Abstract
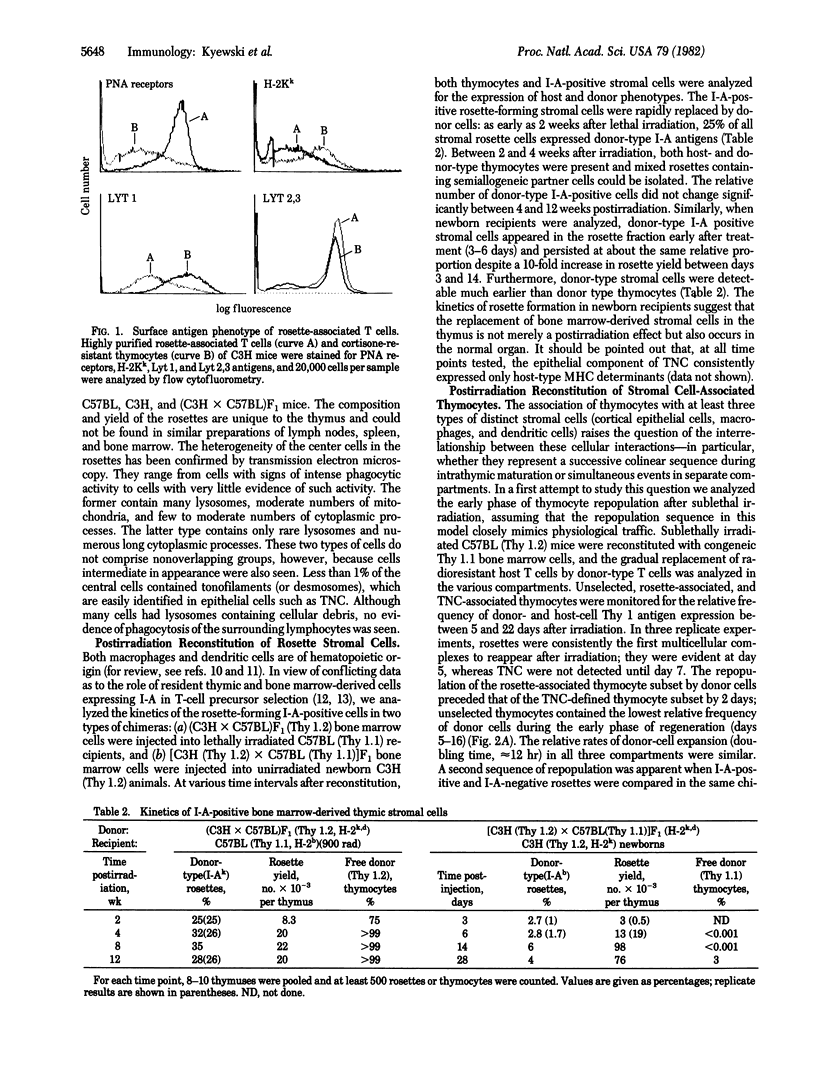
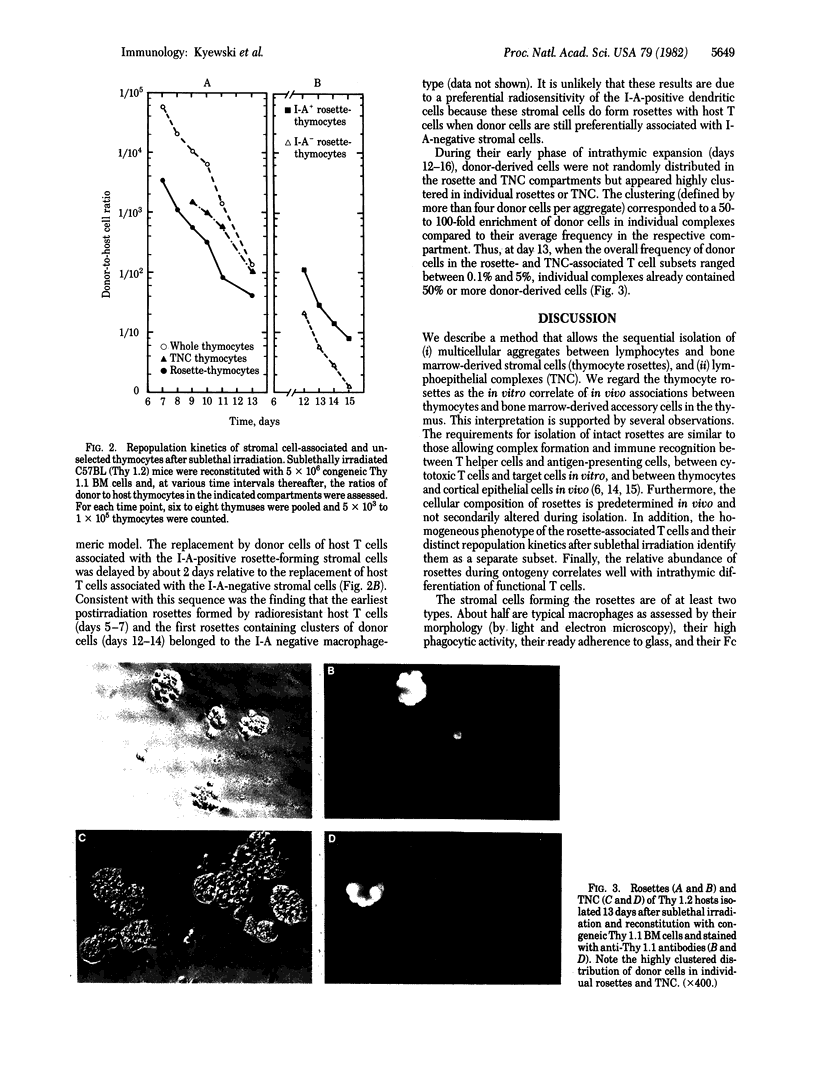
We describe the isolation and purification of multicellular complexes composed of lymphocytes and bone marrow-derived stromal cells ("thymocyte rosettes") from the mouse thymus. These rosettes are the structural in vitro correlate of in vivo associations between lymphoblasts and I-A/E negative macrophages or medullary I-A/E positive dendritic-like cells. Both types of rosettes are preformed in vivo. The rosette-associated thymocytes display a surface antigen phenotype typical of immature thymocytes. In radiation chimeras, replacement of host thymocytes by injected bone marrow cells follows a regular pattern: donor type T cells appear first at day 11 as clusters around I-A negative macrophages and approximately 2 days later as similar clusters associated with either I-A positive cortical epithelial cells or I-A positive medullary dendritic cells. These data suggest (a) a defined sequence of lymphostromal interactions during intrathymic maturation and (b) a rapid proliferation of thymocytes after interaction with stromal cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barclay A. N., Mayrhofer G. Bone marrow origin of Ia-positive cells in the medulla rat thymus. J Exp Med. 1981 Jun 1;153(6):1666–1671. doi: 10.1084/jem.153.6.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan M. J., Fink P. J. The influence of thymus H-2 antigens on the specificity of maturing killer and helper cells. Immunol Rev. 1978;42:3–19. doi: 10.1111/j.1600-065x.1978.tb00256.x. [DOI] [PubMed] [Google Scholar]
- CLARK S. L., Jr The thymus in mice of strain 129/J, studied with the electron microscope. Am J Anat. 1963 Jan;112:1–33. doi: 10.1002/aja.1001120102. [DOI] [PubMed] [Google Scholar]
- Ceredig R., Glasebrook A. L., MacDonald H. R. Phenotypic and functional properties of murine thymocytes. I. Precursors of cytolytic T lymphocytes and interleukin 2-producing cells are all contained within a subpopulation of "mature" thymocytes as analyzed by monoclonal antibodies and flow microfluorometry. J Exp Med. 1982 Feb 1;155(2):358–379. doi: 10.1084/jem.155.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaijman J. J., Micklem H. S., Ledbetter J. A., Dangl J. L., Herzenberg L. A., Herzenberg L. A. T cell ontogeny. Organ location of maturing populations as defined by surface antigen markers is similar in neonates and adults. J Exp Med. 1981 Mar 1;153(3):605–614. doi: 10.1084/jem.153.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerne N. K. The somatic generation of immune recognition. Eur J Immunol. 1971 Jan;1(1):1–9. doi: 10.1002/eji.1830010102. [DOI] [PubMed] [Google Scholar]
- Kyewski B. A., Kaplan H. S. Lymphoepithelial interactions in the mouse thymus: phenotypic and kinetic studies on thymic nurse cells. J Immunol. 1982 May;128(5):2287–2294. [PubMed] [Google Scholar]
- Lipsky P. E., Rosenthal A. S. Macrophage-lymphocyte interaction. I. Characteristics of the antigen-independent-binding of guinea pig thymocytes and lymphocytes to syngeneic macrophages. J Exp Med. 1973 Oct 1;138(4):900–924. doi: 10.1084/jem.138.4.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo D. L., Schwartz R. H. T-cell specificity for H-2 and Ir gene phenotype correlates with the phenotype of thymic antigen-presenting cells. Nature. 1980 Sep 4;287(5777):44–46. doi: 10.1038/287044a0. [DOI] [PubMed] [Google Scholar]
- Mason D. W., Pugh C. W., Webb M. The rat mixed lymphocyte reaction: roles of a dendritic cell in intestinal lymph and T-cell subsets defined by monoclonal antibodies. Immunology. 1981 Sep;44(1):75–87. [PMC free article] [PubMed] [Google Scholar]
- Raviola E., Karnovsky M. J. Evidence for a blood-thymus barrier using electron-opaque tracers. J Exp Med. 1972 Sep 1;136(3):466–498. doi: 10.1084/jem.136.3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse R. V., Weissman I. L. Microanatomy of the thymus: its relationship to T cell differentiation. Ciba Found Symp. 1981;84:161–177. doi: 10.1002/9780470720660.ch9. [DOI] [PubMed] [Google Scholar]
- Sprent J. Role of H-2 gene products in the function of T helper cells from normal and chimeric mice in vivo. Immunol Rev. 1978;42:108–137. doi: 10.1111/j.1600-065x.1978.tb00260.x. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Nussenzweig M. C. Dendritic cells: features and functions. Immunol Rev. 1980;53:127–147. doi: 10.1111/j.1600-065x.1980.tb01042.x. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. The regulatory role of macrophages in antigenic stimulation. Part Two: symbiotic relationship between lymphocytes and macrophages. Adv Immunol. 1981;31:1–136. doi: 10.1016/s0065-2776(08)60919-0. [DOI] [PubMed] [Google Scholar]
- Weissman I. L. Thymus cell maturation. Studies on the origin of cortisone-resistant thymic lymphocytes. J Exp Med. 1973 Feb 1;137(2):504–510. doi: 10.1084/jem.137.2.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wekerle H., Ketelsen U. P., Ernst M. Thymic nurse cells. Lymphoepithelial cell complexes in murine thymuses: morphological and serological characterization. J Exp Med. 1980 Apr 1;151(4):925–944. doi: 10.1084/jem.151.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wekerle H., Ketelsen U. P. Thymic nurse cells--Ia-bearing epithelium involved in T-lymphocyte differentiation? Nature. 1980 Jan 24;283(5745):402–404. doi: 10.1038/283402a0. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]
- van Ewijk W., van Soest P. L., van den Engh G. J. Fluorescence analysis and anatomic distribution of mouse T lymphocyte subsets defined by monoclonal antibodies to the antigens Thy-1, Lyt-1, Lyt-2, and T-200. J Immunol. 1981 Dec;127(6):2594–2604. [PubMed] [Google Scholar]






