Abstract
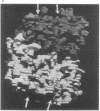
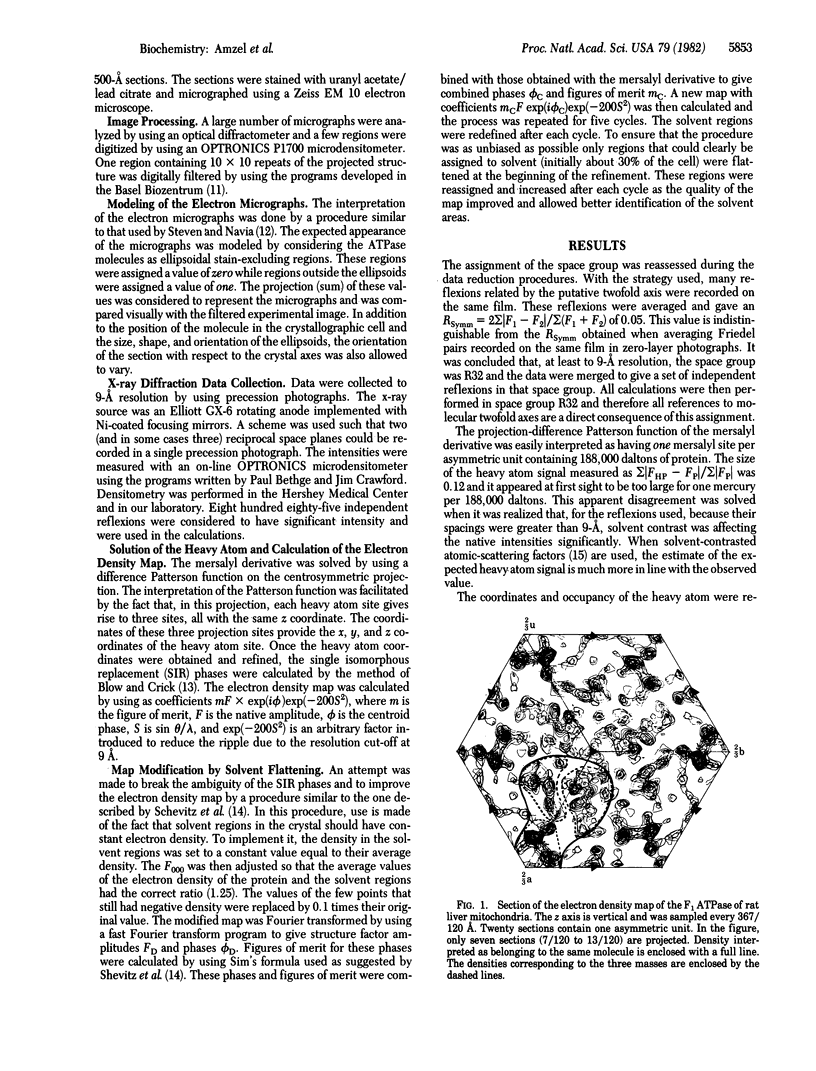
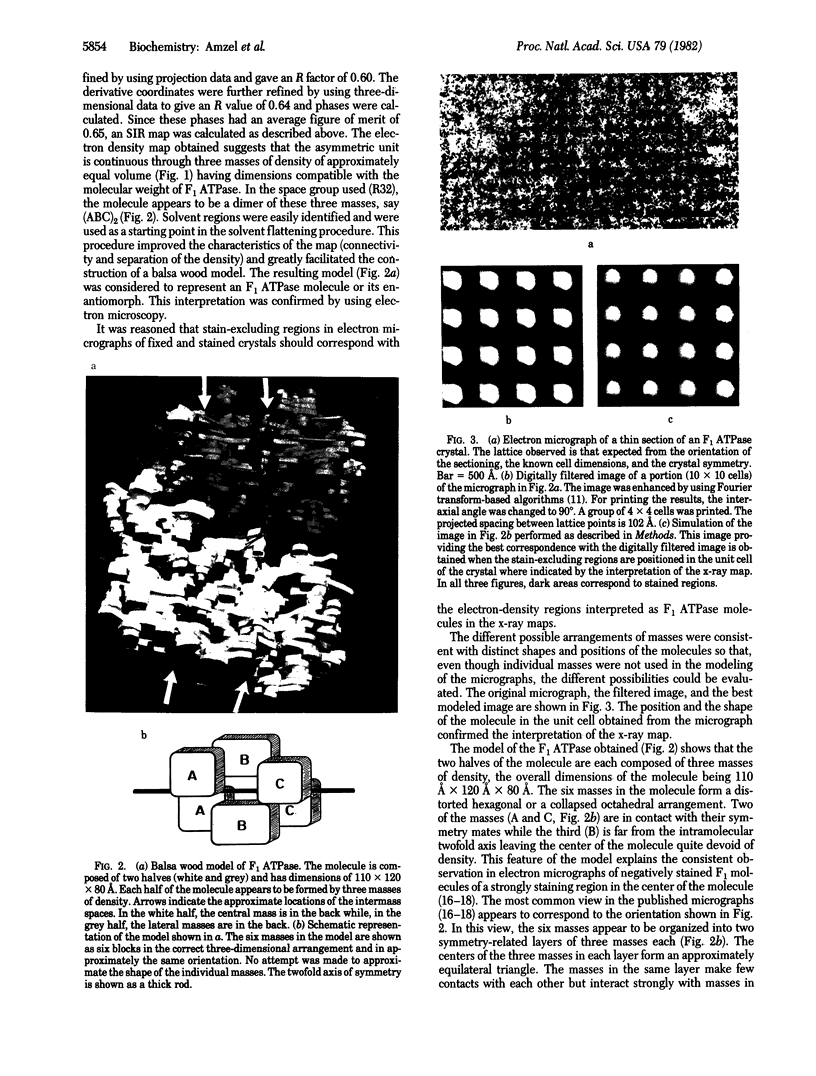
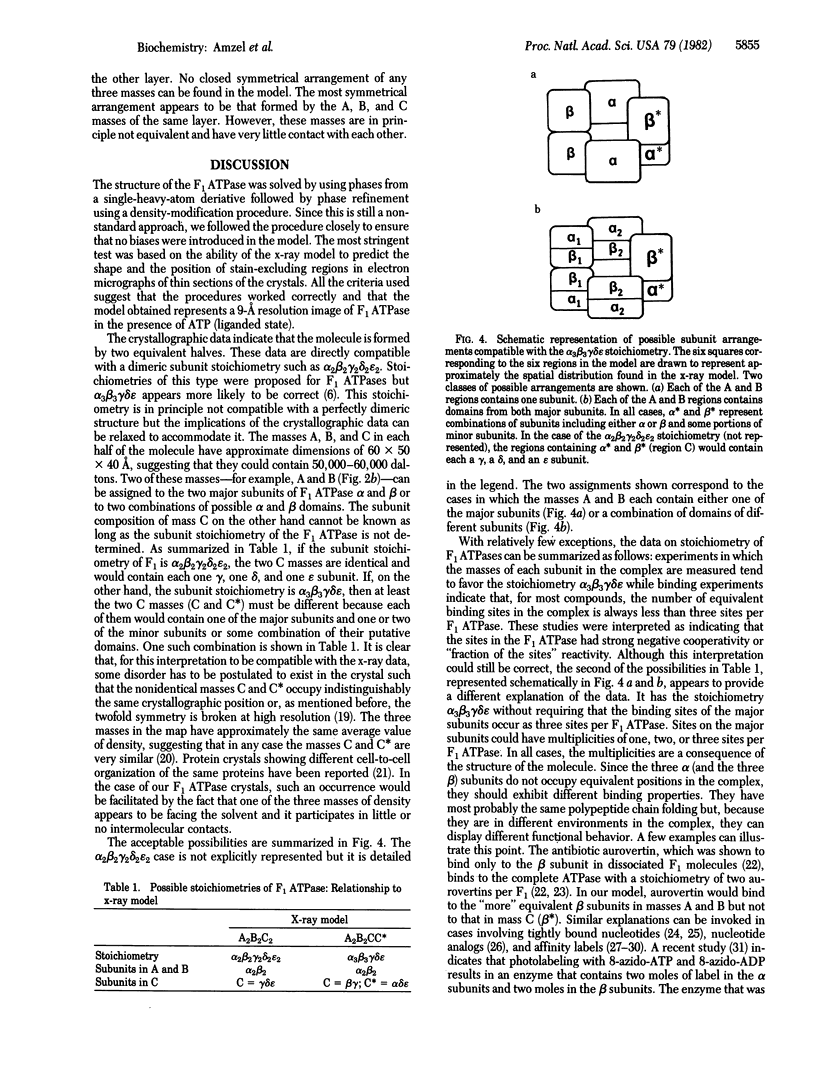
The soluble portion (F1 ATPase) of the mitochondrial ATP-synthesizing system is a multisubunit enzyme of molecular weight 380,000. It is composed of five different subunits, alpha, beta, gamma, and epsilon. The subunit stoichiometry is not known but there are strong suggestions that it is alpha 3 beta 3 gamma delta epsilon. We have determined the three-dimensional structure of the F1 ATPase of rat liver mitochondria to 9-A resolution by using x-ray diffraction techniques. The molecule appears to be formed by two equivalent halves, each formed by three regions of approximately equal size. These regions form a distorted hexagonal or octahedral arrangement. None of the regions form closed symmetrical trimers in the complex. It is proposed that, if the subunit stoichiometry is alpha 3 beta 3 gamma delta epsilon, the major subunits exist in at least two different environments in the complex. In this arrangement, the different copies of the major subunits are functionally not equivalent. This observation appears to offer a natural explanation of the complicated binding and labeling data of F1 ATPases.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adolfsen R., Moudrianakis E. N. Purification and properties of 2 soluble coupling factors of oxidative phosphorylation from Alcaligenes faecalis. Biochemistry. 1971 Jun 8;10(12):2247–2253. doi: 10.1021/bi00788a010. [DOI] [PubMed] [Google Scholar]
- Amzel L. M., Pedersen P. L. Adenosine triphosphatase from rat liver mitochondria. Crystallization and x-ray diffraction studies of the F1-component of the enzyme. J Biol Chem. 1978 Apr 10;253(7):2067–2069. [PubMed] [Google Scholar]
- Amzel L. M. Structure of F1-ATPase. J Bioenerg Biomembr. 1981 Aug;13(3-4):109–121. doi: 10.1007/BF00763833. [DOI] [PubMed] [Google Scholar]
- Baird B. A., Hammes G. G. Chemical cross-linking studies of beef heart mitochondrial coupling factor 1. J Biol Chem. 1977 Jul 10;252(13):4743–4748. [PubMed] [Google Scholar]
- Baird B. A., Hammes G. G. Chemical cross-linking studies of chloroplast coupling factor 1. J Biol Chem. 1976 Nov 25;251(22):6953–6962. [PubMed] [Google Scholar]
- Bragg P. D., Hou C. A cross-linking study of the Ca2+, Mg2+-activated adenosine triphosphatase of Escherichia coli. Eur J Biochem. 1980 May;106(2):495–503. doi: 10.1111/j.1432-1033.1980.tb04596.x. [DOI] [PubMed] [Google Scholar]
- Bragg P. D., Hou C. Subunit composition, function, and spatial arrangement in the Ca2+-and Mg2+-activated adenosine triphosphatases of Escherichia coli and Salmonella typhimurium. Arch Biochem Biophys. 1975 Mar;167(1):311–321. doi: 10.1016/0003-9861(75)90467-1. [DOI] [PubMed] [Google Scholar]
- Bragg P. D., Hou C. Subunit composition, function, and spatial arrangement in the Ca2+-and Mg2+-activated adenosine triphosphatases of Escherichia coli and Salmonella typhimurium. Arch Biochem Biophys. 1975 Mar;167(1):311–321. doi: 10.1016/0003-9861(75)90467-1. [DOI] [PubMed] [Google Scholar]
- Cantley L. C., Hammes G. G. Characterization of sulfhydryl groups on chloroplast coupling factor 1 exposed by heat activation. Biochemistry. 1976 Jan 13;15(1):9–14. doi: 10.1021/bi00646a002. [DOI] [PubMed] [Google Scholar]
- Cantley L. C., Jr, Hammes G. G. Fluorescence energy transfer between ligand binding sites on chloroplast coupling factor 1. Biochemistry. 1975 Jul;14(13):2976–2981. doi: 10.1021/bi00684a028. [DOI] [PubMed] [Google Scholar]
- Cantley L. C., Jr, Hammes G. G. Investigation of quercetin binding sites on chloroplast coupling factor 1. Biochemistry. 1976 Jan 13;15(1):1–8. doi: 10.1021/bi00646a001. [DOI] [PubMed] [Google Scholar]
- Catterall W. A., Coty W. A., Pedersen P. L. Adenosine triphosphatase from rat liver mitochondria. 3. Subunit composition. J Biol Chem. 1973 Nov 10;248(21):7427–7431. [PubMed] [Google Scholar]
- Catterall W. A., Pedersen P. L. Adenosine triphosphatase from rat liver mitochondria. I. Purification, homogeneity, and physical properties. J Biol Chem. 1971 Aug 25;246(16):4987–4994. [PubMed] [Google Scholar]
- Chang T., Penefsky H. S. Aurovertin, a fluorescent probe of conformational change in beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1973 Apr 25;248(8):2746–2754. [PubMed] [Google Scholar]
- Cross R. L. The mechanism and regulation of ATP synthesis by F1-ATPases. Annu Rev Biochem. 1981;50:681–714. doi: 10.1146/annurev.bi.50.070181.003341. [DOI] [PubMed] [Google Scholar]
- Deters D. W., Racker E., Nelson N., Nelson H. Partial resolution of the enzymes catalyzing photophosphorylation. XV. Approaches to the active site of coupling factor I. J Biol Chem. 1975 Feb 10;250(3):1041–1047. [PubMed] [Google Scholar]
- Dunn S. D., Futai M. Reconstitution of a functional coupling factor from the isolated subunits of Escherichia coli F1 ATPase. J Biol Chem. 1980 Jan 10;255(1):113–118. [PubMed] [Google Scholar]
- Fisher R. G., Woods N. E., Fuchs H. E., Sweet R. M. Three-dimensional structures of C-phycocyanin and B-phycoerythrin at 5-A resolution. J Biol Chem. 1980 Jun 10;255(11):5082–5089. [PubMed] [Google Scholar]
- Fitzgerald P. M., Stankiewicz P. J., Smith S. C., McPherson A. Internal dihedral symmetry of alpha-amylase and alpha-mannosidase. J Mol Biol. 1979 Dec 15;135(3):753–756. doi: 10.1016/0022-2836(79)90175-x. [DOI] [PubMed] [Google Scholar]
- Harris D. A., Gomez-Fernandez J. C., Klungsøyr L., Radda G. K. Specificity of nucleotide binding and coupled reactions utilising the mitochondrial ATPase. Biochim Biophys Acta. 1978 Dec 7;504(3):364–383. doi: 10.1016/0005-2728(78)90060-9. [DOI] [PubMed] [Google Scholar]
- Harris D. A., Rosing J., van de Stadt R. J., Slater E. C. Tight binding of adenine nucleotides to beef-heart mitochondrial ATPase. Biochim Biophys Acta. 1973 Aug 31;314(2):149–153. doi: 10.1016/0005-2728(73)90130-8. [DOI] [PubMed] [Google Scholar]
- Kumar G., Kalra V. K., Brodie A. F. Affinity labeling of coupling factor-latent ATPase from Mycobacterium phlei with 2',3'-dialdehyde derivatives of adenosine 5'-triphosphate and adenosine 5'-diphosphate. J Biol Chem. 1979 Mar 25;254(6):1964–1971. [PubMed] [Google Scholar]
- Lee S. H., Kalra V. K., Ritz C. J., Brodie A. F. Binding of nucleotides to purified coupling factor-latent ATPase from Mycobacterium phlei. J Biol Chem. 1977 Feb 10;252(3):1084–1091. [PubMed] [Google Scholar]
- Matsushima M., Marquart M., Jones T. A., Colman P. M., Bartels K., Huber R. Crystal structure of the human Fab fragment Kol and its comparison with the intact Kol molecule. J Mol Biol. 1978 Jun 5;121(4):441–459. doi: 10.1016/0022-2836(78)90393-5. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Vectorial chemiosmotic processes. Annu Rev Biochem. 1977;46:996–1005. doi: 10.1146/annurev.bi.46.070177.005024. [DOI] [PubMed] [Google Scholar]
- Mollinedo F., Larraga V., Coll F. J., Muñoz E. Role of the subunits of the energy-transducing adenosine triphosphatase from Micrococcus lysodeikticus membranes studied by proteolytic digestion and immunological approaches. Biochem J. 1980 Mar 15;186(3):713–723. doi: 10.1042/bj1860713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson N., Kanner B. I., Gutnick D. L. Purification and properties of Mg2+-Ca2+ adenosinetriphosphatase from Escherichia coli. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2720–2724. doi: 10.1073/pnas.71.7.2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen P. L. Adenosine triphosphatase from rat liver mitochondria - evidence for a mercurial-sensitive site for the activating anion bicarbonate. Biochem Biophys Res Commun. 1976 Aug 23;71(4):1182–1188. doi: 10.1016/0006-291x(76)90778-6. [DOI] [PubMed] [Google Scholar]
- Salton M. R., Schor M. T. Subunit structure and properties of two forms of adenosine triphosphatase released from Micrococcus lysodeikticus membranes. Biochem Biophys Res Commun. 1972 Oct 17;49(2):350–357. doi: 10.1016/0006-291x(72)90417-2. [DOI] [PubMed] [Google Scholar]
- Saraste M., Gay N. J., Eberle A., Runswick M. J., Walker J. E. The atp operon: nucleotide sequence of the genes for the gamma, beta, and epsilon subunits of Escherichia coli ATP synthase. Nucleic Acids Res. 1981 Oct 24;9(20):5287–5296. doi: 10.1093/nar/9.20.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. R. An integrated set of computer programs for processing electron micrographs of biological structures. Ultramicroscopy. 1978;3(2):153–160. doi: 10.1016/s0304-3991(78)80021-7. [DOI] [PubMed] [Google Scholar]
- Steven A. C., Navia M. A. Fidelity of structure representation in electron micrographs of negatively stained protein molecules. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4721–4725. doi: 10.1073/pnas.77.8.4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verschoor G. J., van der Sluis P. R., Slater E. C. The binding of aurovertin to isolated beta subunit of F1 (mitochondrial ATPase). Stoicheiometry of beta subunit in F1. Biochim Biophys Acta. 1977 Nov 17;462(2):438–449. doi: 10.1016/0005-2728(77)90141-4. [DOI] [PubMed] [Google Scholar]
- Wagenvoord R. J., Kemp A., Slater E. C. The number and localisation of adenine nucleotide-binding sites in beef-heart mitochondrial ATPase (F1) determined by photolabelling with 8-azido-ATP and 8-azido-ADP. Biochim Biophys Acta. 1980 Dec 3;593(2):204–211. doi: 10.1016/0005-2728(80)90058-4. [DOI] [PubMed] [Google Scholar]
- Wakabayashi T., Kubota M., Yoshida M., Kagawa Y. Structure of ATPase (coupling factor TF1) from a thermophilic bacterium. J Mol Biol. 1977 Dec 5;117(2):515–519. doi: 10.1016/0022-2836(77)90140-1. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Okamoto H., Sone N., Hirata H., Kagawa Y. Reconstitution of thermostable ATPase capable of energy coupling from its purified subunits. Proc Natl Acad Sci U S A. 1977 Mar;74(3):936–940. doi: 10.1073/pnas.74.3.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M., Sone N., Hirata H., Kagawa Y. Reconstitution of adenosine triphosphatase of thermophilic bacterium from purified individual subunits. J Biol Chem. 1977 May 25;252(10):3480–3485. [PubMed] [Google Scholar]