Abstract
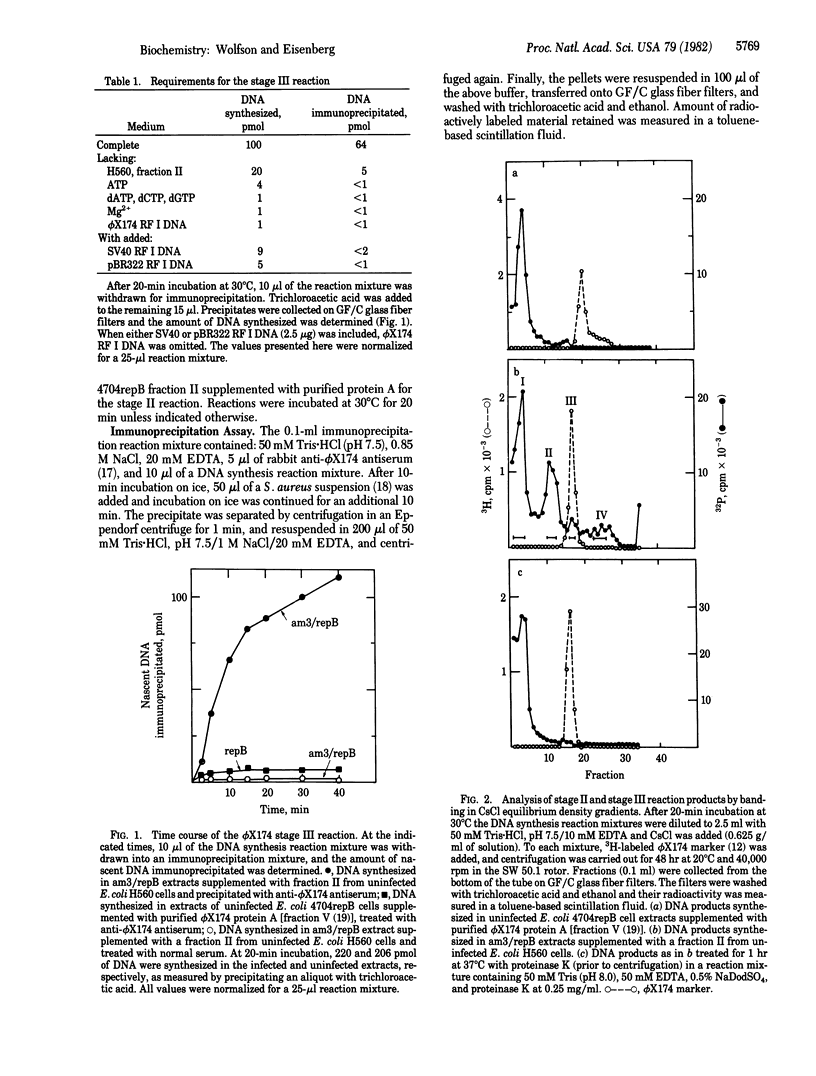
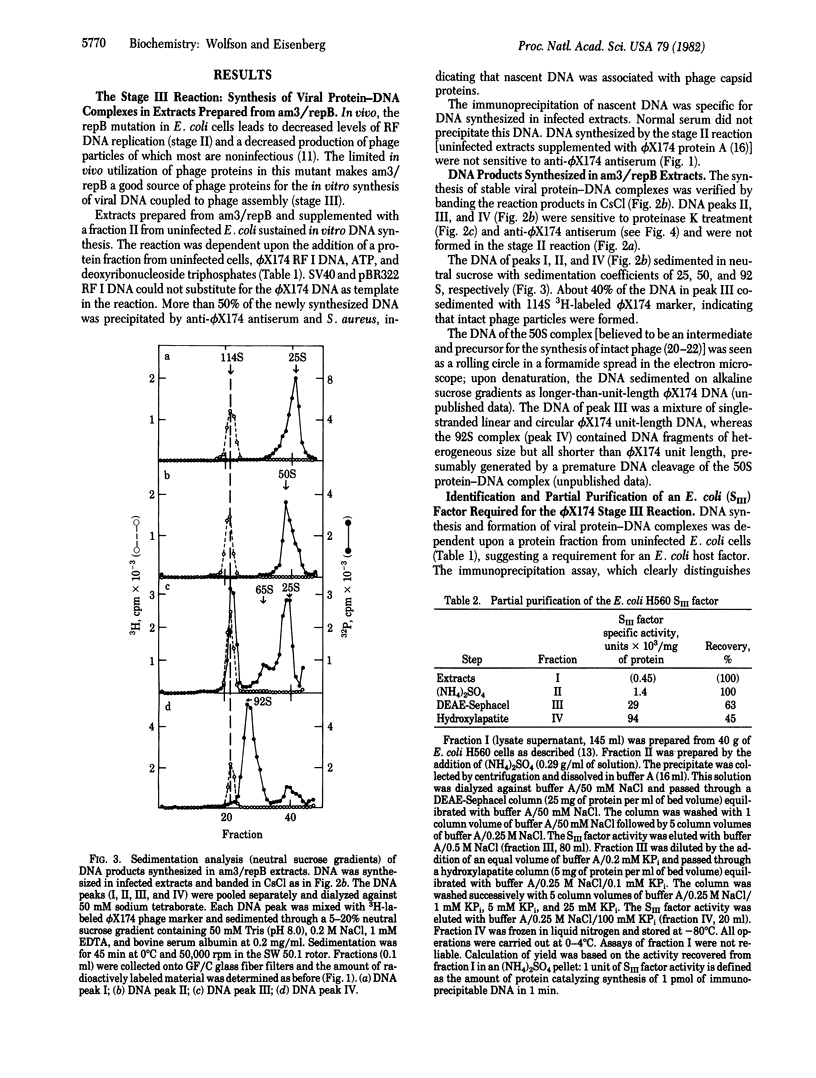
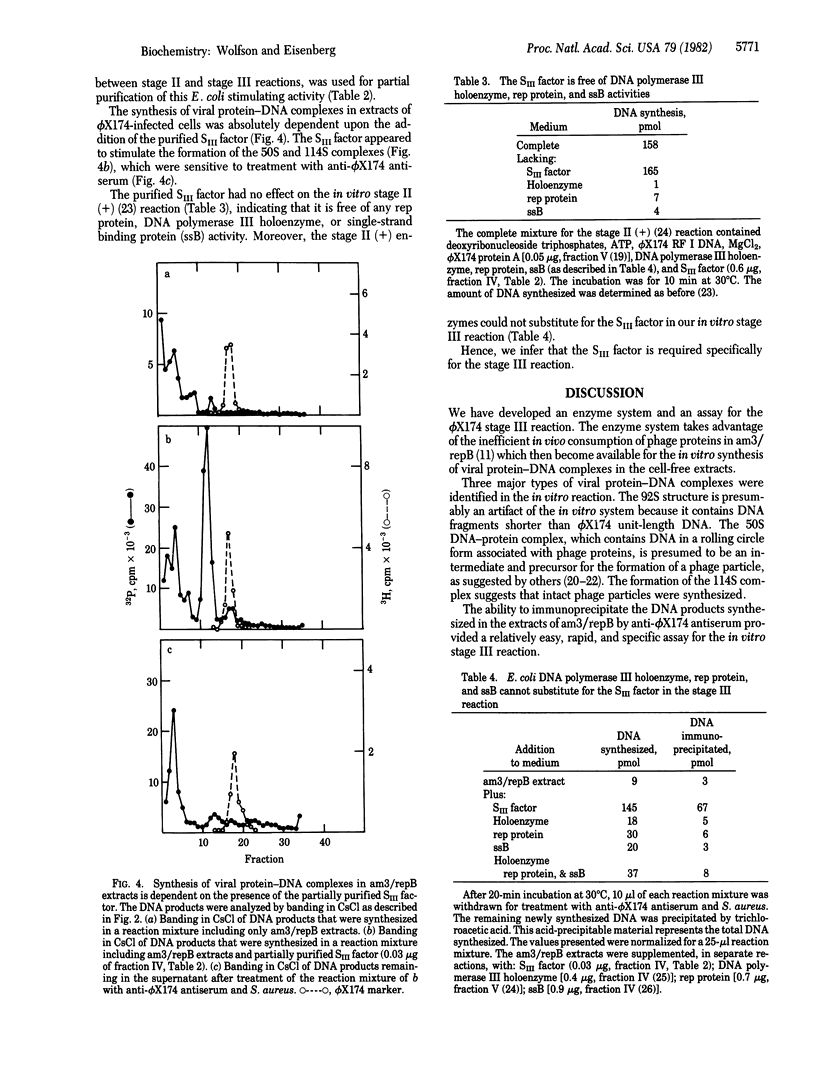
A cell-free extract prepared from phi X174-infected Escherichia coli cells sustained in vitro synthesis of viral DNA (stage III reaction) when supplemented with fraction II from uninfected cells. The reaction was dependent upon deoxyribonucleoside triphosphate, ATP, added phi X174 replicative form I DNA template, and the fraction II from uninfected cells. This reaction differed from the stage II reaction (semiconservative replication of duplex replicative form DNA) by the production of stable viral protein-DNA complexes sensitive to anti-phi X174 antiserum. Three types of protein-DNA complexes were identified, 50S, 92S, and a 114S complex that cobanded in CsCl and cosedimented in neutral sucrose gradients with a phi X174 phage marker. The sensitivity of these complexes to anti-phi X174 antiserum and Staphylococcus aureus provided a relatively rapid biochemical assay for direct measurement of the amount of DNA synthesized by the stage III reaction. With this assay, an E. coli factor (SIII) required specifically for the synthesis of viral protein-DNA complexes was identified and purified 200-fold from uninfected E. coli cells. The partially purified SIII factor was required for the synthesis of DNA and viral protein-DNA complexes in the phi X174-infected cell extracts and could not be replaced by rep protein, single-strand binding protein, or DNA polymerase III holoenzyme.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyama A., Hamatake R. K., Hayashi M. Morphogenesis of phi X174: in vitro synthesis of infectious phage from purified viral components. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7285–7289. doi: 10.1073/pnas.78.12.7285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai N., Polder L., Akai K., Kornberg A. Replication of phi X174 DNA with purified enzymes. II. Multiplication of the duplex form by coupling of continuous and discontinuous synthetic pathways. J Biol Chem. 1981 May 25;256(10):5239–5246. [PubMed] [Google Scholar]
- Denhardt D. T. The single-stranded DNA phages. CRC Crit Rev Microbiol. 1975 Dec;4(2):161–223. doi: 10.3109/10408417509111575. [DOI] [PubMed] [Google Scholar]
- Eisenberg S., Harbers B., Hours C., Denhardt D. T. The mechanism of replication of phiX174 DNA. XII. Non-random location of gaps in nascent phiX174 RF II DNA. J Mol Biol. 1975 Nov 25;99(1):107–123. doi: 10.1016/s0022-2836(75)80162-8. [DOI] [PubMed] [Google Scholar]
- Eisenberg S., Kornberg A. Purification and characterization of phiX174 gene A protein. A multifunctional enzyme of duplex DNA replication. J Biol Chem. 1979 Jun 25;254(12):5328–5332. [PubMed] [Google Scholar]
- Eisenberg S., Scott J. F., Kornberg A. An enzyme system for replication of duplex circular DNA: the replicative form of phage phi X174. Proc Natl Acad Sci U S A. 1976 May;73(5):1594–1597. doi: 10.1073/pnas.73.5.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg S., Scott J. F., Kornberg A. Enzymatic replication of viral and complementary strands of duplex DNA of phage phiX174 proceeds by seprate mechanisms. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3151–3155. doi: 10.1073/pnas.73.9.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg S., Scott J. F., Kronberg A. Enzymatic replication of phiX174 duplex circles: continuous synthesis. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):295–302. doi: 10.1101/sqb.1979.043.01.036. [DOI] [PubMed] [Google Scholar]
- Fujisawa H., Hayashi M. Assembly of bacteriophage phi X174: identification of a virion capsid precursor and proposal of a model for the functions of bacteriophage gene products during morphogenesis. J Virol. 1977 Oct;24(1):303–313. doi: 10.1128/jvi.24.1.303-313.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaya M., Eisenberg S., Bartok K., Denhardt D. T. Mechanism of replication of single-stranded PhiX174 DNA. VII. Circularization of the progeny viral strand. J Virol. 1973 Oct;12(4):808–818. doi: 10.1128/jvi.12.4.808-818.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Koths K., Dressler D. The rolling circle . capsid complex as an intermediate in phi X DNA replication and viral assembly. J Biol Chem. 1980 May 10;255(9):4328–4338. [PubMed] [Google Scholar]
- McHenry C., Kornberg A. DNA polymerase III holoenzyme of Escherichia coli. Purification and resolution into subunits. J Biol Chem. 1977 Sep 25;252(18):6478–6484. [PubMed] [Google Scholar]
- Meyer R. R., Shlomai J., Kobori J., Bates D. L., Rowen L., McMacken R., Ueda K., Kornberg A. Enzymatic conversion of single-stranded phiX174 and G4 circles to duplex forms: discontinuous replication. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):289–293. doi: 10.1101/sqb.1979.043.01.035. [DOI] [PubMed] [Google Scholar]
- Mukai R., Hamatake R. K., Hayashi M. Isolation and identification of bacteriophage phi X174 prohead. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4877–4881. doi: 10.1073/pnas.76.10.4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. F., Kornberg A. Purification of the rep protein of Escherichia coli. An ATPase which separates duplex DNA strands in advance of replication. J Biol Chem. 1978 May 10;253(9):3292–3297. [PubMed] [Google Scholar]
- Shlomai J., Polder L., Arai K., Kornberg A. Replication of phi X174 dna with purified enzymes. I. Conversion of viral DNA to a supercoiled, biologically active duplex. J Biol Chem. 1981 May 25;256(10):5233–5238. [PubMed] [Google Scholar]
- Sumida-Yasumoto C., Hurwitz J. Synthesis of phiX174 viral DNA in vitro depends on phiX replicative form DNA. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4195–4199. doi: 10.1073/pnas.74.10.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumida-Yasumoto C., Ikeda J. E., Benz E., Marians K. J., Vicuna R., Sugrue S., Zipursky S. L., Hurwitz J. Replication of phiX174 DNA: in vitro synthesis of phiX RFI DNA and circular, single-stranded DNA. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):311–329. doi: 10.1101/sqb.1979.043.01.038. [DOI] [PubMed] [Google Scholar]
- Ueda K., McMacken R., Kornberg A. dnaB protein of Escherichia coli. Purification and role in the replication of phiX174 DNA. J Biol Chem. 1978 Jan 10;253(1):261–269. [PubMed] [Google Scholar]
- Weiner J. H., Bertsch L. L., Kornberg A. The deoxyribonucleic acid unwinding protein of Escherichia coli. Properties and functions in replication. J Biol Chem. 1975 Mar 25;250(6):1972–1980. [PubMed] [Google Scholar]
- Wickner W., Brutlag D., Schekman R., Kornberg A. RNA synthesis initiates in vitro conversion of M13 DNA to its replicative form. Proc Natl Acad Sci U S A. 1972 Apr;69(4):965–969. doi: 10.1073/pnas.69.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]


