Abstract
Insulin resistance is a feature of many common disorders including obesity and type 2 diabetes mellitus. In these disorders, the β-cells compensate for the insulin resistance for long periods of time with an increase in secretory capacity, an increase in β-cell mass, or both. To determine whether the β-cell response might relate to a circulating growth factor, we have transplanted normal islets under the kidney capsule of normoglycemic insulin-resistant mice with two different models of insulin resistance: lean mice that have a double heterozygous deletion of the insulin receptor and insulin receptor substrate-1 (DH) or the obese, hyperglycemic ob/ob mice. In the grafts transplanted into both hosts, there was a marked increase in β-cell mitotic activity and islet mass that was comparable with that observed in the endogenous pancreas. By contrast, islets of the DH mouse transplanted into normal mice showed reduced mitotic index. These data suggest the insulin resistance is associated with a circulating islet cell growth factor that is independent of glucose and obesity.
Many common disorders including obesity, type 2 (non-insulin-dependent) diabetes mellitus, various endocrinopathies, hypertension, and hyperlipidemias are associated with insulin resistance (1–4). In patients with type 2 diabetes, the β-cells eventually fail to meet the demand created by the insulin resistance leading to hyperglycemia; however, in most of these disorders, the β-cells compensate for the insulin resistance for long periods of time with an increase in secretory capacity, an increase in β-cell mass, or both (5–7). Rodent models of diabetes, such as the ob/ob and db/db mice and the Zucker fatty rat, and hyperinsulinemic humans with obesity and/or type 2 diabetes exhibit mild to marked islet hyperplasia at varying times of their disorders (8–12). Likewise, several mouse models of insulin resistance, such as those created by inactivation of the gene for insulin receptor substrate-1 (IRS-1) or double heterozygous (DH) knockout of the insulin receptor and IRS-1, exhibit marked islet hyperplasia (7, 13, 14).
The factors contributing to β-cell hyperplasia in insulin-resistant states remain poorly understood (15–17). Glucose itself is known to stimulate β-cell replication (reviewed in ref. 17); however, DH and IRS-1 knockouts and ob/ob and db/db mice all manifest islet hyperplasia before the onset of detectable hyperglycemia (7, 14, 18–20). Furthermore, the hyperplastic response in most cases does not bear a relationship to the level of hyperglycemia, suggesting that factors independent of glucose likely contribute to the islet growth.
The aim of the present study was to examine the hypothesis that insulin resistance induces the growth of islet β-cells via a circulating mitogenic factor that is independent of glucose and obesity. To test this hypothesis, we adopted a transplantation strategy wherein wild-type (WT) islets were transplanted under the kidney capsule into normoglycemic lean DH mice and obese, hyperglycemic ob/ob mice with corresponding controls.
Methods
Animals.
To minimize islet rejection, insulin receptor/insulin receptor substrate-1 DH mice (7) were backcrossed to >98% purity on the C57BL background at Taconic Farms and shipped with littermate WT controls to the Joslin Animal Facility before experiments. ob/ob mice and corresponding C57BL/6J controls were purchased from The Jackson Laboratory. All mice were maintained on a normal light/dark cycle and handled in accordance with Joslin Diabetes Center Animal Care and Use Committee protocols. Mice were fed normal chow ad libitum and were housed separately after transplantation. To avoid the confounding effects of hyperglycemia, we selected DH male mice that were normoglycemic.
Islet Isolation and Transplantation.
Islets from male mice were isolated by using the intraductal collagenase technique (14). Following isolation from 8-week-old mice, 100 size-matched islets (≈80–100 μm) were hand-picked by using a dissecting microscope (GZ7 Stereozoom; Leica, Deerfield, IL), concentrated in a pellet, and kept on ice until transplantation. Surgery was performed under anesthesia by using a 1:1 (wt/vol) mixture of 2,2,2-tribromoethanol and tert-amyl alcohol and diluted 1:50 with PBS (pH 7.4). Mice were injected with the mixture intraperitoneally at a dose of 15 μl/g body weight. Using a retroperitoneal approach, the capsule of one of the kidneys was incised, and the islets were implanted near the upper pole in 10-week-old male mice. The capsule was cauterized, and the mice were allowed to recover on a heating pad.
Glucose, Insulin, and Leptin Levels.
Blood glucose levels were measured at least on two different occasions by using an Elite glucometer (Bayer, Elkhart, IN). Blood was obtained from the tail vein in the fed state in the morning (between 0800 and 1000), collected in chilled heparinized tubes, and immediately centrifuged to obtain plasma, which was stored at −20°C for subsequent insulin and leptin ELISA (Crystal Chem, Chicago, IL).
Harvesting and Sectioning of Pancreas and Islet Grafts.
Eight weeks after transplantation, the animals were injected with BrdUrd (100 mg/kg body weight, at a concentration of 10 mg/ml in sterile PBS, Sigma) and killed after 6 h by administering an overdose of sodium amytal. Host animal pancreas and islet graft were rapidly dissected, fixed in Bouin's solution, and embedded in paraffin.
Islet Mass.
β-Cell mass was evaluated by point counting morphometry on immunoperoxidase-stained sections of pancreas (21, 22). Multiple sections (separated by 100 μm each) were obtained from each pancreas and analyzed systematically by using a grid system covering at least 150 fields per mouse. Separate images were acquired by using a BX60 microscope (Olympus, New Hyde Park, New York) as described earlier (23). Relative volumes were calculated for β-cells, non-β-cells, and exocrine tissue. Contaminating tissue (pancreatic ducts, lymph nodes, adipose, intestine, and exocrine pancreas) was recorded to correct for the pancreatic weight. The β-cell mass was calculated by the following formula: Islet β-Cell Mass = Relative β-cell Volume × Corrected Pancreatic Weight.
Immunohistochemistry and Graft Analysis.
Sections of pancreas and islet grafts were incubated with a mixture of mouse primary anti-BrdUrd antibody (cell proliferation kit, Amersham Pharmacia) and a mixture of antibodies against non-β-cells (mixture of anti-glucagon, anti-somatostatin, and anti-pancreatic polypeptide antibodies) as described elsewhere (14, 23) overnight at 4°C. Sections were incubated with secondary antibody (peroxidase-linked anti-mouse IgG, ICN) at room temperature for 30 min, visualized by using DAB, and counterstained with hematoxylin. At least 1,200 β-cells were counted for labeled nuclei for each islet graft. The surface area of the in situ islet grafts was measured by using a dissecting microscope (GZ7 Stereozoom, Fisher) equipped with an eyepiece bearing a calibrated micrometer disk. The thickness of the islet graft cross sections was measured by using Image Pro Plus (4.0, Media Cybernetics, Silver Spring, MD), and images of the graft sections were captured by using a BX60 microscope (Olympus) equipped with a U-PMTVC video adapter (Olympus) and an HV-C20 TV camera (Hitachi, Tokyo). Three measurements were taken at quarterly intervals for each graft and then averaged to obtain the thickness. Three sections from each graft were evaluated in this manner and averaged to yield one data point. The relative graft volume was calculated by multiplying the graft surface area by the corresponding average graft thickness. Apoptosis was measured by in situ staining of DNA cleavage and chromatin condensation by using chemically labeled nucleotides. Staining was performed by using the ApopTag kit (Intergen, New York). Islet grafts were assessed individually for BrdUrd positive and apoptotic cells by using Image Pro Plus (4.0). At least 1,000 cells were counted in each islet graft, and images of graft cross sections were captured as described above.
Statistical Analysis.
Data were analyzed by paired and unpaired Student's t tests as appropriate by using Sigma Plot (SPSS, Chicago). A P value <0.05 was considered statistically significant. All data, unless otherwise indicated, are presented as means ± SEM.
Results
Body Weight.
Before transplantation, the IR/IRS-1 double heterozygote mice were slightly smaller than controls as previously observed (7), whereas the leptin-deficient ob/ob mice were obese and significantly heavier than controls (Table 1). Over the course of the 8-week posttransplantation period, all of the animals significantly increased their body weights. The increase in weight in each group during the transplantation period was comparable to sham-operated controls and was consistent with the normal growth curves of the mice (data not shown).
Table 1.
Blood glucose, plasma insulin, and body weights in transplant recipients during the pre- and posttransplantation periods
| Islet donor | Tx recipient | Blood glucose, mg/dl
|
Plasma insulin, ng/ml
|
Body weight, g
|
|||
|---|---|---|---|---|---|---|---|
| Pre-Tx | Post-Tx | Pre-Tx | Post-Tx | Pre-Tx | Post-Tx | ||
| WT | WT | 112.9 ± 3.9 | 118.6 ± 3.9 | 1.67 ± 0.2 | 1.9 ± 0.2 | 27.5 ± 0.35 | 29.66 ± 0.37‡ |
| WT | DH | 119.5 ± 3.1 | 118.1 ± 4.0 | 4.55 ± 0.6* | 8.0 ± 1.6*‡ | 26.4 ± 0.4* | 27.82 ± 0.34*‡ |
| WT | ob/ob | 181.1 ± 19.5* | 145.6 ± 5.3* | 42.5 ± 5.7* | 42.3 ± 4.9* | 58.1 ± 1.09* | 68.2 ± 1.35*‡ |
| DH | WT | 128.3 ± 9.9 | 134.2 ± 7.5 | 2.1 ± 0.46 | 2.6 ± 0.5 | 28.82 ± 0.42 | 30.27 ± 0.28‡ |
| DH | DH | 146.4 ± 10.5 | 133.4 ± 9.3 | 7.9 ± 1.4* | 11.6 ± 1.6* | 26.77 ± 0.4* | 28.55 ± 0.39*‡ |
Blood glucose levels were significantly higher in the ob/ob recipient mice compared with the WT and DH groups in the pre- and post-Tx periods; *, P < 0.001 ob/ob vs. WT or DH. In the pre-Tx and post-Tx states, the plasma insulin levels of the DH and ob/ob mice receiving WT islets were significantly higher than controls. *, P < 0.001, WT vs. DH or ob/ob;
, P < 0.01, pre-Tx vs. post-Tx. Similarly, DH mice receiving DH islets showed significantly higher insulin levels than WT recipients. *, P < 0.01 WT vs. DH. The body weights of the DH mice were lighter than the WT controls, whereas the ob/ob mice were significantly heavier than both the WT and DH groups in the pre-Tx and post-Tx periods; *, P < 0.05 WT vs. DH or ob/ob., ‡, P < 0.05 pre-Tx vs. post-Tx. Post-Tx values were obtained eight weeks after transplantation. All values are means ± SEM. WT, n = 6–19; DH, n = 5–21; ob/ob, n = 9–21.
Blood Glucose, Plasma Insulin, and Leptin Levels in Recipient Mice.
Blood glucose levels during the pretransplantation period were comparable between the WT and DH-recipient mice, whereas the ob/ob group showed moderate hyperglycemia with glucose levels elevated 1.6-fold above normal (Table 1). As expected based on the relatively small number of islets used for the transplants, none of the mice receiving normal WT islet grafts exhibited a statistically significant alteration in blood glucose levels throughout the 8-week transplantation period. The DH and the ob/ob recipient mice displayed a slight, albeit insignificant, downward trend in glucose levels. It is possible that this reflects a temporary alteration in insulin sensitivity. There was no significant difference in blood glucose levels in the WT or DH mice receiving DH islets throughout the 8-week posttransplant period (Table 1).
In both the pretransplantation and posttransplantation states, the plasma insulin levels of the insulin-resistant DH and ob/ob mice were significantly higher than control levels (Table 1). Although posttransplantation insulin levels of the WT and ob/ob mice receiving WT islets were not different from their pretransplantation values, the posttransplantation insulin levels of the DH mice receiving WT islets were elevated ≈2-fold above pretransplantation levels, similar to the trend observed in DH mice receiving DH islets. This rise in circulating insulin is consistent with the age-dependent increase in insulin resistance observed in the double heterozygous knockout mice (7). Circulating leptin levels were significantly lower in the DH mice compared with the WT controls, as a consequence of the reduced fat mass in the double heterozygotes [7.2 ± 1.1 (WT) vs. 4.4 ± 0.8 (DH) ng/ml, P < 0.05, n = 7–9].
Islet Graft Growth.
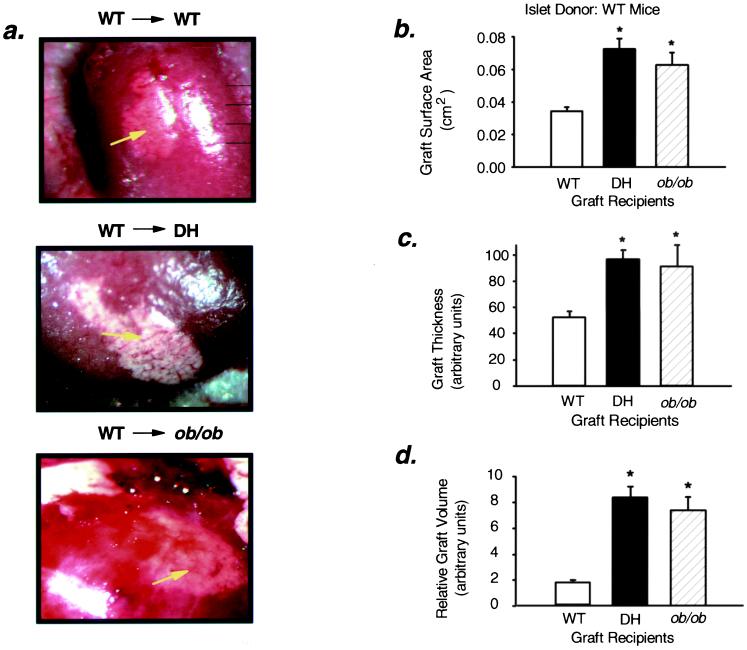
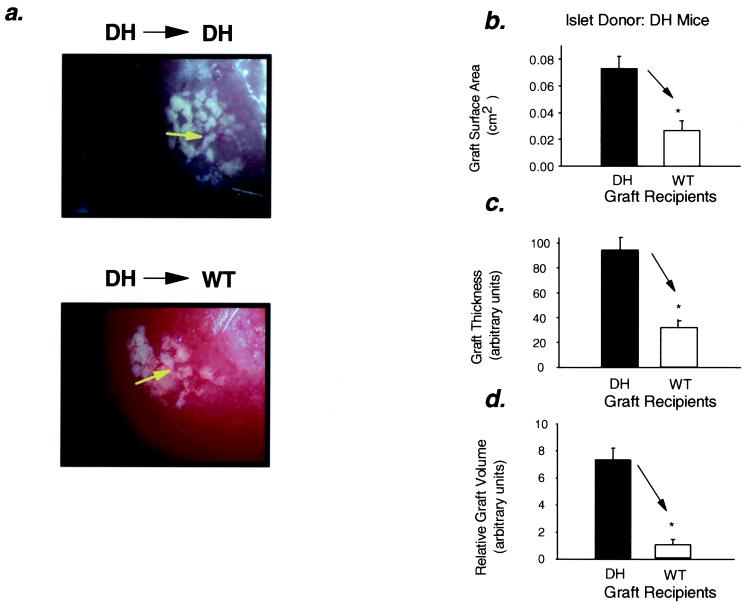
After 8 weeks within the recipient, the islet grafts appeared intact and well vascularized (Figs. 1a and 2a). Under the dissecting microscope equipped with an eyepiece bearing a calibrated micrometer disk, the average surface area of WT islet grafts in DH and ob/ob recipients was ≈110 and 81% larger than that of grafts in control WT recipients (Fig. 1b). Likewise, the surface area of DH islet grafts placed in DH recipients was ≈170% greater than the surface area of similar grafts in WT recipients (Fig. 2b). The thickness of the WT islet grafts in DH and ob/ob recipients was 1.9-fold and 1.8-fold greater, respectively, than that of WT islet grafts in WT recipients (Fig. 1c), and the mean thickness of DH islet grafts in DH recipients was 2.9-fold greater than that of similar islets in control recipients (Fig. 2c). Thus, the relative graft volume of the WT grafts in the DH and ob/ob recipients was 4.9-fold and 4.3-fold greater than the grafts in WT recipients (Fig. 1d), and the graft volume was ≈8-fold greater when DH islets were placed in DH recipients as compared with WT recipients (Fig. 2d).
Figure 1.
Surface area, thickness, and relative volume of islet grafts are increased in DH and ob/ob recipients. (a) Representative images depicting intact WT islet grafts transplanted into WT (Top), DH (Middle), and ob/ob (Bottom) mice are shown (20× magnification). The white graft tissue can be distinguished from the surrounding kidney tissue (dark red), and blood vessels (arrows) infiltrating the graft indicate vascularization. (b) Relative surface area: *, P < 0.0001, WT vs. DH, n = 17–19; *, P < 0.0001, WT vs. ob/ob, n = 6–19. (c) Thickness (expressed in arbitrary units): *, P < 0.0001, WT vs. DH, n = 17–18; *, P < 0.01, WT vs. ob/ob, n = 6–18. (d) Relative graft volume: *, P < 0.0001, WT vs. DH, n = 16–18; *, P < 0.0001, WT vs. ob/ob, n = 5–18. All values are means ± SEM.
Figure 2.
Surface area, thickness, and relative volume of islet grafts are increased in DH recipients and reduced in WT recipients. (a) Representative images depicting intact DH islet grafts transplanted into DH (Upper) and WT (Lower) mice are shown (25× magnification). The white graft tissue can be distinguished from the surrounding kidney tissue (dark red), and blood vessels infiltrating the graft indicate vascularization. (b) Relative surface area: *, P < 0.01, WT vs. DH, n = 5–6. (c) Thickness (expressed in arbitrary units): *, P < 0.01, WT vs. DH, n = 4–5. (d) Relative graft volume: *, P < 0.001, WT vs. DH, n = 4–5. All values are means ± SEM.
Assessment of β-cell mass in the endogenous pancreas of the recipient mice was also performed by using quantitative morphometry at the end of the transplantation period. This revealed a 2.5-fold and 3.6-fold increase in the DH and ob/ob mice compared with wild-type controls (2.67 ± 0.4 (WT) vs. 6.67 ± 1.7 (DH) and 9.58 ± 1.4 (ob/ob) mg; P < 0.05 WT vs. DH or ob/ob, n = 3–4).
β-Cell Mitosis and Apoptosis.
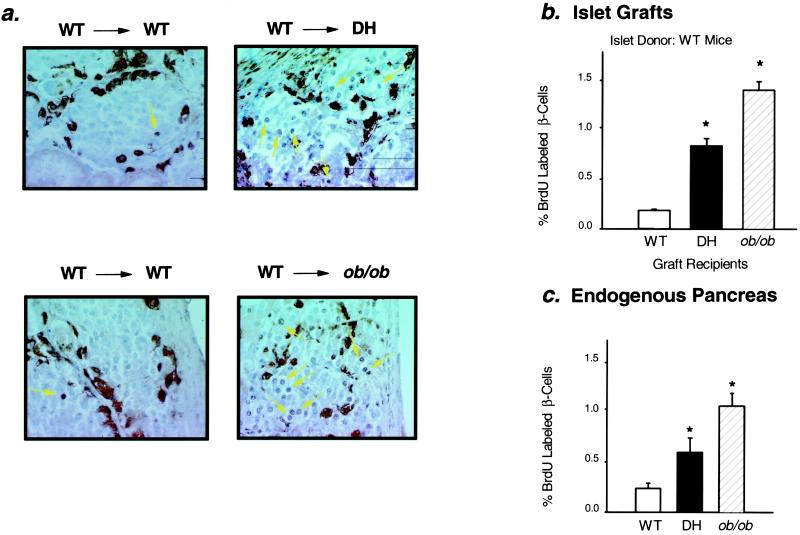
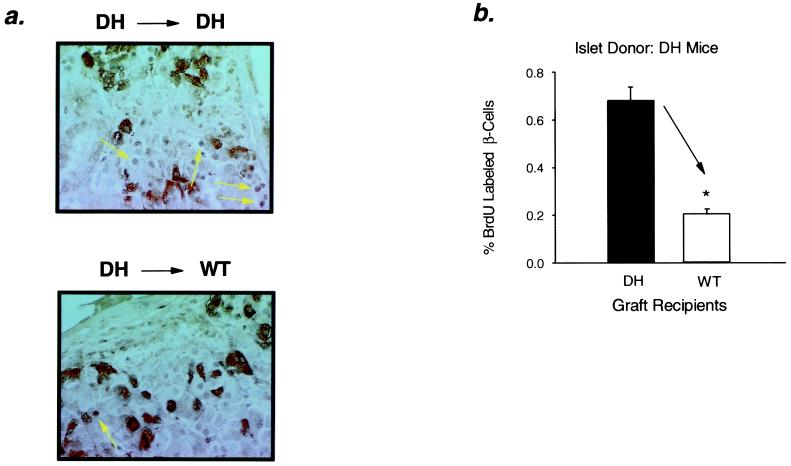
Islet mass in the pancreas is maintained by a balance between β-cell proliferation, β-cell apoptosis, and neogenesis of islets from pancreatic ductal tissue (15–17). Mitotic activity in the transplanted islets was evaluated by bromodeoxyuridine (BrdUrd) labeling. The percentage of BrdUrd-labeled nuclei in WT islets transplanted into DH and ob/ob recipients was increased by 4.4-fold and 7.4-fold, respectively, as compared with controls (Fig. 3 a and b). The increase in mitotic index in the transplanted islets in the insulin-resistant recipients was similar to that present in the endogenous islets of the host animal (Fig. 3c). BrdUrd incorporation was also increased in DH islets transplanted into DH recipients, whereas mitotic index was decreased in DH islets transplanted into WT controls (Fig. 4).
Figure 3.
Increased BrdUrd labeling of β-cell nuclei in WT islet grafts transplanted into DH and ob/ob mice and endogenous pancreas of insulin-resistant mice. (a) Cross sections of WT islet grafts dissected from WT (Left), DH (Right Upper), and ob/ob (Right Lower) recipients were stained for BrdUrd and mixture antibody as described under Methods. BrdUrd positive nuclei are stained black (arrows), and non-β-cells are stained brown-black. Representative sections are shown. (b) Mitotic index of β-cells in islet grafts: *, P < 0.0001, WT vs. DH, n = 12–13; *, P < 0.0001, WT vs. ob/ob, n = 6–13. All values are means ± SEM. (c) Mitotic index of β-cells in endogenous pancreas: *, P < 0.05 WT vs. DH or ob/ob, n = 3–4. All values are means ± SEM.
Figure 4.
Reduced BrdUrd labeling of β-cell nuclei in DH islet grafts transplanted into WT mice. (a) Cross sections of DH islet grafts dissected from DH (Upper) and WT (Lower) hosts were stained for BrdUrd and mixture antibody as described under Methods. Representative sections are shown. (b) Mitotic index of β-cells in islet grafts: *, P < 0.0001, WT vs. DH, n = 5–6. All values are means ± SEM.
To evaluate whether the islet mass is influenced by alterations in cell death, apoptosis was measured in the islet grafts by using the ApopTag kit. By contrast to mitosis, WT or DH islets showed a similar number of apoptotic cells when transplanted into WT and DH mice (17 ± 2 vs. 20 ± 3 positive cells for WT islets and 27 ± 3 vs. 23 ± 3 positive cells for DH islets; both P = nonsignificant, n = 4). These data suggest that the islet growth effects are largely mediated by an increase in β-cell mitosis rather than by an alteration in β-cell death.
Discussion
Insulin resistance is associated with a compensatory hyperinsulinemic response. In most naturally occurring, as well as genetically engineered, rodent models of type 2 diabetes, there is a strong correlation between the hyperinsulinemia and islet hyperplasia. In the present study, we have shown this islet hyperplasia is most likely due to the presence of a circulating mitogenic growth factor for islets. Thus, when islets from normal WT mice are transplanted under the kidney capsule of insulin-resistant DH or ob/ob mice, there is an increase in mitotic activity compared with that in WT recipients. As a consequence, there is a significant increase in the graft thickness and graft surface area, resulting in an overall increase in graft volume. This parallels the increase in the β-cell mass in the endogenous islets in the insulin-resistant recipients.
Although pancreatic ductal cells may serve as precursors of islet cells (16, 17, 24, 25), this factor appears to act directly on β-cells, as there are no ductal cells in the transplanted islets. Although fibroblast growth factor (26) and the pancreatic homeodomain transcription factor PDX-1 (27) have been implicated in β-cell growth, they are likely involved in terminal differentiation and maturation of β-cells. However, it is possible that they act in concert with the insulin resistance-induced islet growth factor. The factor also appears to stimulate growth by producing an increase in β-cell mitosis rather than reducing the rate of β-cell apoptosis. The fact that islets transplanted under the kidney capsule in insulin-resistant states become hyperplastic rules out the possibility of a neural signal.
The exact nature of the circulating factor is unknown, but it appears to be present in both the lean, genetically insulin-resistant DH mouse and in the obese insulin-resistant ob/ob mouse. Furthermore, although glucose has been shown to stimulate β-cell mitosis in vitro and in vivo (reviewed in refs. 17, 28, and 29), glucose does not appear to be the major mitogen in insulin-resistant states because the increase in islet mass of both endogenous and transplanted islets occurs in models that are normoglycemic; even in hyperglycemic models, islet hyperplasia has been observed before the onset of detectable hyperglycemia (7–10, 13). The mitotic activity in both the endogenous and transplanted islets is higher in the ob recipients than that in the DH mice, however, suggesting that glucose or obesity may provide additional stimulatory effects above those due to insulin resistance alone.
Among circulating growth factors, both GH and IGF-1 receptors have been demonstrated on β-cells (30, 31), and IGF-1 is known to promote DNA synthesis and prevent apoptosis of β-cells (reviewed in ref. 17). However, plasma levels of GH and IGF-1 are either normal or low in IRS-1 knockouts and in ob/ob mice (32, 33), both of which manifest islet hyperplasia. A local autocrine role for IGF-1 (34) cannot be excluded in these models. Prolactin and placental lactogen can stimulate β-cell proliferation and have been implicated in the insulin resistance of pregnancy (17), but they seem unlikely candidates in the current studies, which have been performed exclusively in male mice. Leptin has also been suggested to promote β-cell growth (35); however, this could not be a factor in the ob/ob mouse, which has genetic leptin deficiency (36), or in the DH mice that have significantly lower circulating leptin levels.
Another question that remains to be answered is what tissue serves as the source of the insulin resistance-related β-cell growth factor. In mice in which we have created liver-specific or muscle-specific insulin resistance by conditional inactivation of the insulin receptor (LIRKO and MIRKO mice), we find marked islet hyperplasia in response to hepatic insulin resistance but no islet response to the isolated muscle insulin resistance (23, 37), suggesting a possible hepatic origin of the islet growth factor. Another possibility is that the β-cell growth factor is insulin itself, as insulin levels are elevated in most states of insulin resistance, including the LIRKO mouse, but are not elevated in the MIRKO mouse. Recent studies have shown that all of the components of the insulin signaling pathway are present in β-cells and may play an important role in islet growth and function (38–41). Although IRS-1 null mice exhibit islet hyperplasia (14), IRS-2 null mice show a defect in early β-cell development (41). Mice deficient in insulin receptors in the β-cell only (βIRKO) are born with normal-size islets but fail to show the normal increase in β-cell mass observed in aging mice (39). In preliminary experiments, it appears βIRKO mice bred with LIRKO mice also fail to demonstrate an islet hyperplastic response to insulin resistance.‡ Further studies will be needed to determine the specific β-cell growth factor and the potential role of insulin in mediating or modifying this important biological feedback mechanism. Identification of this factor might be ofgreat therapeutic potential in reversing β-cell loss in type 1 diabetes or stimulating islet growth for transplantation into humans with diabetes.
Acknowledgments
We thank Ed Boschetti for help with the initial transplantation experiments, Drs. Myra Lipes and Susan Bonner-Weir for assistance with graft analysis, and Shannon E. Curtis for help with the maintenance of the animal colony. This research was supported by the Juvenile Diabetes Foundation Center for Islet Transplantation at Harvard Medical School (60-086-9293-2), National Institutes of Health Grant DK-55545 (to C.R.K.), the Joslin Diabetes Endocrinology Research Care Grant P30 DK-36836 (to C.R.K), and the Joslin Advanced Microscopy Core. R.N.K. was supported by National Institutes of Health, National Research Service Award DK-09825-02.
Abbreviations
- IRS-1
insulin receptor substrate-1
- DH
insulin receptor/insulin receptor substrate-1 double heterozygous
- WT
wild type
Footnotes
Kulkarni, R. N., Michael, M. D., Curtis, S. E., Magnuson, M. A. & Kahn, C. R., Endocrine Society, 82nd Annual Meeting, June 21–24, 2000, Toronto, Canada.
References
- 1.Reaven G M. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 2.Martin B C, Warram J H, Krolewski A S, Bergman R N, Soeldner J S, Kahn C R. Lancet. 1992;340:925–929. doi: 10.1016/0140-6736(92)92814-v. [DOI] [PubMed] [Google Scholar]
- 3.Lillioja S, Mott D M, Spraul M, Ferraro R, Foley J E, Ravussin E, Knowler W C, Bennett P H, Bogardus C. N Engl J Med. 1992;329:1988–1992. doi: 10.1056/NEJM199312303292703. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg RB. Med Clin North Am. 2000;84:81–93. doi: 10.1016/s0025-7125(05)70208-x. [DOI] [PubMed] [Google Scholar]
- 5.Kahn C R. Diabetes. 1994;43:1066–1084. doi: 10.2337/diab.43.8.1066. [DOI] [PubMed] [Google Scholar]
- 6.DeFronzo R A. Diabetes Rev. 1997;5:177–269. [Google Scholar]
- 7.Bruning J C, Winnay J, Bonner-Weir S, Taylor S I, Accili D, Kahn C R. Cell. 1997;88:561–572. doi: 10.1016/s0092-8674(00)81896-6. [DOI] [PubMed] [Google Scholar]
- 8.Gapp D A, Leiter E H, Coleman D L, Schwizer R W. Diabetologia. 1983;25:439–443. doi: 10.1007/BF00282525. [DOI] [PubMed] [Google Scholar]
- 9.Tomita T, Doull V, Pollock H G, Krizsan D. Pancreas. 1992;7:367–375. doi: 10.1097/00006676-199205000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Tokuyama Y, Sturis J, Depaoli A M, Takeda J, Stoffel M, Tang J, Sun X, Polonsky K S, Bell G I. Diabetes. 1995;44:1447–1457. doi: 10.2337/diab.44.12.1447. [DOI] [PubMed] [Google Scholar]
- 11.Kloppel G, Lohr M, Habich K, Oberholzer M, Heitz P U. Surv Synth Pathol Res. 1985;4:110–125. doi: 10.1159/000156969. [DOI] [PubMed] [Google Scholar]
- 12.Pinar H, Pinar T, Singer D B. Pediatr Dev Pathol. 2000;3:48–52. doi: 10.1007/s100240050006. [DOI] [PubMed] [Google Scholar]
- 13.Araki E, Lipes M A, Patti M E, Brüning J C, Haag B L, III, Johnson R S, Kahn C R. Nature (London) 1994;372:186–190. doi: 10.1038/372186a0. [DOI] [PubMed] [Google Scholar]
- 14.Kulkarni R N, Winnay J N, Daniels M, Bruning J C, Flier S N, Hanahan D, Kahn C R. J Clin Invest. 1999;104:R69–R75. doi: 10.1172/JCI8339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vinik A I, Pittenger G, Rafaeloff R, Rosenberg L, Duguid W. Diabetes Rev. 1996;4:235–263. [Google Scholar]
- 16.Bonner-Weir S. Endocrinology. 2000;141:1926–1929. doi: 10.1210/endo.141.6.7567. [DOI] [PubMed] [Google Scholar]
- 17.Bonner-Weir S, Smith F E. Trends Endocrinol Metab. 1994;5:60–64. doi: 10.1016/1043-2760(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 18.Edvell A, Lindström P. Endocrinology. 1999;140:778–783. doi: 10.1210/endo.140.2.6514. [DOI] [PubMed] [Google Scholar]
- 19.Dubuc P U. Metabolism. 1976;25:1567–1574. doi: 10.1016/0026-0495(76)90109-8. [DOI] [PubMed] [Google Scholar]
- 20.Chick W L, Like A A. Diabetologia. 1970;6:243–251. doi: 10.1007/BF01212233. [DOI] [PubMed] [Google Scholar]
- 21.Weibel E R. In: Stereological Methods. Weibel E R, editor. London: Academic; 1979. pp. 101–161. [Google Scholar]
- 22.Bonner-Weir S, Deery D, Leahy J L, Weir G C. Diabetes. 1989;38:49–53. doi: 10.2337/diab.38.1.49. [DOI] [PubMed] [Google Scholar]
- 23.Michael M D, Kulkarni R N, Postic C, Previs S F, Shulman G I, Magnuson M A, Kahn C R. Mol Cell. 2000;6:87–97. [PubMed] [Google Scholar]
- 24.Bonner-Weir S, Taneja M, Weir G C, Tatarkiewiez K, Song K, Sharma A, O'Neil J J. Proc Natl Acad Sci USA. 2000;97:7999–8004. doi: 10.1073/pnas.97.14.7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernard C, Berthault M F, Saulnier C, Ktorza A. FASEB J. 1999;13:1195–1205. doi: 10.1096/fasebj.13.10.1195. [DOI] [PubMed] [Google Scholar]
- 26.Hart A W, Baeza N, Apelqvist A, Edlund H. Nature (London) 2000;408:864–868. doi: 10.1038/35048589. [DOI] [PubMed] [Google Scholar]
- 27.Slack J M. Development (Cambridge, UK) 1995;121:1569–1580. doi: 10.1242/dev.121.6.1569. [DOI] [PubMed] [Google Scholar]
- 28.Swenne I. Diabetologia. 1992;35:193–201. doi: 10.1007/BF00400917. [DOI] [PubMed] [Google Scholar]
- 29.Rhodes C J. J Mol Endocrinol. 2000;24:303–311. doi: 10.1677/jme.0.0240303. [DOI] [PubMed] [Google Scholar]
- 30.Moldrup A, Billestrup N, Thorn N A, Lernmark A, Nielsen J H. Mol Endocrinol. 1989;3:1173–1182. doi: 10.1210/mend-3-8-1173. [DOI] [PubMed] [Google Scholar]
- 31.Van Schravendijk C F, Foriers A, Van den Brande J L, Pipeleers D G. Endocrinology. 1987;121:1784–1788. doi: 10.1210/endo-121-5-1784. [DOI] [PubMed] [Google Scholar]
- 32.Pete G, Fuller C R, Oldham J M, Smith D R, D'Ercole A J, Kahn C R, Lund P K. Endocrinology. 1999;140:5478–5487. doi: 10.1210/endo.140.12.7219. [DOI] [PubMed] [Google Scholar]
- 33.Cascieri M A, Slater E E, Vicario P P, Green B G, Bayne M L, Saperstein R. Diabetologia. 1989;32:342–347. doi: 10.1007/BF00277256. [DOI] [PubMed] [Google Scholar]
- 34.Smith F E, Rosen K M, Villa-Komaroff L, Bonner-Weir S. Proc Natl Acad Sci USA. 1991;88:6152–6156. doi: 10.1073/pnas.88.14.6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanabe K, Okuya S, Tanizawa Y, Matsutani A, Oka Y. Biochem Biophys Res Commun. 1997;241:765–768. doi: 10.1006/bbrc.1997.7894. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman J M. Nature (London) 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 37.Bruning J C, Michael M D, Winnay J N, Hayashi T, Horsch D, Accili D, Goodyear L J, Kahn C R. Mol Cell. 1998;2:559–569. doi: 10.1016/s1097-2765(00)80155-0. [DOI] [PubMed] [Google Scholar]
- 38.Aspinwall C A, Lakey J R, Kennedy R T. J Biol Chem. 1999;274:6360–6365. doi: 10.1074/jbc.274.10.6360. [DOI] [PubMed] [Google Scholar]
- 39.Kulkarni R N, Bruning J C, Winnay J N, Postic C, Magnuson M A, Kahn C R. Cell. 1999;96:329–339. doi: 10.1016/s0092-8674(00)80546-2. [DOI] [PubMed] [Google Scholar]
- 40.Leibiger I B, Leibiger B, Moede T, Berggren P O. Mol Cell. 1998;1:933–938. doi: 10.1016/s1097-2765(00)80093-3. [DOI] [PubMed] [Google Scholar]
- 41.Withers D J, Gutierrez J S, Towery H, Burks D J, Ren J M, Previs S, Zhang Y, Bernal D, Pons S, Shulman G I, et al. Nature (London) 1998;391:900–904. doi: 10.1038/36116. [DOI] [PubMed] [Google Scholar]