino N., Chance B., Silver I. A. Optical measurements of intracellular oxygen concentration of rat heart in vitro. Arch Biochem Biophys. 1978 Nov;191(1):8–22. doi: 10.1016/0003-9861(78)90062-0. [DOI] [PubMed] [Google Scholar]
- Thurman R. G., McKenna W. R., McCaffrey T. B. Pathways responsible for the adaptive increase in ethanol utilization following chronic treatment with ethanol: inhibitor studies with the hemoglobin-free perfused rat liver. Mol Pharmacol. 1976 Jan;12(1):156–166. [PubMed] [Google Scholar]
- Videla L., Bernstein J., Israel Y. Metabolic alterations produced in the liver by chronic ethanol administration. Increased oxidative capacity. Biochem J. 1973 Jun;134(2):507–514. doi: 10.1042/bj1340507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuki T., Thurman R. G. The swift increase in alcohol metabolism. Time course for the increase in hepatic oxygen uptake and the involvement of glycolysis. Biochem J. 1980 Jan 15;186(1):119–126. doi: 10.1042/bj1860119. [DOI] [PMC free article] [PubMed] [Google Scholar]



