Abstract
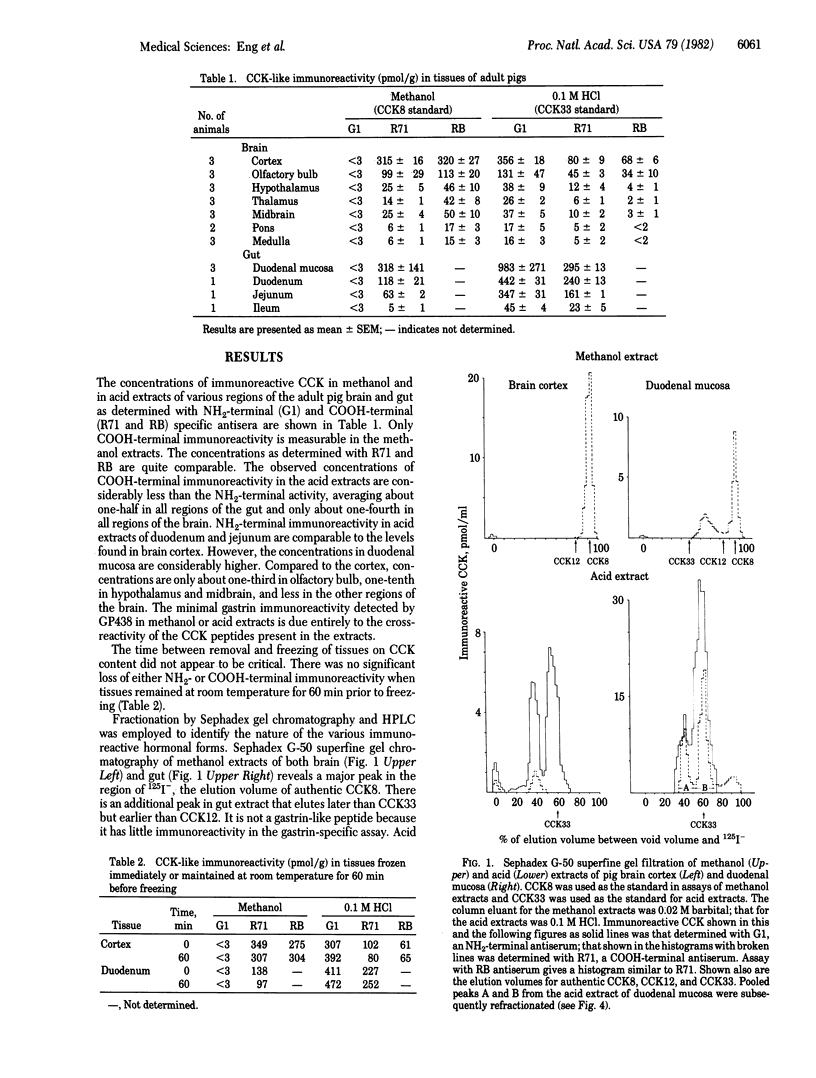
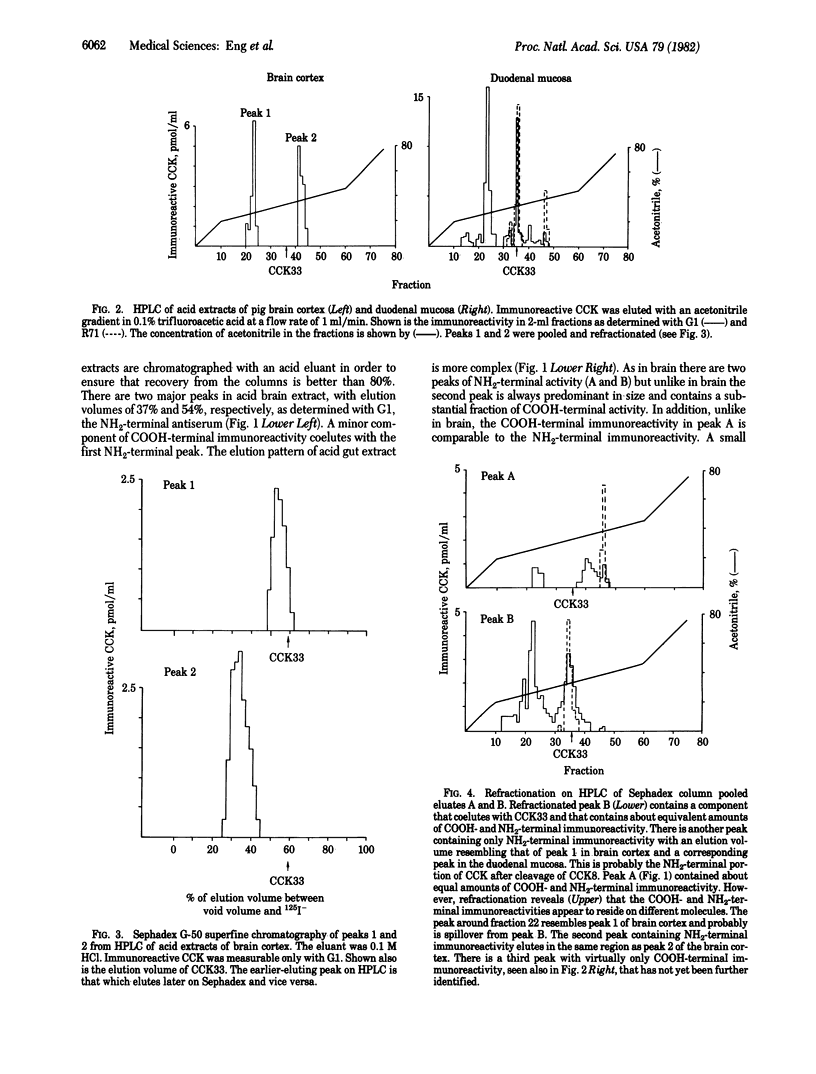
A sequential extraction method employing methanol extraction of the COOH-terminal fragments of cholecystokinin (CCK) from pig tissues followed by HCl extraction of intact CCK and its NH2-terminal fragments is described. Radioimmunoassay of extracts and their fractionation by Sephadex chromatography and HPLC demonstrate that the distributions of COOH-terminal and NH2-terminal immunoreactivities among various regions of brain are similar and independent of the concentrations in individual regions. The distribution in gut differs from that in brain. Greatest concentrations of CCK immunoreactivity are located in cortical tissue in the brain and in duodenal mucosa in gut. Both brain and gut contain CCK octapeptide (CCK8) and an NH2-terminal fragment that is likely to be desoctapeptide-CCK33. Intact CCK33 is extractable from gut but not from brain. Brain contains another NH2-terminal immunoreactive molecule lacking COOH-terminal immunoreactivity that may be a peptide with a COOH-terminal extension, as has been described for gastrin, or one that may not be derived from a CCK33-like precursor. This peptide is much less prominent in gut, or may be nonexistent there. The failure to find CCK33 in the brain and the presence in the brain of this as-yet-uncharacterized NH2-terminal peptide raises the question as to whether the differences between neuronal and mucosal tissues are a consequence of differences in post-translational processing or in the DNA templates.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anastasi A., Erspamer V., Endean R. Isolation and amino acid sequence of caerulein, the active decapeptide of the skin of hyla caerulea. Arch Biochem Biophys. 1968 Apr;125(1):57–68. doi: 10.1016/0003-9861(68)90638-3. [DOI] [PubMed] [Google Scholar]
- Beinfeld M. C., Meyer D. K., Brownstein M. J. Cholecystokinin octapeptide in the rat hypothalamo-neurohypophysial system. Nature. 1980 Nov 27;288(5789):376–378. doi: 10.1038/288376a0. [DOI] [PubMed] [Google Scholar]
- Beinfeld M. C., Meyer D. K., Eskay R. L., Jensen R. T., Brownstein M. J. The distribution of cholecystokinin immunoreactivity in the central nervous system of the rat as determined by radioimmunoassay. Brain Res. 1981 May 11;212(1):51–57. doi: 10.1016/0006-8993(81)90031-7. [DOI] [PubMed] [Google Scholar]
- Calam J., Ellis A., Dockray G. J. Identification and measurement of molecular variants of cholecystokinin in duodenal mucosa and plasma. Diminished concentrations in patients with celiac disease. J Clin Invest. 1982 Jan;69(1):218–225. doi: 10.1172/JCI110433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockray G. J. Cholecystokinin-like peptides in avian brain and gut. Experientia. 1979 May 15;35(5):628–630. doi: 10.1007/BF01960363. [DOI] [PubMed] [Google Scholar]
- Dockray G. J. Cholecystokinins in rat cerebral cortex: identification, purification and characterization by immunochemical methods. Brain Res. 1980 Apr 21;188(1):155–165. doi: 10.1016/0006-8993(80)90564-8. [DOI] [PubMed] [Google Scholar]
- Dockray G. J., Gregory R. A. Does the C-terminal tetrapeptide of gastrin and CCK exist as an entity? Nature. 1980 Aug 14;286(5774):742–742. doi: 10.1038/286742a0. [DOI] [PubMed] [Google Scholar]
- Dockray G. J., Gregory R. A., Hutchison J. B., Harris J. I., Runswick M. J. Isolation, structure and biological activity of two cholecystokinin octapeptides from sheep brain. Nature. 1978 Aug 17;274(5672):711–713. doi: 10.1038/274711a0. [DOI] [PubMed] [Google Scholar]
- Dockray G. J. Immunochemical evidence of cholecystokinin-like peptides in brain. Nature. 1976 Dec 9;264(5586):568–570. doi: 10.1038/264568a0. [DOI] [PubMed] [Google Scholar]
- Dockray G. J. Immunoreactive component resembling cholecystokinin octapeptide in intestine. Nature. 1977 Nov 24;270(5635):359–361. doi: 10.1038/270359a0. [DOI] [PubMed] [Google Scholar]
- Geola F. L., Hershman J. M., Warwick R., Reeve J. R., Walsh J. H., Tourtellotte W. W. Regional distribution of cholecystokinin-like immunoreactivity in the human brain. J Clin Endocrinol Metab. 1981 Aug;53(2):270–275. doi: 10.1210/jcem-53-2-270. [DOI] [PubMed] [Google Scholar]
- Glowinski J., Iversen L. L. Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain. J Neurochem. 1966 Aug;13(8):655–669. doi: 10.1111/j.1471-4159.1966.tb09873.x. [DOI] [PubMed] [Google Scholar]
- Holmquist A. L., Dockray G. J., Rosenquist G. L., Walsh J. H. Immunochemical characterization of cholecystokinin-like peptides in lamprey gut and brain. Gen Comp Endocrinol. 1979 Apr;37(4):474–481. doi: 10.1016/0016-6480(79)90028-5. [DOI] [PubMed] [Google Scholar]
- Lamers C. B., Morley J. E., Poitras P., Sharp B., Carlson H. E., Hershman J. M., Walsh J. H. Immunological and biological studies on cholecystokinin in rat brain. Am J Physiol. 1980 Sep;239(3):E232–E235. doi: 10.1152/ajpendo.1980.239.3.E232. [DOI] [PubMed] [Google Scholar]
- Larsson L., Rehfeld J. F. A peptide resembling COOH-terminal tetrapeptide amide of gastrin from a new gastrointestinal endocrine cell type. Nature. 1979 Feb 15;277(5697):575–578. doi: 10.1038/277575a0. [DOI] [PubMed] [Google Scholar]
- Muller J. E., Straus E., Yalow R. S. Cholecystokinin and its COOH-terminal octapeptide in the pig brain. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3035–3037. doi: 10.1073/pnas.74.7.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutt V. Chemistry, isolation and purification of gastrointestinal hormones. Biochem Soc Trans. 1980 Feb;8(1):11–14. doi: 10.1042/bst0080011. [DOI] [PubMed] [Google Scholar]
- Noyes B. E., Mevarech M., Stein R., Agarwal K. L. Detection and partial sequence analysis of gastrin mRNA by using an oligodeoxynucleotide probe. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1770–1774. doi: 10.1073/pnas.76.4.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehfeld J. F., Goltermann N. R. Immunochemical evidence of cholecystokinin tetrapeptides in hog brain. J Neurochem. 1979 Apr;32(4):1339–1341. doi: 10.1111/j.1471-4159.1979.tb11065.x. [DOI] [PubMed] [Google Scholar]
- Rehfeld J. F. Immunochemical studies on cholecystokinin. II. Distribution and molecular heterogeneity in the central nervous system and small intestine of man and hog. J Biol Chem. 1978 Jun 10;253(11):4022–4030. [PubMed] [Google Scholar]
- Rehfeld J. F., Larsson L. I. The predominating molecular form of gastrin and cholecystokinin in the gut is a small peptide corresponding to their COOH-terminal tetrapeptide amide. Acta Physiol Scand. 1979 Jan;105(1):117–119. doi: 10.1111/j.1748-1716.1979.tb06320.x. [DOI] [PubMed] [Google Scholar]
- Robberecht P., Deschodt-Lanckman M., Vanderhaeghen J. J. Demonstration of biological activity of brain gastrin-like peptidic material in the human: its relationship with the COOH-terminal octapeptide of cholecystokinin. Proc Natl Acad Sci U S A. 1978 Jan;75(1):524–528. doi: 10.1073/pnas.75.1.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder S. W., Eng J., Straus E., Yalow R. S. Alkaline extraction of cholecystokinin-immunoreactivity from rat brain. Biochem Biophys Res Commun. 1980 May 30;94(2):704–709. doi: 10.1016/0006-291x(80)91289-9. [DOI] [PubMed] [Google Scholar]
- Ryder S. W., Eng J., Straus E., Yalow R. S. Extraction and immunochemical characterization of cholecystokinin-like peptides from pig and rat brain. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3892–3896. doi: 10.1073/pnas.78.6.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider B. S., Monahan J. W., Hirsch J. Brain cholecystokinin and nutritional status in rats and mice. J Clin Invest. 1979 Nov;64(5):1348–1356. doi: 10.1172/JCI109591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus E., Ryder S., Eng J., Yalow R. S. Nature of immunoreactive CCK in rat and pig brain. Peptides. 1981;2 (Suppl 2):89–92. doi: 10.1016/0196-9781(81)90017-6. [DOI] [PubMed] [Google Scholar]
- Straus E., Yalow R. S. Species specificity of cholecystokinin in gut and brain of several mammalian species. Proc Natl Acad Sci U S A. 1978 Jan;75(1):486–489. doi: 10.1073/pnas.75.1.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhaeghen J. J., Signeau J. C., Gepts W. New peptide in the vertebrate CNS reacting with antigastrin antibodies. Nature. 1975 Oct 16;257(5527):604–605. doi: 10.1038/257604a0. [DOI] [PubMed] [Google Scholar]


