Abstract
Objectives To determine whether the routine use of intraoperative cholangiography can improve survival from complications related to bile duct injuries.
Design Population based cohort study.
Setting Prospectively collected data from the Swedish national registry of gallstone surgery and endoscopic retrograde cholangiopancreatography, GallRiks. Multivariate analysis done by Cox regression.
Population All cholecystectomies recorded in GallRiks between 1 May 2005 and 31 December 2010.
Main outcome measures Evidence of bile duct injury, rate of intended use of intraoperative cholangiography, and rate of survival after cholecytectomy.
Results During the study, 51 041 cholecystectomies were registered in GallRiks and 747 (1.5%) iatrogenic bile duct injuries identified. Patients with bile duct injuries had an impaired survival compared with those without injury (mortality at one year 3.9% v 1.1%). Kaplan-Meier analysis showed that early detection of a bile duct injury, during the primary operation, improved survival. The intention to use intraoperative cholangiography reduced the risk of death after cholecystectomy by 62% (hazard ratio 0.38 (95% confidence interval 0.31 to 0.46)).
Conclusions The high incidence of bile duct injury recorded is probably from GallRiks’ ability to detect the entire range of injury severities, from minor ductal lesions to complete transections of major ducts. Patients with bile duct injury during cholecystectomy had impaired survival, and early detection of the injury improved survival. The intention to perform an intraoperative cholangiography reduced the risk of death after cholecystectomy.
Introduction
Bile duct injury during cholecystectomy is a serious surgical complication that can have devastating consequences, including a significant risk of early death.1 2 Bile duct injury also has a major effect on healthcare costs, since injured patients frequently need prolonged and repeated hospital stay, multiple reinterventions, and long sick leave.3
Many efforts have been made to determine which factors affect the risk of death after bile duct injury. Advanced age and severe comorbidity have been clearly established as causally linked to poor survival.1 2 4 Indeed, the most intensely debated factor is the use of intraoperative cholangiography; previous studies by our group2 and other researchers1 have shown an association between the performance of intraoperative cholangiography and improved survival. However, since all previous results have been based on data for intraoperative cholangiography actually performed, skeptics have validly argued that this association might be due to bias through differential misclassification.
In anatomically difficult cholecystectomies, which probably carry a high risk of bile duct injury, it is difficult to perform a successful intraoperative cholangiogram, thus reducing the cholangiography rate in cholecystectomies prone to bile duct injury. In the present study, we have the advantage, compared with previous studies, of using data that are validated, prospectively collected, and population based, not only on actually performed intraoperative cholangiography but also on intended but failed procedures. The main aim of this study was to determine the effect of all intended intraoperative cholangiograms on survival. Our hypothesis was that the routine performance of intraoperative cholangiography is causally linked to improved survival.
Other aims of the study were to determine whether other exposures affect survival after bile duct injury, as previously proposed—such as hospital and surgeon yearly volume of cholecystectomy and preoperative versus delayed detection of bile duct injury. Finally, we aimed to validly measure the incidence of bile duct injury, because most previous studies could have underestimated the incidence owing to a certain inability to detect less severe bile duct injuries in their methodology.4 5 6
Methods
GallRiks
The study was based on data from GallRiks, the Swedish registry for gallstone surgery and endoscopic retrograde cholangiopancreatography (ERCP). The registry was founded by the Swedish Surgical Society together with the Swedish Society of Upper Abdominal Surgery and the Swedish Society of Laparoscopic Surgery in 2005, and is financially supported by the Swedish national health authorities. Registration in GallRiks started in May 2005. The aim of the registry is to obtain a complete national registration of gallstone interventions and to provide continuously updated information regarding indications, treatment methods, and complications, as well as patient satisfaction with reference to the given treatment. GallRiks covers laparoscopic and open surgery of the gallbladder as well as endoscopic interventions of the bile ducts and pancreas, and uses an internet platform for online registration. The surgeon or endoscopist reports patient characteristics, indications, and choice of surgical method as well as detailed information of immediate complications. After 30 days, the medical records are reviewed and late complications reported by a local coordinator.
Since the registry’s founding in 2005, the number of participating hospitals has steadily increased, and GallRiks is considered to be completely nationwide, including all Swedish centres performing biliary interventions, from 2009 and onwards. Comparison with data for registered cholecystectomies in the Swedish Outpatient register, where all diagnosis codes and surgical intervention codes from the international classification of diseases (ICD) are registered, shows that the coverage rate has increased rapidly since the start of registration in 2005. The coverage rate was about 65% in 2006 and has steadily increased since. In 2009 and 2010, 90% of Swedish cholecystectomies were registered in GallRiks.7
The data collected in GallRiks were validated on a regular basis by an independent audit group that regularly compared registered data with local patient records, both validating the data themselves as well as auditing to ensure that adequate resources were assigned for registration and follow-up at each centre. Each hospital was visited once every three years, and a sample of medical records were compared with entries of the registry database. GallRiks’ annual report presented the results from hospital visits, and the review of the first 1207 medical records at 67 different hospitals showed 98% correct registrations.8
Definition and classification of bile duct injuries in GallRiks
We defined bile duct injury at cholecystectomy as any tissue damage to the wall of any bile duct in the biliary tree detected during the cholecystectomy or diagnosed postoperatively as a result of a bile leak or biliary obstruction not caused by a stone. This definition included all types of postoperative bile leaks, including leakage from the cystic duct.
Bile duct injuries detected during the primary cholecystectomy were registered in relation to their anatomical location in the bile duct tree. The extent of the injury was graded and registered as a lesion smaller than a third of the duct circumference, a lesion larger than a third of the circumference, or a complete transection of the duct. Bile duct injuries detected after the primary operation (that is, delayed detection), and within the 30 day span of the registry follow-up were usually discovered from a bile leak or obstruction not caused by a stone. The leak or obstruction, which could in turn be caused by stricture or a clipping or suture, was classified as a bile duct injury with delayed detection if a reoperation, imaging (such as endoscopically performed cholangiography), or bile leakage indicated a bile duct lesion at the 30 day follow-up.
There is no international consensus regarding the definition of severe and less severe bile duct injury. However, lesions requiring reconstructive surgery with hepatico-enteric anastomosis (major strictures, transectional injuries, and injuries above the hepatic confluence) are usually considered to be severe, whereas partial wall injuries or leakages from the cystic duct or small ducts in the liver bed are regarded as less severe. We used this definition.
Statistical analysis
We did statistical analyses using Stata 11 software. We extracted available data for all 51 041 cholecystectomies in GallRiks between 1 May 2005 and 31 December 2010. The data included dates of death during the study period, collected from the National Population Registry. We calculated annual incidence of bile duct injury detected perioperatively and postoperatively, and those with delayed detection. Survival among patients having undergone cholecystectomy, both with and without bile duct injury, as well as at detection, was assessed by the Kaplan-Meier method.
To estimate the effect of bile duct injury on survival, as well as to identify risk factors affecting survival, we used Cox proportional hazards regression to calculate hazard ratios with 95% confidence intervals. The endpoint was all cause mortality. We introduced sex, age, American Society of Anesthesiologists (ASA) classification, planned or emergency operation, hospital annual caseload, surgeon annual caseload, bile duct injury, and the use of intraoperative cholangiography into the model as potential risk factors or confounders of survival. We examined the proportional hazards assumption using Shoenfeld’s partial residuals.9 The presence or absence of bile duct injury, sex, ASA classification, planned or emergency operation, and intraoperative cholangiography did not completely fulfill the proportional hazards assumption. Therefore, we entered these variables into the model using the time varying covariate option for the main exposure variable, and the strata option for potential confounders—that is, by assuming a different baseline hazard for each combination of those variables.
Follow-up was from the date of cholecystectomy till date of death or end of follow-up on 31 December 2010. We analyzed age as a continuous variable (yearly increase). Comorbidity, measured using the ASA classification, was categorized as healthy (ASA 1), mild disease (ASA 2), or severely impaired health (ASA 3-5). The annual hospital caseload of cholecystectomies was classified into low volume or high volume using the close to mean 200 annual cholecystectomies (mean 206, median 178). We also categorized surgeon annual caseload into fewer or more than 14 (mean 21, median 14) cholecystectomies per year. Use of intraoperative cholangiography was dichotomized into performed or attempted procedures as one category, and those procedures not attempted as the other, thus using an intention-to-do approach. To estimate the effect of intraoperative cholangiography on bile duct injury occurrence, we used a logistic regression model, adjusting for the same possible confounders as the Cox model.
Regarding missing data, the relative numbers were very low. In the 51 041 records, the following variables analyzed had missing data: sex (n=24), age (n=120), and intraoperative cholangiography (n=626). Analyses including or excluding the missing records did not differ significantly.
Results
Incidence of bile duct injury
Between 1 May 2005 and 31 December 2010, 51 041 cholecystectomies were registered in the Swedish Registry for Gallstone Surgery and ERCP, GallRiks. In total, 747 bile duct injuries, according to our definition, were detected, corresponding to a cumulative incidence of 1.5%. There was no significant difference in incidence of bile duct injury between the sexes, but patients with a bile duct injury were slightly older than those patients without an injury (mean 55.2 v 50.7 years).
We detected 170 (23%) bile duct injuries during the primary cholecystectomy and 577 (77%) during the postoperative period. Table 1 presents annual distribution of registered cholecystectomies in GallRiks, together with perioperative and delayed detected bile duct injuries.
Table 1.
Cholecystectomies and annual incidence of bile duct injury, recorded in GallRiks
| Year | No of cholecystectomies | No (%) of bile duct injuries | ||
|---|---|---|---|---|
| Early detection* | Delayed detection† | Total | ||
| 2005‡ | 1113 | 1 (0.1) | 7 (0.6) | 8 (0.7) |
| 2006 | 7680 | 36 (0.5) | 81 (1.1) | 117 (1.5) |
| 2007 | 8931 | 21 (0.2) | 94 (1.1) | 115 (1.3) |
| 2008 | 10 350 | 35 (0.3) | 135 (1.3) | 170 (1.6) |
| 2009 | 11 823 | 44 (0.4) | 126 (1.1) | 170 (1.6) |
| 2010 | 11 144 | 33 (0.3) | 134 (1.2) | 167 (1.5) |
| Total | 51 041 | 170 (0.3) | 577 (1.1) | 747 (1.5) |
*Detected during cholecystectomy.
†Detected after cholecystectomy.
‡1 May to 31 December 2005.
Severity of bile duct injuries
Among 55 severe bile duct injuries, 41 (75%) were detected during the primary cholecystectomy. Among perioperatively detected lesions, 41 (24%) were correspondingly classified as severe. A majority of bile duct injuries detected postoperatively were discovered as bile leakage, either from the cystic duct (46%) or from small ducts in the liver bed (18%), whereas only 14 (2%) injuries with delayed detection were classified as severe. Table 2 summarizes distribution and severity of bile duct injuries detected perioperatively and postoperatively.
Table 2.
Bile duct injuries among 51 041 cholecystectomies recorded in GallRiks, according to detection timing
| No of bile duct injuries | |
|---|---|
| Detected during primary operation | |
| Partial injury to common bile duct or hepatic duct | 119 |
| Transection of common bile duct or hepatic duct* | 13 |
| Lesion above hepatic duct confluence* | 28 |
| Injury not classifiable | 10 |
| Subtotal (%) | 170 (23) |
| Delayed detection† | |
| Cystic duct leakage | 265 |
| Leakage from small ducts in the liver bed | 106 |
| Partial injury to common bile duct or hepatic duct | 11 |
| Transection of common bile duct or hepatic duct* | 3 |
| Lesion above the hepatic duct confluence* | 4 |
| Postoperative major stricture* | 7 |
| Unknown cause or injury not classifiable | 181 |
| Subtotal (%) | 577 (77) |
| Total | 747 |
*Classified as severe bile duct injuries.
†Injury detected postoperatively, within 30 days of cholecystectomy.
Survival after cholecystectomy with and without bile duct injury
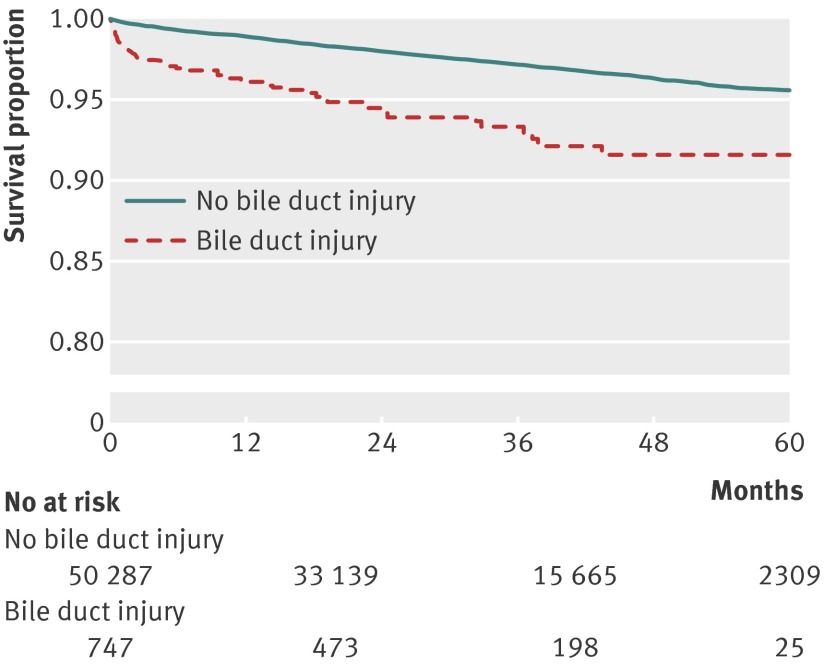
Figure 1 shows the Kaplan-Meier survival curves of study patients. Patients with bile duct injury had a significantly lower overall survival than non-injured patients after cholecystectomy (mortality at one year 3.9% v 1.1%).
Fig 1 Survival in patients after cholecystectomy, with and without bile duct injury
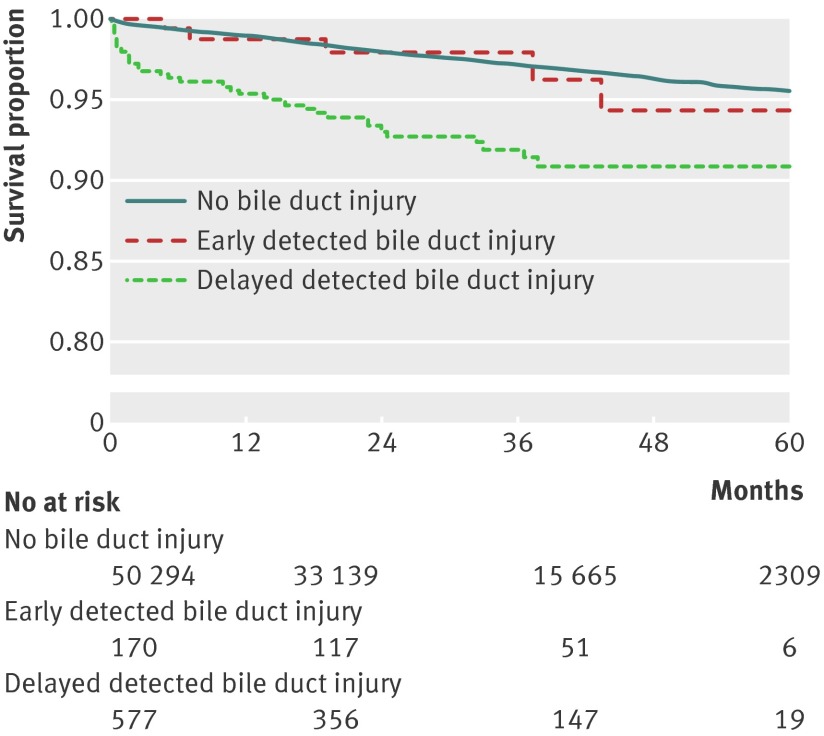
Compared with patients with a bile duct injury detected during surgery, Kaplan-Meier survival curves showed impaired survival in those patients with delayed detection of bile duct injury. Survival in patients with perioperatively detected injuries did not differ significantly from that in non-injured patients undergoing cholecystectomy (fig 2). In survival analysis with respect to the severity of bile duct injury, the statistical power was insufficient for analysis with acceptable precision (data not shown).
Fig 2 Survival in patients after cholecystectomy, in relation to no injury and early and delayed detection of bile duct injury
We used Cox regression analysis to investigate the relative risk of death, adjusted for sex, age, ASA classification, planned or emergency operation, and the use of intraoperative cholangiography. We found a close to doubled risk of death among patients with a bile duct injury during the first year after surgery, compared with those without (hazard ratio 1.92 (95% confidence interval 1.24 to 2.97)). When considering detection time, injuries with delayed detection had a significantly doubled risk of death compared with no injuries (1.95 (1.12 to 3.37)). Risk of death in patients with bile duct injuries detected perioperatively did not differ from the risk in patients with no injury after cholecystectomy.
Increased age, a high ASA score, and emergency operation were all associated with impaired survival after cholecystectomy in the multivariate model (table 3). However, surgery at a hospital with annual volumes of more than 200 cholecystectomies was associated with improved survival. A surgeon’s annual caseload of cholecystectomies did not influence the survival rate. We used the median of 14 annual cholecystectomies per surgeon in the model, owing to reasons relating to study power, but a complementary analysis comparing a high volume of annual operations (>40) with a low volume (<10) did not show significant differences in survival (data not shown). The intention to use intraoperative cholangiography significantly reduced the risk of death after cholecystectomy by 62% (hazard ratio 0.38 (95% confidence interval 0.31 to 0.46)).
Table 3.
Cox proportional hazard model of survival and factors influencing survival after cholecystectomy
| Variables | Hazard ratio (95% CI) | |
|---|---|---|
| Crude | Adjusted§ | |
| Age (per yearly increase) | 1.10 (1.09 to 1.10) | 1.07 (1.07 to 1.08) |
| Sex | ||
| Male | 1.0 (reference) | 1.0 (reference) |
| Female | 0.48 (0.43 to 0.54) | 0.85 (0.72 to 1.01) |
| ASA score* | ||
| 1 | 1.0 (reference) | 1.0 (reference) |
| 2 | 5.04 (4.29 to 5.92) | 2.65 (2.11 to 3.34) |
| 3-5 | 23.46 (19.89 to 27.67) | 9.76 (7.17 to 13.28) |
| Surgery* | ||
| Planned | 1.0 (reference) | 1.0 (reference) |
| Emergency | 2.49 (2.23 to 2.78) | 2.05 (1.69 to 2.49) |
| Surgeon’s annual caseload† | ||
| <14 cholecystectomies/surgeon | 1.0 (reference) | 1.0 (reference) |
| >14 cholecystectomies/surgeon | 0.89 (0.80 to 1.00) | 0.90 (0.82 to 1.01) |
| Hospital annual caseload‡ | ||
| <200 cholecystectomies/year | 1.0 (reference) | 1.0 (reference) |
| >200 cholecystectomies/year | 0.77 (0.69 to 0.86) | 0.86 (0.76 to 0.97) |
| Bile duct injury* | ||
| No injury | 1.0 (reference) | 1.0 (reference) |
| With injury | 2.57 (1.91 to 3.46) | 1.92 (1.24 to 2.97) |
| Early detection of injury | 1.17 (0.49 to 2.82) | 0.71 (0.21 to 2.40) |
| Delayed detection of injury | 3.02 (2.20 to 4.14) | 1.95 (1.12 to 3.37) |
| Intraoperative cholangiogram* | ||
| Not performed | 1.0 (reference) | 1.0 (reference) |
| Performed | 0.42 (0.37 to 0.48) | 0.38 (0.31 to 0.45) |
| Attempted but interrupted | 0.51 (0.36 to 0.70) | 0.36 (0.23 to 0.54) |
| Intended | 0.44 (0.38 to 0.50) | 0.38 (0.31 to 0.46) |
*Variables with sign of non-proportional hazards according to Schoenfeld’s residuals; Thus, variables were treated as time varying, and hazard ratios should be interpreted as the effect during the first year after surgery.
†Median: 14 annual cholecystectomies/surgeon.
‡Mean: 201 cholecystectomies/year.
§Derived from a Cox regression model, mutually adjusted for variables listed in the table.
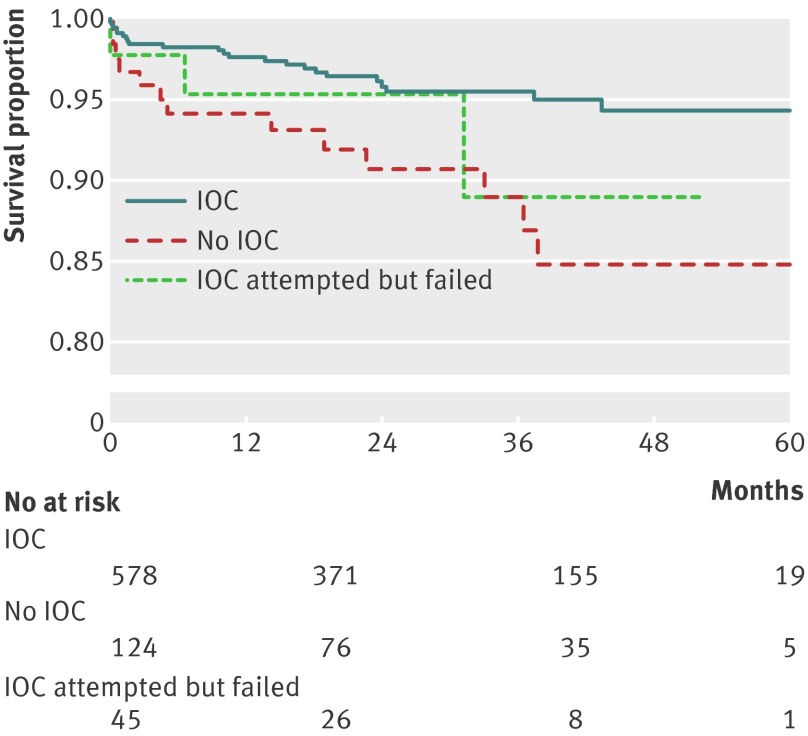
We analyzed the cohort of patients with bile duct injury using the Kaplan-Meier method. We found that injured patients who were operated with the use of intraoperative cholangiography had a significantly improved survival compared with injured patients for whom intraoperative cholangiography was not attempted. Even an unsuccessful attempt to use intraoperative cholangiography showed better outcome than no use at all (fig 3).
Fig 3 Survival in patients with iatrogenic bile duct injury during cholecystectomy, according to use of intraoperative cholangiography (IOC)
Bile duct injury and use of intraoperative cholangiography
The incidence of a bile duct injury was 29% lower when intraoperative cholangiography was successfully performed or attempted (odds ratio 0.71 (95% confidence interval 0.58 to 0.87)), after multivariate adjustment for the variables in table 3. Intraoperative cholangiography was intended in 157 (92%) of 170 injuries detected during the primary operation compared with 466 (81%) of 577 injuries with delayed detection. When intraoperative cholangiography was intended to be used, 157 (25%) of 623 injuries were detected during the primary operation compared with only 13 (11%) of 121 in the non-intraoperative cholangiography group.
Discussion
This study, using data from the highly valid GallRiks, the national Swedish registry for gallstone surgery and ERCP, confirms that survival among patients with bile duct injury during cholecystectomy is significantly impaired, compared with patients without bile duct injury. Furthermore, our study indicates that survival after bile duct injury is impaired by the failure to detect injury intraoperatively, and that the intention to use intraoperative cholangiography during cholecystectomy improves survival significantly.
Strengths and limitations of the study
The population based design, with prospectively collected data from GallRiks, was a major strength of this study. From the start of the registry in May 2005, a continuous validation process has disclosed a high validity of registered data. Annual reports7 8 deal with coverage and accuracy of registered data, and present web based summaries that are easily available to participating hospitals and the community at large.
Self reported registries are always prone to a risk of selection bias, in which the reporting clinician might fail to report negative events related to the intervention, thereby generating a falsely low rate of complications. To minimize losses in validity by data being self reported in GallRiks, a local non-physician coordinator at each hospital reviewed complications and interventions 30 days after surgery. The review would look at pharmacological treatment, percutaneous drainage, endoscopic interventions, or reoperations.
There might be some concern about how we used data at the start of the registry, even though the registry did not have nationwide coverage until 2009. However, the annual reports showed that data were valid, even for registration early in the period. Moreover, the risk of selection bias imposed by low coverage was largely relieved by the fact that coverage, even in the registry’s early years, was high at each participating hospital, and that the coverage increase during the period 2005-09 has predominantly been by the addition of new hospitals and not by a gradual increase in coverage within each hospital. Since the Swedish healthcare system is largely based on hospitals with defined catchment areas, it is reasonable to consider the registry to be population based from the start of registration, since coverage has always been high within each catchment area.
A study validating registrations of complications in GallRiks was presented in the registry’s annual report of 2010,8 comparing registration in the Swedish health insurance system with GallRiks data. All health related injuries associated with a cholecystectomy reported to the insurance system between 1 June 2005 and 31 May 2010 were cross checked with GallRiks. All cases of bile duct injury reported to the Swedish patient insurance were also documented as a bile duct injury in GallRiks, indicating a good coverage of these cases in the registry throughout the study. Furthermore, 90% of cholecystectomies in the Swedish patient registry were also registered in GallRiks.
Another strength of this study was the ability to detect the entire spectrum of injuries, ranging from less severe bile leakages after surgery (such as the cystic duct or lesions to peripheral ducts in the liver bed) to severe injuries (with transections and tissue loss located at or above the hepatic confluence). The vast majority of studies on this topic have focused either on severe bile duct injury, referred to and treated by tertiary hepato-biliary centers,1 2 4 5 10 or on a selection of cases accessible to endoscopic treatment.11 12 13 Larger, population based studies originating from administrative national registries with injury identification through ICD diagnosis or procedure codes have substantial difficulties with the identification of less severe bile duct injuries, owing to the inconsequent registration of complication codes. A detailed registration of localization and extent of detected lesions in GallRiks enables a classification of injuries according to existing international classification systems.14 15 16 Unfortunately, data for concomitant vascular injuries were not registered in GallRiks, which could have understaged the severity of combined bile duct injury and vascular injury, since the frequency of such injuries has been reported to be as high as 11-32%17 18 19 in severe bile duct injuries.
There also might be arguments against including minor, postoperative bile leakages into the definition of bile duct injury, since these are often managed in conservatively, and only a minority of these leakages have been properly diagnosed and therefore eligible for bile duct injury classification. These injuries are rarely included in published incidence data of bile duct injuries, although they are clearly defined as bile duct injuries in the majority of internationally accepted classification systems.15 16
Comparison with previous studies
In this study, we found a cumulative incidence of bile duct injury of 1.5%, which widely exceeds previously reported rates of 0.15-1.09%.1 4 5 20 In a large, population based study of 152 776 cholecystectomies in the Swedish inpatient registry, Waage and colleagues5 estimated the incidence of reconstructed iatrogenic bile duct injury to be 0.4-0.5% in 1987-2001, using ICD codes for biliary reconstruction as a proxy for bile duct injury. This notable difference is most likely due to the unique ability of procedure based quality registries, such as GallRiks, in detecting less severe lesions that cause postoperative bile leakage. If we were to exclude these instances of postoperative leakage, the incidence would have dropped to 0.3%, which is highly comparable to data for bile duct injury based on reconstruction identification methodology, reflecting a selection of the most severe cases. Without the use of quality registries based on validated procedures, less severe bile duct injuries would indeed be difficult to study, and previously reported incidence rates of bile duct injury should be interpreted with this factor in mind.
We found 77% of bile duct injuries that were detected postoperatively, mainly as a bile leakage from small ducts in the liver bed or cystic duct. This proportion accords with previous findings that about three quarters of bile duct injuries are detected after the primary cholecystectomy.20 21 22 Only a minority of bile duct injuries with delayed detection manifested as biliary strictures, but this is not surprising because strictures usually develop during a period longer than the 30 days of follow-up in GallRiks. Therefore, the true incidence of bile duct injury could be even higher than the estimated 1.5%.
Among patients with a bile duct injury during cholecystectomy, we found a doubled hazard of death during the first year compared with patients with no injuries. Using data from the Swedish inpatient registry from 1965 to 2005, we investigated impaired survival after reconstructed bile duct injury, and found a 3.7 hazard ratio of death within one year compared with non-injured patients.2 However, in conformity with other studies1 4 using reconstruction codes to identify bile duct injury, these data represented a selection of severe cases of bile duct injury, without including the entire range of less severe injuries captured in GallRiks.
Using a stratified Cox model, we analyzed factors influencing the risk of death after bile duct injury. Increased age and presence of comorbid disease were unsurprisingly negative prognostic factors for survival, which is consistent with previous studies.1 2 Moreover, cholecystectomy at a hospital with annual volumes exceeding 200 operations had a favorable survival outcome compared with low volume hospitals. The association between adverse outcome and volumes of common surgical procedures has not been established in previous larger studies,23 24 but is well known in more complex procedures such as pancreatectomy25 and esophagectomy.26
Furthermore, our results strongly suggest that bile duct injury detected postoperatively, even if it is a less severe injury, is associated with substantially impaired survival, with twice the hazard of death compared with injuries detected during the cholecystectomy. Since delayed detection is largely associated with bile leakage, the bile leakage could be a substantial negative prognostic factor, causing chemical peritonitis and often secondarily generalized infection.
The intention to use intraoperative cholangiography was significantly associated with improved survival after cholecystectomy compared with operations without such intention of use. Additionally, the overall risk of bile duct injury fell by 29% if the intraoperative cholangiogram was intended compared with not used. Furthermore, our data showed that injuries were more frequently detected during the cholecystectomy when intraoperative cholangiography was used, which seems important bearing in mind the survival benefit of early detection that our data also indicate.
The routine use of intraoperative cholangiography has been widely debated in past decades, with diverging recommendations.5 27 28 29 Large, population based studies have predominantly shown a reduced risk of bile duct injury after intraoperative cholangiography. Flum and colleagues28 showed a 42% reduction in risk of reconstructed bile duct injury with the use of intraoperative cholangiography; their data were supported by Waage and colleague’s study,5 which used records from the Swedish inpatient registry with identical methodology. On the other hand, in the Swiss Association of Laparoscopic and Thoracoscopic Surgery study,27 Giger and colleagues did not find evidence of decreased risk of bile duct injury after intraoperative cholangiography was used.
The major drawback in previous studies on this topic is that the reasons for not performing intraoperative cholangiography might be a selection of cases in which the procedure was not technically possible, with an increased risk of negative outcomes (including bile duct injury) caused by generally difficult surgical circumstances. This problem is solved with GallRiks registering data not only on the actual use of intraoperative cholangiography, but also on the intention to use the procedure, whether successful or not. Additionally, there are surgeons who rarely perform intraoperative cholangiography except when an injury might have occurred, which includes a disproportionally larger number of bile duct injuries in the intraoperative cholangiography group. This factor would dilute any favorable association between intraoperative cholangiography use and bile duct injury; therefore, the observed survival benefit could be even larger than that recorded in our study.
Conclusions and policy implications
To our knowledge, this study is the first to use data for intention-to-do intraoperative cholangiography to analyze the effect of intraoperative cholangiography on survival after cholecystectomy. The significant advantage in survival among patients for whom intraoperative cholangiography was intended suggests that routine use during cholecystectomy might be beneficial.
In conclusion, in this study using data from the highly valid GallRiks registry, we found the incidence of bile duct injury to be 1.5%, which included the entire range of injuries, from minor to major cases. Survival after bile duct injury decreased with increased age and comorbidity. Our study also strongly suggests that survival is impaired by failure to detect iatrogenic bile duct injury during cholecystectomy, and that use of intraoperative cholangiography significantly improved survival after cholecystectomy.
What is already known on this topic
Iatrogenic bile duct injury is a serious complication to cholecystectomy
The use of intraoperative cholangiography to prevent injury has been widely debated
Previous studies have had methodological limitations, often with insufficient information on the use of intraoperative cholangiography from registers
What this study adds
GallRiks is a high quality registry for gallstone surgery and endoscopic retrograde cholangiopancreatography in Sweden
With GallRiks data, early detection of a bile duct injury and the intention to use intraoperative cholangiography were found to improve survival after cholecystectomy
Survival after bile duct injury decreased with increased age and comorbidity
Contributors: All authors have participated in the conception and design of this study. BT performed the data analysis, but all the authors participated in the interpretation of analysed data and the drafting and critical revision of the article before submission. MN is the study guarantor.
Funding: No external funding was used for this project.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: The regional research ethics committee at Karolinska Institutet, Stockholm, Sweden, approved the study.
Data sharing: Dataset can be made available after contact with the corresponding author at bjorn.tornqvist@karolinska.se.
Cite this as: BMJ 2012;345:e6457
References
- 1.Flum DR, Cheadle A, Prela C, Dellinger EP, Chan L. Bile duct injury during cholecystectomy and survival in medicare beneficiaries. JAMA 2003;290:2168-73. [DOI] [PubMed] [Google Scholar]
- 2.Tornqvist B, Zheng Z, Ye W, Waage A, Nilsson M. Long-term effects of iatrogenic bile duct injury during cholecystectomy. Clin Gastroenterol Hepatol 2009;7:1013-8. [DOI] [PubMed] [Google Scholar]
- 3.Savader SJ, Lillemoe KD, Prescott CA, Winick AB, Venbrux AC, Lund GB, et al. Laparoscopic cholecystectomy-related bile duct injuries: a health and financial disaster. Ann Surg 1997;225:268-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dolan JP, Diggs BS, Sheppard BC, Hunter JG. Ten-year trend in the national volume of bile duct injuries requiring operative repair. Surg Endosc 2005;19:967-73. [DOI] [PubMed] [Google Scholar]
- 5.Waage A, Nilsson M. Iatrogenic bile duct injury: a population-based study of 152 776 cholecystectomies in the Swedish Inpatient Registry. Arch Surg 2006;141:1207-13. [DOI] [PubMed] [Google Scholar]
- 6.Flum DR, Dellinger EP, Cheadle A, Chan L, Koepsell T. Intraoperative cholangiography and risk of common bile duct injury during cholecystectomy. JAMA 2003;289:1639-44. [DOI] [PubMed] [Google Scholar]
- 7.Persson G, Enochsson L, Sandblom G. [GallRiks’ annual report 2010]. GallRiks Board, 2011.
- 8.Persson G, Enochsson L, Sandblom G. [GallRiks’ annual report 2009]. GallRiks Board, 2010.
- 9.Schoenfeld D. Partial residuals for the proportional hazards regression-model. Biometrika 1982;69:239-41. [Google Scholar]
- 10.Lillemoe KD, Martin SA, Cameron JL, Yeo CJ, Talamini MA, Kaushal S, et al. Major bile duct injuries during laparoscopic cholecystectomy. Follow-up after combined surgical and radiologic management. Ann Surg 1997;225:459-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisenstein S, Greenstein AJ, Kim U, Divino CM. Cystic duct stump leaks: after the learning curve. Arch Surg 2008;143:1178-83. [DOI] [PubMed] [Google Scholar]
- 12.Tzovaras G, Peyser P, Kow L, Wilson T, Padbury R, Toouli J. Minimally invasive management of bile leak after laparoscopic cholecystectomy. HPB (Oxford) 2001;3:165-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinkas H, Brady PG. Biliary leaks after laparoscopic cholecystectomy: time to stent or time to drain. Hepatobiliary Pancreat Dis Int 2008;7:628-32. [PubMed] [Google Scholar]
- 14.Bismuth H, Majno PE. Biliary strictures: classification based on the principles of surgical treatment. World J Surg 2001;25:1241-4. [DOI] [PubMed] [Google Scholar]
- 15.Strasberg SM, Hertl M, Soper NJ. An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J Am Coll Surg 1995;180:101-25. [PubMed] [Google Scholar]
- 16.Bektas H, Schrem H, Winny M, Klempnauer J. Surgical treatment and outcome of iatrogenic bile duct lesions after cholecystectomy and the impact of different clinical classification systems. Br J Surg 2007;94:1119-27. [DOI] [PubMed] [Google Scholar]
- 17.Siewert JR, Ungeheuer A, Feussner H. [Bile duct lesions in laparoscopic cholecystectomy]. Chirurg 1994;65:748-57. [PubMed] [Google Scholar]
- 18.Stewart L, Robinson TN, Lee CM, Liu K, Whang K, Way LW. Right hepatic artery injury associated with laparoscopic bile duct injury: incidence, mechanism, and consequences. J Gastrointest Surg 2004;8:523-30. [DOI] [PubMed] [Google Scholar]
- 19.Alves A, Farges O, Nicolet J, Watrin T, Sauvanet A, Belghiti J. Incidence and consequence of an hepatic artery injury in patients with postcholecystectomy bile duct strictures. Ann Surg 2003;238:93-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gouma DJ, Go PM. Bile duct injury during laparoscopic and conventional cholecystectomy. J Am Coll Surg 1994;178:229-33. [PubMed] [Google Scholar]
- 21.Connor S, Garden OJ. Bile duct injury in the era of laparoscopic cholecystectomy. Br J Surg 2006;93:158-68. [DOI] [PubMed] [Google Scholar]
- 22.Richardson MC, Bell G, Fullarton GM. Incidence and nature of bile duct injuries following laparoscopic cholecystectomy: an audit of 5913 cases. West of Scotland Laparoscopic Cholecystectomy Audit Group. Br J Surg 1996;83:1356-60. [DOI] [PubMed] [Google Scholar]
- 23.Murphy MM, Ng SC, Simons JP, Csikesz NG, Shah SA, Tseng JF. Predictors of major complications after laparoscopic cholecystectomy: surgeon, hospital, or patient? J Am Coll Surg 2010;211:73-80. [DOI] [PubMed] [Google Scholar]
- 24.Khuri SF, Daley J, Henderson W, Hur K, Hossain M, Soybel D, et al. Relation of surgical volume to outcome in eight common operations: results from the VA National Surgical Quality Improvement Program. Ann Surg 1999;230:414-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Birkmeyer JD, Warshaw AL, Finlayson SR, Grove MR, Tosteson AN. Relationship between hospital volume and late survival after pancreaticoduodenectomy. Surgery 1999;126:178-83. [PubMed] [Google Scholar]
- 26.Patti MG, Corvera CU, Glasgow RE, Way LW. A hospital’s annual rate of esophagectomy influences the operative mortality rate. J Gastrointest Surg 1998;2:186-92. [DOI] [PubMed] [Google Scholar]
- 27.Giger UF, Michel JM, Opitz I, Th Inderbitzin D, Kocher T, Krahenbuhl L. Risk factors for perioperative complications in patients undergoing laparoscopic cholecystectomy: analysis of 22,953 consecutive cases from the Swiss Association of Laparoscopic and Thoracoscopic Surgery database. J Am Coll Surg 2006;203:723-8. [DOI] [PubMed] [Google Scholar]
- 28.Flum DR, Koepsell T, Heagerty P, Sinanan M, Dellinger EP. Common bile duct injury during laparoscopic cholecystectomy and the use of intraoperative cholangiography: adverse outcome or preventable error? Arch Surg 2001;136:1287-92. [DOI] [PubMed] [Google Scholar]
- 29.Archer SB, Brown DW, Smith CD, Branum GD, Hunter JG. Bile duct injury during laparoscopic cholecystectomy: results of a national survey. Ann Surg 2001;234:549-58. [DOI] [PMC free article] [PubMed] [Google Scholar]