Abstract
The antidiabetic intestinal L cell hormone glucagon-like peptide-1 (GLP-1) enhances glucose-dependent insulin secretion and inhibits gastric emptying. GLP-1 secretion is stimulated by luminal oleic acid (OA), which crosses the cell membrane by an unknown mechanism. We hypothesized that L cell fatty acid transport proteins (FATPs) are essential for OA-induced GLP-1 release. Therefore, the murine GLUTag L cell model was used for immunoblotting, [3H]OA uptake assay, and GLP-1 secretion assay as determined by radioimmunoassay following treatment with OA ± phloretin, sulfo-N-succinimidyl oleate, or siRNA against FATP4. FATP4−/− and cluster-of-differentiation 36 (CD36)−/− mice received intraileal OA, and plasma GLP-1 was measured by sandwich immunoassay. GLUTag cells were found to express CD36, FATP1, FATP3, and FATP4. The cells demonstrated specific 3H[OA] uptake that was dose-dependently inhibited by 500 and 1,000 μM unlabeled OA (P < 0.001). Cell viability was not altered by treatment with OA. Phloretin and sulfo-N-succinimidyl oleate, inhibitors of protein-mediated transport and CD36, respectively, also decreased [3H]OA uptake, as did knockdown of FATP4 by siRNA transfection (P < 0.05–0.001). OA dose-dependently increased GLP-1 secretion at 500 and 1,000 μM (P < 0.001), whereas phloretin, sulfo-N-succinimidyl oleate, and FATP4 knockdown decreased this response (P < 0.05–0.01). FATP4−/− mice displayed lower plasma GLP-1 at 60 min in response to intraileal OA (P < 0.05), whereas, unexpectedly, CD36−/− mice displayed higher basal GLP-1 levels (P < 0.01) but a normal response to intraileal OA. Together, these findings demonstrate a key role for FATP4 in OA-induced GLP-1 secretion from the murine L cell in vitro and in vivo, whereas the precise role of CD36 remains unclear.
Keywords: carrier-mediated transport, cluster of differentiation 36, fatty acid uptake, GLUTag, monounsaturated fatty acid
the intestinal l cell hormone glucagon-like peptide-1 (GLP-1) is secreted following nutrient ingestion, leading to glucose-dependent insulin release as well as inhibition of gastric emptying, glucagon secretion, and food intake (1, 39, 46, 48). The antidiabetic properties of this hormone have led to the use of both GLP-1 mimetics and GLP-1 degradation inhibitors in the clinic to treat patients with type 2 diabetes (33). Although nutrients such as sugars and peptones are known to stimulate L cell secretion (15, 20, 35), fats in particular are potent GLP-1 secretagogues (10, 18, 21, 23, 24). Furthermore, only fats appear to transit the intestine to the ileum (3, 24, 28), which has the highest density of L cells in the intestine (11).
Monounsaturated fatty acids (MUFAs) such as oleic acid (OA) are known to induce beneficial metabolic effects, and therefore, arguments have been made in favor of the Mediterranean diet, which is rich in OA-containing olive oil (14). Indeed, insulin-resistant patients placed on a diet enriched in MUFAs display increased plasma GLP-1 levels and improved glycemic control (34). A study in rats has also linked dietary OA to increased GLP-1 secretion and GLP-1-dependent improvements in glycemic tolerance (37). Furthermore, OA has been demonstrated to directly increase GLP-1 secretion from the intestinal L cell, as observed in the murine GLUTag (23, 37), human NCI-H716 (35), and primary fetal rat intestinal culture (23) L cell models.
Several G protein-coupled receptors (GPCRs), such as GPR40, GRP120, and GPR119, have been implicated as long-chain fatty acid receptors on the L cell, responding to saturated fatty acids, polyunsaturated fatty acids, and the fatty acid derivative oleoylethanolamide, respectively (10, 21, 26). In contrast, OA is known to increase GLP-1 secretion through a mechanism that is dependent on the atypical isozyme protein kinase C (PKC)ζ (23, 24). Although OA can directly activate this enzyme in vitro (31), whether, and if so, how, it crosses the plasma membrane to permit direct interaction with PKCζ in the intestinal L cell is currently unknown.
Although the topic of fatty acid transport has remained controversial, it is generally believed that the predominant mechanisms underlying fatty acid uptake consist of passive diffusion and a saturable, protein-mediated process (17, 41, 43). Candidates for L cell fatty acid transport proteins include the class B scavenger receptor cluster-of-differentiation 36 (CD36)/fatty acid translocase as well as isoforms of the fatty acid transport protein (FATP) family. CD36 is widely expressed in the body but is also involved in intestinal absorption of fatty acids, including OA in the proximal gut (8, 32, 40). The isoforms of the FATP family also demonstrate broad expression, with FATP4 being the most abundant isoform in the small intestine (45). Previous studies have identified mRNA transcripts for CD36 as well as for FATP1, FATP3, and FATP4 in the murine GLUTag L cell line (24). The GLUTag cells have been validated extensively as an L cell model, demonstrating appropriate GLP-1 secretion in response to a wide variety of known secretagogues (4, 15, 23, 26, 37). Furthermore, the GLUTag cells have been shown to take up the OA analog C1-Bodipy-C12 (24), consistent with an ability of these cells to internalize fatty acids. Therefore, we hypothesized that one or more of the intestinal L cell FATPs plays a role in OA uptake and subsequent GLP-1 secretion.
METHODS
Cells.
Murine GLUTag cells were grown in medium [Dulbecco's modified Eagle's medium (DMEM); Gibco Invitrogen, Burlington, ON, Canada] containing 25 mM glucose and 10% fetal bovine serum. Cells were plated in six- or 24-well plates coated with poly-d-lysine (Sigma Chemical, St. Louis, MO) and allowed to recover for 24 h for uptake assay or 48 h for immunoblot, transfection, and secretion experiments. Cell viability following treatment with OA was assessed by uptake of neutral red during the last hour of a 2-h incubation (36).
Small interfering RNA transfection.
Preliminary attempts to knockdown FATP4 were conducted using small interfering RNA (siRNA) from Ambion (Austin, TX). However, this led to a maximum 20% reduction in FATP4 protein levels despite numerous attempts to optimize the approach (data not shown). Therefore, subsequent studies were performed by transfection of cells using SMARTpool siRNA, a mixture of four targeted FATP4 siRNA sequences (SMARTpool; Dharmacon, Lafayette, CO), or scrambled control in Opti-MEM I medium (Gibco Invitrogen) (16). The SMARTpool siRNA approach is designed to reduce “off-target” effects by ≤90% by reducing the concentration of each of the individual siRNA sequences. After optimization of the approach, based upon protein expression levels, all experiments were conducted in cells that were incubated with the siRNA (50 nM with 2.25 μl of DharmaFECT-4 transfection reagent; Dharmacon) for 5 h, washed twice, and allowed to recover for 48 h.
Immunoblot.
Cells or mouse duodenum (positive control) were collected into radioimmunoprecipitation assay buffer. One-hundred micrograms of protein (measured by Bradford assay; Bio-Rad, Hercules, CA) was run on a 10% gel, transferred onto a polyvinyl difluoride membrane, and probed with rabbit anti-FATP1, -3, or -4 (1:1,000), rabbit anti-CD36 (1:1,000; Cayman Chemicals, Ann Arbor, MI), and rabbit anti-actin (1:4,000; Sigma Chemical), followed by detection using horseradish peroxidise-linked goat anti-rabbit IgG (1:2,000; Cell Signaling Technology, Beverly, MA) and electrochemiluminescence Western blotting detection reagent (Amersham GE Healthcare, Baie d'Urfe, QC, Canada).
[3H]oleic acid uptake assay.
Cells were starved in serum-free medium overnight. [3H]OA (3.0 μCi/ml; specific activity 1.96 × 1012 Bq/mmol) and [14C]mannitol (0.6 μCi/ml; specific activity 2.04 × 109 Bq/mmol; Moravek Biochemicals, Brea, CA) were added to CaCl2-free medium containing 0.5% fatty acid-free bovine serum albumin (Sigma Chemical). In some experiments, 500 or 1,000 μM unlabeled OA (100 mM stock solution in ethanol; Sigma Chemical), 200 μM phloretin [a non-specific inhibitor of carrier-mediated transport (49), 20 mM stock solution in ethanol; Sigma Chemical], or 400 μM sulfo-N-succinimidyl oleate [SSO; a CD36 inhibitor (6), 0.4 M stock solution in DMSO; Toronto Research Chemicals, North York, ON, Canada] was added. The maximum final concentrations of ethanol and DMSO in the medium were 1.6 and 0.1%, respectively. Finally, CaCl2 was added back to the medium to a final concentration of 1.8 mM. Cells treated with phloretin (200 μM) or SSO (400 μM) were preincubated with medium containing only phloretin or SSO, respectively, for 30 min at 37°C prior to the start of the uptake assay.
Immediately preceding the assay, cells were briefly washed twice with 500 μl of Hanks' balanced salt solution before receiving 130 μl of treatment medium and incubation at 37°C. Because preliminary uptake assays revealed that 62% of the total uptake observed over 120 min occurred during the first 60 min, and we have shown previously that 70% of GLP-1 secretion occurs within the same time frame (27), all further uptake studies were conducted using a 60-min incubation period. Hence, at various time points between t = 5 and t = 60 min, the medium was removed, and the cells were briefly washed twice with Hanks' balanced salt solution containing 0.5% fatty acid-free bovine serum albumin to remove any tracer bound nonspecifically to the cell membrane. Ice-cold 1.0 M KOH (Sigma Chemical) was then added to the cells, and an aliquot was used to measure radioactivity in a β-counter with the isotope windows set at 3H = 0–8 keV and 14C = 35–156 keV to avoid signal overlap, as determined in preliminary studies. The remaining sample was used to determine protein concentration by Bradford assay.
GLP-1 secretion assay.
All treatments were made up in CaCl2-free DMEM (Gibco Invitrogen) containing 0.5% fatty acid-free bovine serum albumin (Sigma Chemical), and then CaCl2 was added at a final concentration of 1.8 mM. Cells were washed twice with Hanks' balanced salt solution and then treated with medium containing 1 μM phorbol 12-myristate 13-acetate (PMA; 100 μM stock solution in ethanol, positive control; Sigma Chemical), 150–1,000 μM OA (from a 40-mM stock solution in 0.5 M NaOH; Sigma Chemical), or vehicle alone (negative control). Some cells were pretreated for 30 min with 200 μM phloretin (20 mM stock solution in ethanol; Sigma Chemical) or 400 μM SSO (0.4 M stock solution in DMSO; Toronto Research Chemicals) or for 48 h with siRNA (or scrambled control, as described above). Cells were then incubated with treatments for 2 h, including phloretin or SSO in the medium, as appropriate. At the end of the incubation period the medium was collected into 1% trifluoroacetic acid, whereas cells were scraped into 1 N hydrochloric acid containing 5% formic acid, 1% trifluoroacetic acid, and 1% sodium chloride. Peptides from both medium and cell samples were collected by reversed-phase extraction using C18 Sep-Pak cartridges (Waters Associates, Milford, MA), as validated previously (4, 9, 23, 26, 37). Samples were then subjected to a radioimmunoassay using an antibody that recognized the carboxy terminal of GLP-17–36NH2 (Enzo Life Sciences, Farmingdale, NY) (4, 9, 23, 26, 37). GLP-1 secretion was calculated as the amount of GLP-1 detected in the medium, normalized to total GLP-1 in the medium and cells combined, and expressed as percent of negative control, as reported previously (4, 9, 23, 26, 37). Total GLP-1 cell content (medium plus cells) of cells treated with vehicle was 381 ± 60 pg/ml (n = 10) and did not differ with any of the treatments.
Immunocytochemistry.
Cells were grown on glass coverslips until 80% confluent and then treated for 1 h with vehicle or OA, as described above. Cells were then rinsed and incubated overnight at 4°C with rabbit anti-mouse/human FATP4 antiserum (1/500; Abnova/Cedarlane Laboratories, Burlington, ON, Canada) followed by Cy3-coupled donkey anti-rabbit IgG (1/400; Jackson ImmunoResearch/Cedarlane Laboratories) for 1 h at 20°C, rinsed, and mounted with 4,6-diamidino-2-phenylindole for visualization using a Zeiss AxioPlan microscope with AxioPlan software (Carl Zeiss Canada, Don Mills, ON, Canada). Images along the z-axis were taken at 1-μm intervals.
In vivo experiments.
All animal protocols were approved by the Animal Care Committee at the University of Toronto. Fatp4−/−;Ivl-Fatp4tg/+ (FATP4-null) mice (30) were on a mixed 129/B6/CBA background. Transgenic reexpression of FATP4 in the skin of the FATP4−/− mice via Ivl-Fatp4tg/+ is required to prevent the neonatal lethality of the whole body FATP4 knockout. These mice have been reported to display no compensatory upregulation of other FATP isoforms in the intestine (42). Both Fatp4+/−;Ivl-Fatp4tg/+ mice and Fatp4+/−mice were used as control mice, and the results were combined since they did not differ between genotypes (data not shown). The study was conducted using female and male littermates at 9–24 wk of age, and the results were combined. CD36−/− mice, a generous gift from Dr. Kevin Kain (University of Toronto, Toronto, ON, Canada) and derived originally by Dr. Maria Febbraio (13), were on a C57BL/6 background. FATP4 levels are not altered in the small intestine of these animals (42). Age-matched C57BL/6 mice (Charles River, St. Constant, QC, Canada) were used as controls. The study was conducted using both female and male mice at 8–25 wk of age, and the results were combined.
Following an overnight fast, mice were anesthetized with isofluorane, and blood samples (50–100 μl) were obtained from the saphenous vein. After a laparotomy, 200 μl of 125 mM OA in 125 mM Tween-80 (Sigma Chemical) was injected directly into the lumen of the ileum in an oral direction (24); Tween-80 was used to solubilize the OA rather than bile acids due to the ability of bile acids to directly stimulate GLP-1 release (47), whereas we have shown previously that Tween-80 does not affect GLP-1 release from the rat ileal L cell in vivo (24, 26). Blood samples were then obtained via a cardiac puncture at either 15 or 60 min. All blood samples were collected into a 10% volume of trasylol:EDTA;diprotin A (5,000 kallikrein inhibitory units/0.03 M/0.1 M), and plasma was stored at −80°C. Plasma GLP-1 levels were determined using the Total GLP-1 Assay Kit (Meso Scale Discovery, Gaithersburg, MD), with a detection limit of 0.98 pg/ml. Ileal tissue sections (2 cm) were collected into radioimmunoprecipitation assay buffer, protein concentrations were measured by Bradford assay, and levels of FATP4 or CD36 were determined by immunoblot, as described above.
Statistical analysis.
All results are expressed as means ± SE. Statistical analysis was performed with SAS software (SAS Institute, Cary, NC) using Student's t-test or one- or two-way ANOVA followed by Student's t-test or one-way ANOVA as appropriate. Some data were log10 transformed to normalize variances. Significance of data was assumed at P < 0.05.
RESULTS
GLUTag cells express fatty acid transport proteins.
To confirm expression of the fatty acid transport proteins CD36, FATP1, FATP3, and FATP4 in the murine GLUTag L cell model, immunoblot was carried out, using mouse duodenum as a control (Fig. 1). Bands were detected consistently for all four proteins. However, interestingly, although there was a clear band of CD36 immunoreactivity at ∼55 kDa, consistent with intracellular localization of CD36, little to no expression of the heavily glycosylated, high-molecular weight cell surface form of CD36 was detected in either the cells or the tissue.
Fig. 1.
Expression of fatty acid transport proteins in the L cell. Immunoblot for cluster of differentiation 36 (CD36) (55 kDa: nonglycosylated intracellular form; 88 kDa: glycosylated membrane form; A), fatty acid transport protein (FATP)1 (63 kDa; B), FATP3 (72 kDa; C), and FATP4 (72 kDa; D) in murine GLUTag L cells (n = 3). Actin (42 kDa) was used as the loading control and murine duodenum as a positive (+ve) control.
OA is taken up by GLUTag cells and stimulates GLP-1 secretion.
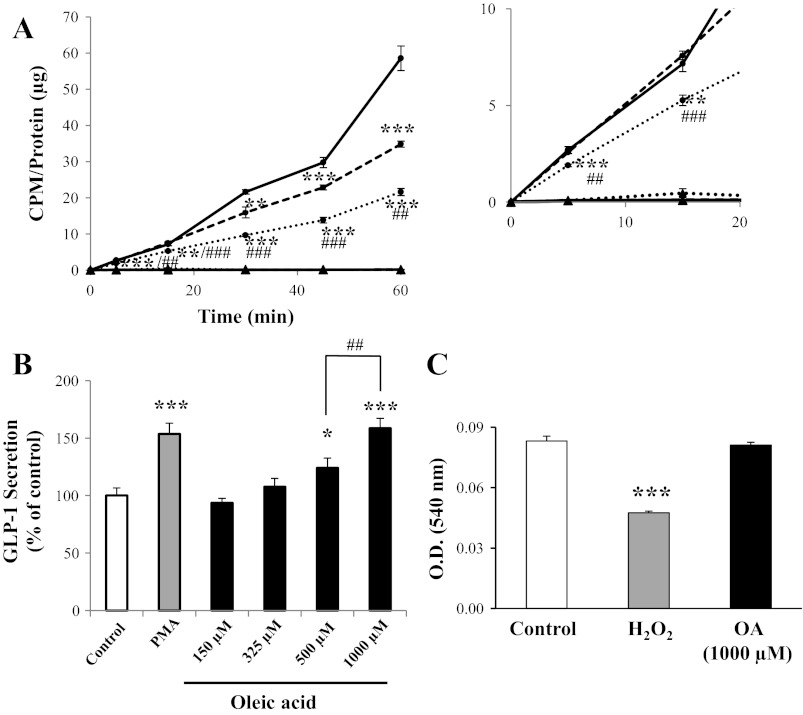
GLUTag cells demonstrated uptake of [3H]OA for ≤60 min (Fig. 2A). No significant uptake of the cell integrity control [14C]mannitol was observed in any of the treatment groups. Uptake of [3H]OA was competitively inhibited in a dose-dependent manner by 500 and 1,000 μM unlabeled OA to 60 ± 2 and 37 ± 2%, respectively, of control levels at t = 60 min (P < 0.001 vs. control and P < 0.01 vs. each other). Independent experiments that included an additional time point between 45 and 60 min (i.e., t = 52 min) confirmed the linearity of the response between 45 and 60 min (r2 = 0.999, n = 8; data not shown). The absolute uptake of [3H]OA by the GLUTag cells over 60 min was 3.4 × 10−12 nmol·min−1·cell−1. Furthermore, a combination of the vehicle-only (control) data from multiple experiments (including the data shown in Figs. 2A, 3A, and 4A, as well as additional studies to make n = 7, with each experiment conducted in at least triplicate) revealed that the slope of the line of the [3H]OA uptake curve increased at t = 45 min from 0.52 ± 0.06 (at t = 0–45 min) to 1.60 ± 0.22 (at t = 45–60 min, P < 0.01). This increase in slope was not observed in paired cells treated with 1,000 μM unlabeled OA, which demonstrated a straight line from t = 0–60 min (slope = 0.27 ± 0.03; r2 = 0.99).
Fig. 2.
Oleic acid (OA) uptake in the L cell and the effect of OA on glucagon-like peptide-1 (GLP-1) secretion. A: GLUTag cells were incubated with [3H]OA and treated with vehicle control (solid line) or 500 (dashed line) or 1,000 μM (dotted line) unlabeled OA, followed by determination of [3H]OA uptake (●). [14C]mannitol was used as a cell integrity control in each treatment group (▲). Counts per minute (cpm) were normalized to total protein (inset: expanded scale) (n = 6). B: GLUTag cells were treated with vehicle (control) or increasing concentrations of OA for 2 h, and secretion of GLP-1 was determined by radioimmunoassay (n = 6–11). Basal secretion was 8.6 ± 0.8% of total cell content. C: GLUTag cells were treated for 2 h with vehicle alone (control), 5 mM H2O2, or 1,000 μM OA for 2 h, followed by determination of viability using neutral red uptake, as assessed by optical density (OD) at 540 nm (n = 8). *P < 0.05, **P < 0.01, and ***P < 0.001 vs. control; ##P < 0.01 and ###P < 0.001 for 500 vs. 1,000 μM unlabeled OA or as indicated.
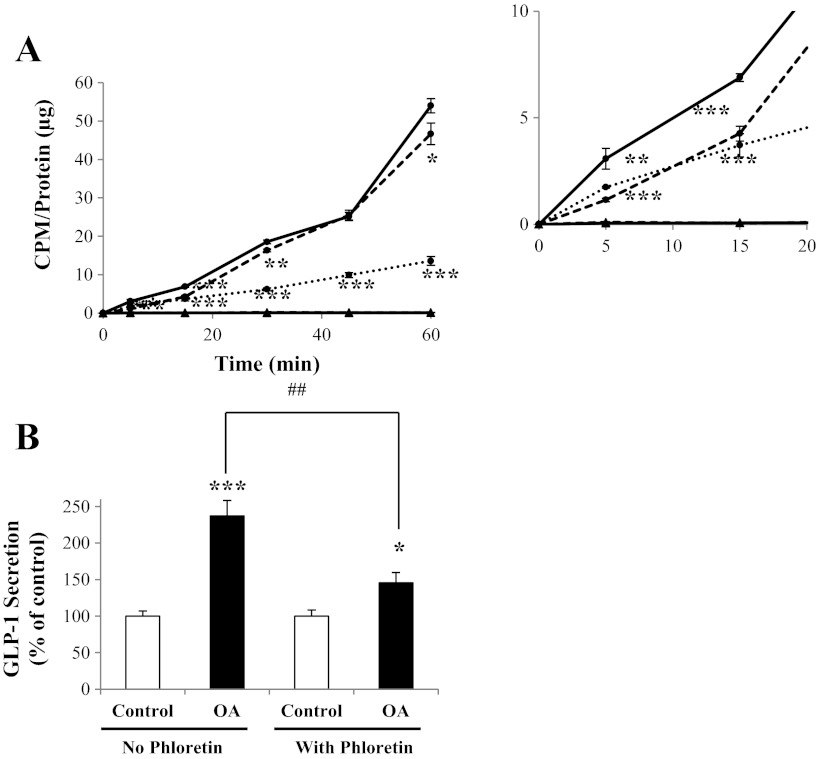
Fig. 3.
Effect of phloretin on L cell OA uptake and GLP-1 secretion. A: GLUTag cells were incubated with [3H]OA and treated with vehicle control (solid line), 1,000 μM unlabeled OA (dotted line), or 200 μM phloretin (dashed line), followed by determination of [3H]OA uptake (●). [14C]mannitol was used as a cell integrity control in each treatment group (▲). CPM were normalized to total protein (inset: expanded scale) (n = 6). B: GLUTag cells were treated with either vehicle control or 200 μM phloretin and incubated further with or without 1,000 μM OA. GLP-1 secretion was determined by radioimmunoassay (n = 11–12). Basal secretion was 8.5 ± 1.4% of total cell content. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. respective control; ##P < 0.01 as indicated.
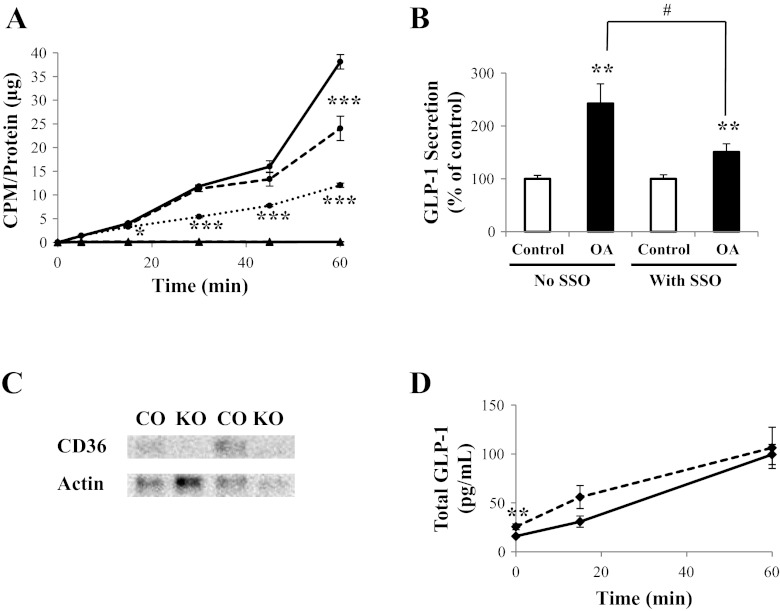
Fig. 4.
Role of CD36 in L cell OA uptake and GLP-1 secretion. A: GLUTag cells were incubated with [3H]OA and treated with vehicle control (solid line), 1,000 μM unlabeled OA (dotted line), or 400 μM SSO (dashed line), followed by determination of [3H]OA uptake (●). [14C]mannitol was used as a cell integrity control in each treatment group (▲). CPM were normalized to total protein (n = 6). B: GLUTag cells were treated with either vehicle control or 400 μM SSO and were incubated further with or without 1,000 μM OA. GLP-1 secretion was determined by radioimmunoassay (n = 11–12). Basal secretion was 8.1 ± 0.8% of total cell content. C: immunoblot for CD36 (55 kDa) and actin (42 kDa; loading control) in the ileum of control (CO) and CD36-null (KO) mice (representative of n = 5–6). D: OA (125 mM in 125 mM Tween-80) was injected directly into the ileum of control (solid line) and CD36-null (dashed line) mice, and blood samples were collected at t = 0 and 15 min or at t = 0 and 60 min. Total GLP-1 levels were determined in the collected plasma using a sandwich immunoassay (n = 9–19). *P < 0.05, **P < 0.01, and ***P < 0.001 vs. respective control; #P < 0.05 as indicated.
Treatment of GLUTag cells with increasing concentrations of OA also led to a dose-dependent increase in GLP-1 secretion such that 500 and 1,000 μM OA increased GLP-1 release to 124 ± 9 and 159 ± 9% of control cells, respectively (P < 0.05–0.001 vs. control and P < 0.01 vs. each other; Fig. 2B). The positive control PMA increased GLP-1 secretion to 154 ± 9% of controls (P < 0.001). Importantly, cell viability assay demonstrated no effect of incubation with the highest concentration of OA for 2 h (Fig. 2C).
Phloretin decreases OA uptake and GLP-1 secretion by GLUTag cells.
Treatment of GLUTag cells with the nonspecific inhibitor of carrier-mediated transport phloretin decreased the uptake of [3H]OA by 38 ± 4% at t = 15 min (P < 0.001) and by 14 ± 4% at t = 60 min (P < 0.05; Fig. 3A). As in previous uptake assays, unlabeled OA (1,000 μM) decreased [3H]OA uptake at t = 5–60 min (P < 0.01–0.001), whereas no significant uptake of [14C]mannitol was observed in any of the treatment groups. As found previously, incubation of the cells with OA (1,000 μM) increased GLP-1 secretion by 137 ± 21% (Fig. 3B). Pre- and coincubation with phloretin (200 μM) markedly reduced OA-induced GLP-1 secretion by 67 ± 14% but did not abrogate the effect of OA (P < 0.01). Basal secretion in the presence of phloretin alone was 85.3 ± 10.2% of control values [P = not significant (NS)]. Control experiments demonstrated no effect of phloretin treatment on PMA-induced GLP-1 release (secretion was 104.9 ± 22.7% of that found in the absence of phoretin, P = NS; data not shown).
CD36 plays a role in the L cell in vitro but not in vivo.
Treatment of GLUTag cells with the CD36 inhibitor SSO reduced [3H]OA uptake by 36 ± 8% at t = 60 min (P < 0.001; Fig. 4A). As observed previously, unlabeled OA (1,000 μM) decreased [3H]OA uptake at t = 15–60 min (P < 0.05–0.001), and no significant uptake of [14C]mannitol was observed in any of the treatment groups. Basal secretion in the presence of SSO alone was increased to 221.9 ± 43.5% of control (P < 0.05). However, SSO treatment decreased OA-induced GLP-1 secretion by GLUTag cells from 243 ± 37% of control values to 151 ± 15% (P < 0.05; Fig. 4B). Control experiments demonstrated no effect of SSO treatment on PMA-induced GLP-1 release (secretion was 80.7 ± 12.5%, which was found in the absence of SSO; P = NS). Therefore, the role of CD36 was examined in vivo using the CD36-null mouse. Immunoblotting confirmed the absence of CD36 in the ileum of CD36−/− mice (Fig. 4C). To determine the effect of OA on plasma GLP-1 levels, 125 mM OA was injected directly into the ileum of anesthetized control and CD36-null mice, and blood samples were collected at t = 0 and 15 min or at t = 0 and 60 min in a paired fashion for determination of total plasma GLP-1 levels. The CD36-null mice were found to have increased basal GLP-1 plasma levels by 61.3 ± 19.4% compared with control animals (P < 0.01; Fig. 4D). However, no differences in GLP-1 plasma levels were observed between the two groups of mice at t = 15 or 60 min following intraileal injection of OA, when the absolute values were compared (Fig. 4D), or following determination of the change from basal levels (data not shown).
FATP4 plays a role in the L cell in vitro and in vivo.
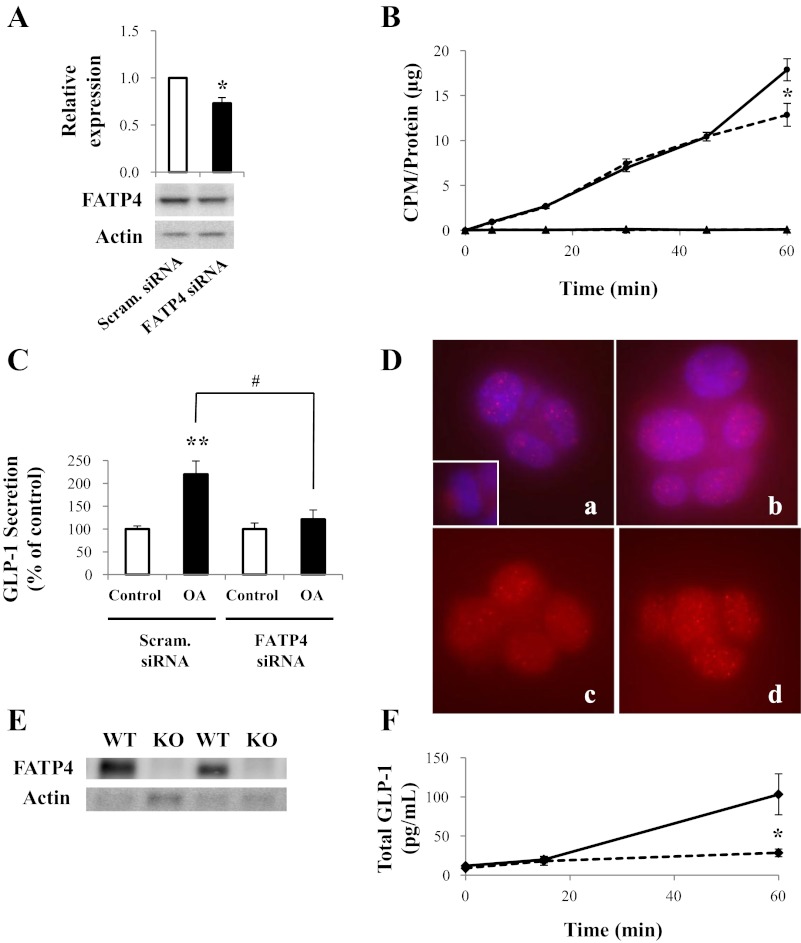
Because there is no specific inhibitor for FATP4, this protein was knocked down in the GLUTag cells using FATP4-targeting siRNA, leading to a maximum 27 ± 6% reduction in protein levels (P < 0.05; Fig. 5A). Nonetheless, knockdown of FATP4 reduced [3H]OA uptake at t = 60 min by 28 ± 7% (P < 0.05; Fig. 5B). Basal GLP-1 secretion in the presence of FATP4 siRNA alone was not different from control values (146.1 ± 21.3% of control, P > 0.05). In contrast, FATP4 knockdown completely abrogated OA-induced GLP-1 secretion, decreasing release from 220 ± 29 to 121 ± 21% of control values (P < 0.05; Fig. 5C). Control experiments showed no effect of FATP4 knockdown on PMA-induced GLP-1 release (secretion was 101.9 ± 7.8% of that found for PMA with the scrambled control, P = NS; data not shown). Immunocytochemistry for FATP4 immunoreactivity revealed a lack of membrane localization, with the majority of the staining appearing in the cytoplasm and/or perinuclear area of both vehicle- and OA-treated cells (Fig. 5D).
Fig. 5.
Role of FATP4 in L cell OA uptake and GLP-1 secretion. A: GLUTag cells were treated with scrambled or FATP4 small interfering RNA (siRNA), and FATP4 and actin (loading control) levels were detected by immunoblot (representative of n = 3). B: [3H]OA uptake was determined in GLUTag cells treated with scrambled (solid line) or FATP4 siRNA (dashed line) (●). [14C]mannitol was used as a cell integrity control in each treatment group (▲). CPM were normalized to total protein (n = 4–5). C: GLUTag cells were treated with scrambled or FATP4 siRNA and were incubated further with or without 1,000 μM OA. GLP-1 secretion was determined by radioimmunoassay (n = 7–8). Basal secretion was 6.7 ± 0.5% of total cell content. D: immunocytochemistry for FATP4 (red) in vehicle- (images a and c) and OA-treated GLUTag cells (images b and d); Blue represents nuclei (images a and b). Images a and b represent whole cell images, whereas the sections shown in images c and d were taken from 3-dimensional “z-stack” analyses at approximately the mid-cell level. E: immunoblot for FATP4 (72 kDa) and actin (42 kDa; loading control) in the ileum of wild-type (WT) and FATP4-null (KO) mice (representative of n = 5). F: OA (125 mM in 125 mM Tween-80) was injected directly into the ileum of WT (solid line) and KO (dashed line) mice, and blood samples were collected at t = 0 and 15 min or at t = 0 and 60 min. Total GLP-1 levels were determined in the collected plasma using an immunoassay system (n = 7–22). *P < 0.05 and **P < 0.01 vs. respective control; #P < 0.05 as indicated.
To further explore the role of FATP4 in OA-induced GLP-1 secretion, the FATP4-null mouse model was utilized. Immunoblotting confirmed the absence of FATP4 in the ileum of FATP4-null mice (Fig. 5E). As for the CD36-null mice, 125 mM OA was injected directly into the ileum of anesthetized wild-type and FATP4-null mice. Although no differences were seen between the two groups of animals at t = 0 and 15 min, plasma GLP-1 levels were markedly lower in the FATP4-null mice at t = 60 min by 72.3 ± 4.8% compared with control animals (P < 0.05; Fig. 5F).
DISCUSSION
The antidiabetic hormone GLP-1 is released from the intestinal L cell upon nutrient ingestion. Fatty acids, and OA in particular, are potent GLP-1 secretagogues, exerting direct effects on the intestinal L cell in vitro as well as increasing GLP-1 release in both humans and rodents (2, 23, 24, 35, 37). However, the mechanism underlying OA-induced GLP-1 secretion has not been fully elucidated, with the only essential component identified to date being the isozyme PKCζ (23, 24). The results of the current study demonstrate that the L cell specifically takes up OA via a carrier-mediated process and that FATP4 plays a key role in OA-induced GLP-1 secretion both in vitro and in vivo.
In keeping with a requirement for FATP4 in OA uptake by the intestinal L cell, FATP4 mRNA (24) and protein are expressed in GLUTag cells. Furthermore, both phloretin treatment and a 27% knockdown of FATP4 reduced OA uptake by up to 28%, consistent with reports of parallel decreases in protein levels and OA uptake in enterocytes after FATP4 knockdown (45). However, our findings in the GLUTag cells differ from those made in endothelial cells, in which a 50% knockdown of FATP4 completely abrogated the ability of vascular endothelial growth factor B to induce OA uptake (16), and from brain microvessel endothelial cells, in which 50% knockdown did not alter OA uptake at all (29a). Hence, the response to modulation of FATP4 expression appears to be highly cell specific, with the intestinal L cell behaving in a fashion similar to the gut absorptive cells rather than to the unrelated adipocytes and endothelial cells.
Consistent with a role for FATP4 in the regulation of OA-induced GLP-1 secretion, both phloretin treatment and FATP4 knockdown markedly reduced stimulated GLP-1 release in vitro, and knockout of FATP4 completely abrogated the intestinal L cell secretory response to OA in vivo. However, somewhat unexpectedly, the effects of both phloretin treatment and FATP4 knockdown to reduce OA uptake by the GLUTag cells were relatively modest compared with their ability to prevent OA-induced GLP-1 release. A similar dissociation between OA uptake and insulin secretion has been noted in mouse insulinoma MIN6 cells such that knockdown of the adipose differentiation-related protein that coats lipid droplets decreases OA uptake by 17% but reduces insulin secretion by more than 50% (12). This discrepancy was attributed to impaired lipid metabolism, although the exact mechanisms were not investigated. We have also observed in GLUTag cells that, despite only a 23% knockdown of the receptor GPR119, there was a much greater effect (e.g., 45% decrease) on GLP-1 secretion induced by the OA derivative oleoylethanolamide (26). Hence, even relatively low levels of knockdown of proteins that mediate fat handling by the intestinal L cell appear to be sufficient to impair GLP-1 release.
Interestingly, immunocytochemistry for FATP4 in the GLUTag cells revealed a predominance of staining in the cytoplasm and/or perinuclear region under both basal and OA-stimulated conditions, with no immunoreactivity detectable in the cell membrane. We have reported previously that PKCζ is required for the effects of OA on GLP-1 secretion, and we have found that this enzyme translocates to the cell membrane upon stimulation with OA (23, 24). However, preliminary data have indicated that the majority of the PKCζ immunoreactivity following treatment with OA is localized to the perinuclear compartment of the GLUTag cell (Iakoubov R and Brubaker PL, unpublished observations). These findings are consistent with the demonstration that, in addition to its reported plasma membrane expression (45), FATP4 also localizes to the endoplasmic reticulum, where its expression drives fatty acid uptake through its ability to act as long-chain acyl-CoA synthetase (29). Hence, intracellular compartmentalization of this enzyme in the L cell could explain the relatively late effect of FATP4 knockdown on fatty acid uptake (e.g., at 60 min only) compared with that of phloretin (e.g., at both 5–30 and 60 min). Finally, the absolute uptake of [3H]OA by the GLUTag cells over 60 min was found to be 3.4 × 10−12 nmol·min−1·cell−1. Although markedly lower than the uptake of OA reported for enterocytes (1.2 × 10−7 nmol·min−1·cell−1, determined over 4 min), FATP4 accounts for ∼50% of the uptake in enterocytes, the major function of which is absorption of ingested fatty acids (45). Therefore, when taken together, the results of the present study indicate that FATP4 plays a key role in both OA uptake and OA-induced GLP-1 secretion in the enteroendocrine L cell in vitro and in vivo likely through delivery of the fatty acid to its effector, PKCζ.
In contrast to the findings on FATP4, a role for CD36 in GLP-1 secretion was found in vitro but not in vivo. Hence, the CD36 inhibitor SSO decreased both [3H]OA uptake and OA-induced GLP-1 release in the GLUTag cells, whereas CD36 knockout did not prevent the effects of OA on GLP-1 secretion in vivo. SSO has been reported to be a specific CD36 inhibitor, reducing fatty acid uptake by up to 70% in a wide variety of tissues (6). However, a recent report showing that SSO also inhibits complex III of the mitochondrial respiratory chain has called the specificity of SSO into question (7). Such a role for CD36 would be consistent with the finding of only the lower-molecular weight, intracellular form of this protein (22) in the GLUTag cells. Hence, the finding that CD36-null mice exhibit a normal GLP-1 secretory response to intraluminal OA suggests that CD36 does not a play an essential role in the effects of OA on the intestinal L cell, whereas it appears to be relatively more important in the immortalized GLUTag cells. Nonetheless, the finding of higher basal GLP-1 levels in the null animals, compared with control mice, implies either that CD36 plays a minor role in the regulation of GLP-1 release under fasting conditions, which seems unlikely, or that the mice have undergone compensatory responses to the global loss of CD36.
Interestingly, the effects of both FATP4 knockdown and CD36 inhibition on OA uptake by the GLUTag cells were found to occur at t = 60 min only, compared with the inhibition of uptake caused by phloretin as well as by unlabeled OA, at early (t = 5–30/45 min) as well as late (t = 60 min) time points. These findings are also consistent with the observation of a change in the rate of OA uptake at t = 45–60 min, although studies of a longer duration may be useful in examining this phenomenon further. Nonetheless, these findings support the notion that multiple uptake mechanisms may be taking place in the L cell over the course of the 60-min assay, including possible roles for FATP1 and -3. Additionally, transport proteins, including CD36 and FATP1, are known to translocate to the plasma membrane from subcellular locations (5, 44). Therefore, it is possible that there is an upregulation of plasma membrane fatty acid transport proteins after t = 45 min, which would explain the increased rate of OA uptake at this time point. Further studies will clearly be necessary to elucidate the specific roles of all of these proteins in the intestinal L cell.
Finally, increasing concentrations of OA increased GLP-1 secretion from the murine GLUTag L cell model in a dose-dependent manner. Although there is evidence that accumulation of free fatty acids in tissues can lead to lipotoxicity and cell dysfunction (38), the highest dose used in this current study (1,000 μM OA) is well below the physiological concentration of OA reached in the ileum (∼105 mM), as determined by measurement of the OA concentration in chyme following oral gavage of olive oil (24). Furthermore, exposure of the GLUTag cells to 1,000 μM OA had no effect on cell viability, consistent with our previous findings using 500 μM OA (23). However, because the luminal concentration of fat, as well as the aboral distance transited by ingested fat, is dependent upon the load of fat ingested (28), the absolute concentration of OA to which the intestinal L cell is exposed will vary depending upon the meal.
The findings of the present study indicate a role for fatty acid transport proteins, and specifically FATP4, in OA-induced GLP-1 secretion by the intestinal L cell. Endogenous GLP-1 production has been shown to be elevated upon stimulation with MUFAs such as OA (24, 37) and has been implicated in the improved glycemic control observed in insulin-resistant patients placed on a MUFA-rich diet (34). Although FATP4 plays a role in mediating the effects of OA on the L cell, FATP4 is not likely to be a therapeutic target due to its widespread distribution throughout the body, including the enterocytes and skin (13, 45). Instead, this signaling pathway, including the essential isozyme PKCζ (23, 24), should be explored further to identify suitable therapeutic targets that could be manipulated to increase endogenous GLP-1 secretion in patients with type 2 diabetes. Finally, mutations in FATP4 were described recently in patients with ichthyosis prematurity syndrome, a condition characterized by premature birth with the infant covered in thick, caseous skin and having respiratory complications, followed by lifelong dry, thick skin (25). Whether these patients exhibit reduced GLP-1 release and a subsequent impairment in glycemic control has not been explored. Nonetheless, an essential role for FATP4 has been established in the skin in these patients as well as in FATP4-null mice, and it now appears that FATP4 additionally plays a key role in mediating OA-induced GLP-1 secretion from the intestinal L cell.
GRANTS
M. A. Poreba was supported by graduate studentships from the Canadian Institutes of Health Research and the Banting and Best Diabetes Centre (BBDC), University of Toronto; C. X. Dong was supported by summer studentships from the Endocrine Society and the BBDC; S. K. Li was supported by a Graduate Stimulus Award from the Department of Physiology, University of Toronto; and P. L. Brubaker was supported by the Canada Research Chairs program. This work was supported by operating grants from the Canadian Diabetes Association (no. 2973 to P. L. Brubaker) and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIH R01-AR-049269 to J. H. Miner).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.A.P. and P.L.B. did the conception and design of the research; M.A.P., C.X.D., S.K.L., and P.L.B. performed the experiments; M.A.P., C.X.D., S.K.L., and P.L.B. analyzed the data; M.A.P., C.X.D., and P.L.B. interpreted the results of the experiments; M.A.P., C.X.D., and P.L.B. prepared the figures; M.A.P. and P.L.B. drafted the manuscript; M.A.P., C.X.D., A.S., J.H.M., and P.L.B. edited and revised the manuscript; M.A.P., C.X.D., S.K.L., A.S., J.H.M., and P.L.B. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We are grateful to Z. Lu and Dr. K. Kain, University of Toronto, for the gift of CD36−/− mice and to Mr. A. Izzo, University of Toronto, for technical assistance.
REFERENCES
- 1. Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 132: 2131–2157, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Beglinger S, Drewe J, Schirra J, Göke B, D'Amato M, Beglinger C. Role of fat hydrolysis in regulating glucagon-like Peptide-1 secretion. J Clin Endocrinol Metab 95: 879–886, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Borgstrom B, Dahlquist A, Lundh G, Sjovall J. Studies of intestinal digestion and absorption in the human. J Clin Invest 36: 1521–1536, 1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brubaker PL, Schloos J, Drucker DJ. Regulation of glucagon-like peptide-1 synthesis and secretion in the GLUTag enteroendocrine cell line. Endocrinology 139: 4108–4114, 1998 [DOI] [PubMed] [Google Scholar]
- 5. Chabowski A, Gorski J, Luiken JJ, Glatz JF, Bonen A. Evidence for concerted action of FAT/CD36 and FABPpm to increase fatty acid transport across the plasma membrane. Prostaglandins Leukot Essent Fatty Acids 77: 345–353, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Coort SL, Willems J, Coumans WA, van der Vusse GJ, Bonen A, Glatz JF, Luiken JJ. Sulfo-N-succinimidyl esters of long chain fatty acids specifically inhibit fatty acid translocase (FAT/CD36)-mediated cellular fatty acid uptake. Mol Cell Biochem 239: 213–219, 2002 [PubMed] [Google Scholar]
- 7. Drahota Z, Vrbacký M, Nůsková H, Kazdová L, Zídek V, Landa V, Pravenec M, Houstek J. Succinimidyl oleate, established inhibitor of CD36/FAT translocase inhibits complex III of mitochondrial respiratory chain. Biochem Biophys Res Commun 391: 1348–1351, 2010 [DOI] [PubMed] [Google Scholar]
- 8. Drover VA, Nguyen DV, Bastie CC, Darlington YF, Abumrad NA, Pessin JE, London E, Sahoo D, Phillips MC. CD36 mediates both cellular uptake of very long chain fatty acids and their intestinal absorption in mice. J Biol Chem 283: 13108–13115, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Drucker DJ, Jin T, Asa SL, Young TA, Brubaker PL. Activation of proglucagon gene transcription by protein kinase A in a novel mouse enteroendocrine cell line. Mol Endocrinol 8: 1646–1655, 1994 [DOI] [PubMed] [Google Scholar]
- 10. Edfalk S, Steneberg P, Edlund H. Gpr40 is expressed in enteroendocrine cells and mediates FFA stimulation of incretin secretion. Diabetes 57: 2280–2287, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eissele R, Göke R, Willemer S, Harthus HP, Vermeer H, Arnold R, Göke B. Glucagon-like peptide-1 cells in the gastrointestinal tract and pancreas of rat, pig and man. Eur J Clin Invest 22: 283–291, 1992 [DOI] [PubMed] [Google Scholar]
- 12. Faleck DM, Ali K, Roat R, Graham MJ, Crooke RM, Battisti R, Garcia E, Ahima RS, Imai Y. Adipose differentiation-related protein regulates lipids and insulin in pancreatic islets. Am J Physiol Endocrinol Metab 299: E249–E257, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Febbraio M, Abumrad NA, Hajjar DP, Sharma K, Cheng W, Pearce SF, Silverstein RL. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J Biol Chem 274: 19055–19062, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Gillingham LG, Harris-Janz S, Jones PJ. Dietary monounsaturated fatty acids are protective against metabolic syndrome and cardiovascular disease risk factors. Lipids 46: 209–228, 2011 [DOI] [PubMed] [Google Scholar]
- 15. Gribble FM, Williams L, Simpson AK, Reimann F. A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line. Diabetes 52: 1147–1154, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Hagberg CE, Falkevall A, Wang X, Larsson E, Huusko J, Nilsson I, van Meeteren LA, Samen E, Lu L, Vanwildemeersch M, Klar J, Genove G, Pietras K, Stone-Elander S, Claesson-Welsh L, Yla-Herttuala S, Lindahl P, Eriksson U. Vascular endothelial growth factor B controls endothelial fatty acid uptake. Nature 464: 917–921, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Hamilton JA. New insights into the roles of proteins and lipids in membrane transport of fatty acids. Prostaglandins Leukot Essent Fatty Acids 77: 355–361, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Hansen KB, Rosenkilde MM, Knop FK, Wellner N, Diep TA, Rehfeld JF, Andersen UB, Holst JJ, Hansen HS. 2-Oleoyl glycerol is a GPR119 agonist and signals GLP-1 release in humans. J Clin Endocrinol Metab 96: E1409–E1417, 2011 [DOI] [PubMed] [Google Scholar]
- 20. Hira T, Mochida T, Miyashita K, Hara H. GLP-1 secretion is enhanced directly in the ileum but indirectly in the duodenum by a newly identified potent stimulator, zein hydrolysate, in rats. Am J Physiol Gastrointest Liver Physiol 297: G663–G671, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med 11: 90–94, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Hoosdally SJ, Andress EJ, Wooding C, Martin CA, Linton KJ. The human scavenger receptor CD36: glycosylation status and its role in trafficking and function. J Biol Chem 284: 16277–16288, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Iakoubov R, Izzo A, Yeung A, Whiteside CI, Brubaker PL. Protein kinase Czeta is required for oleic acid-induced secretion of glucagon-like peptide-1 by intestinal endocrine L cells. Endocrinology 148: 1089–1098, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Iakoubov R, Lauffer L, Ahmed A, Bazinet RP, Brubaker PL. Essential role for protein kinase C zeta in oleic-acid induced glucagon-like peptide-1 secretion in vivo in the rat. Endocrinology 152: 1244–1252, 2011 [DOI] [PubMed] [Google Scholar]
- 25. Klar J, Schweiger M, Zimmerman R, Zechner R, Li H, Torma H, Vahlquist A, Bouadjar B, Dahl N, Fischer J. Mutations in the fatty acid transport protein 4 gene cause the ichthyosis prematurity syndrome. Am J Hum Genet 85: 248–253, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lauffer LM, Iakoubov R, Brubaker PL. GPR119 is essential for oleoylethanolamide-induced glucagon-like peptide-1 secretion from the intestinal enteroendocrine L-cell. Diabetes 58: 1058–1066, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lim GE, Xu M, Sun J, Jin T, Brubaker PL. The rho guanosine 5′-triphosphatase, cell division cycle 42, is required for insulin-induced actin remodeling and glucagon-like peptide-1 secretion in the intestinal endocrine L cell. Endocrinology 150: 5249–5261, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Lin HC, Zhao XT, Wang L. Fat absorption is not complete by midgut but is dependent on load of fat. Am J Physiol Gastrointest Liver Physiol 271: G62–G67, 1996 [DOI] [PubMed] [Google Scholar]
- 29. Milger K, Herrmann T, Becker C, Gotthardt D, Zickwolf J, Ehehalt R, Watkins PA, Stremmel W, Fullekrug J. Cellular uptake of fatty acids driven by the ER-localized acyl-CoA synthetase FATP4. J Cell Sci 119: 4678–4688, 2006 [DOI] [PubMed] [Google Scholar]
- 29a. Mitchell RW, On NH, Del Bigio MR, Miller DW, Hatch GM. Fatty acid transport protein expression in human brain and potential role in fatty acid transport across human brain microvessel endothelial cells. J Neurochem 117: 735–746, 2011 [DOI] [PubMed] [Google Scholar]
- 30. Moulson CL, Lin MH, White JM, Newberry EP, Davidson NO, Miner JH. Keratinocyte-specific expression of fatty acid transport protein 4 rescues the wrinkle-free phenotype in Slc27a4/Fatp4 mutant mice. J Biol Chem 282: 15912–15920, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Nakanishi H, Exton JH. Purification and characterization of the zeta isoform of protein kinase C from bovine kidney. J Biol Chem 267: 16347–16354, 1992 [PubMed] [Google Scholar]
- 32. Nassir F, Wilson B, Han X, Gross RW, Abumrad NA. CD36 is important for fatty acid and cholesterol uptake by the proximal but not distal intestine. J Biol Chem 282: 19493–19501, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Nauck M, Smith U. Incretin-based therapy: how do incretin mimetics and DPP-4 inhibitors fit into treatment algorithms for type 2 diabetic patients? Best Pract Res Clin Endocrinol Metab 23: 513–523, 2009 [DOI] [PubMed] [Google Scholar]
- 34. Paniagua JA, de la Sacristana AG, Sánchez E, Romero I, Vidal-Puig A, Berral FJ, Escribano A, Moyano MJ, Peréz-Martinez P, López-Miranda J, Pérez-Jiménez F. A MUFA-rich diet improves posprandial glucose, lipid and GLP-1 responses in insulin-resistant subjects. J Am Coll Nutr 26: 434–444, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Reimer RA, Darimont C, Gremlich S, Nicolas-Metral V, Ruegg UT, Mace K. A human cellular model for studying the regulation of glucagon-like peptide-1 secretion. Endocrinology 142: 4522–4528, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Repetto G, del Peso A, Zurita JL. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat Protoc 3: 1125–1131, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Rocca AS, LaGreca J, Kalitsky J, Brubaker PL. Monounsaturated fatty acid diets improve glycemic tolerance through increased secretion of glucagon-like peptide-1. Endocrinology 142: 1148–1155, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Schaffer JE. Lipotoxicity: when tissues overeat. Curr Opin Lipidol 14: 281–287, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Schirra J, Katschinski M, Weidmann C, Schafer T, Wank U, Arnold R, Goke B. Gastric emptying and release of incretin hormones after glucose ingestion in humans. J Clin Invest 97: 92–103, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schwartz GJ, Fu J, Astarita G, Li X, Gaetani S, Campolongo P, Cuomo V, Piomelli D. The lipid messenger OEA links dietary fat intake to satiety. Cell Metab 8: 281–288, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schwenk RW, Holloway GP, Luiken JJ, Bonen A, Glatz JF. Fatty acid transport across the cell membrane: regulation by fatty acid transporters. Prostaglandins Leukot Essent Fatty Acids 82: 149–154, 2010 [DOI] [PubMed] [Google Scholar]
- 42. Shim J, Moulson CL, Newberry EP, Lin MH, Xie Y, Kennedy SM, Miner JH, Davidson NO. Fatty acid transport protein 4 is dispensable for intestinal lipid absorption in mice. J Lipid Res 50: 491–500, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stahl A. A current review of fatty acid transport proteins (SLC27). Pflugers Arch 447: 722–727, 2004 [DOI] [PubMed] [Google Scholar]
- 44. Stahl A, Evans JG, Pattel S, Hirsch D, Lodish HF. Insulin causes fatty acid transport protein translocation and enhanced fatty acid uptake in adipocytes. Dev Cell 2: 477–488, 2002 [DOI] [PubMed] [Google Scholar]
- 45. Stahl A, Hirsch DJ, Gimeno RE, Punreddy S, Ge P, Watson N, Patel S, Kotler M, Raimondi A, Tartaglia LA, Lodish HF. Identification of the major intestinal fatty acid transport protein. Mol Cell 4: 299–308, 1999 [DOI] [PubMed] [Google Scholar]
- 46. Szayna M, Doyle ME, Betkey JA, Holloway HW, Spencer RG, Greig NH, Egan JM. Exendin-4 decelerates food intake, weight gain, and fat deposition in Zucker rats. Endocrinology 141: 1936–1941, 2000 [DOI] [PubMed] [Google Scholar]
- 47. Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M, Pellicciari R, Auwerx J, Schoonjans K. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab 10: 167–177, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JP, Smith DM, Ghatei MA, Herbert J, Bloom SR. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 379: 69–72, 1996 [DOI] [PubMed] [Google Scholar]
- 49. Verkman AS, Solomon AK. A stepwise mechanism for the permeation of phloretin through a lipid bilayer. J Gen Physiol 80: 557–581, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]