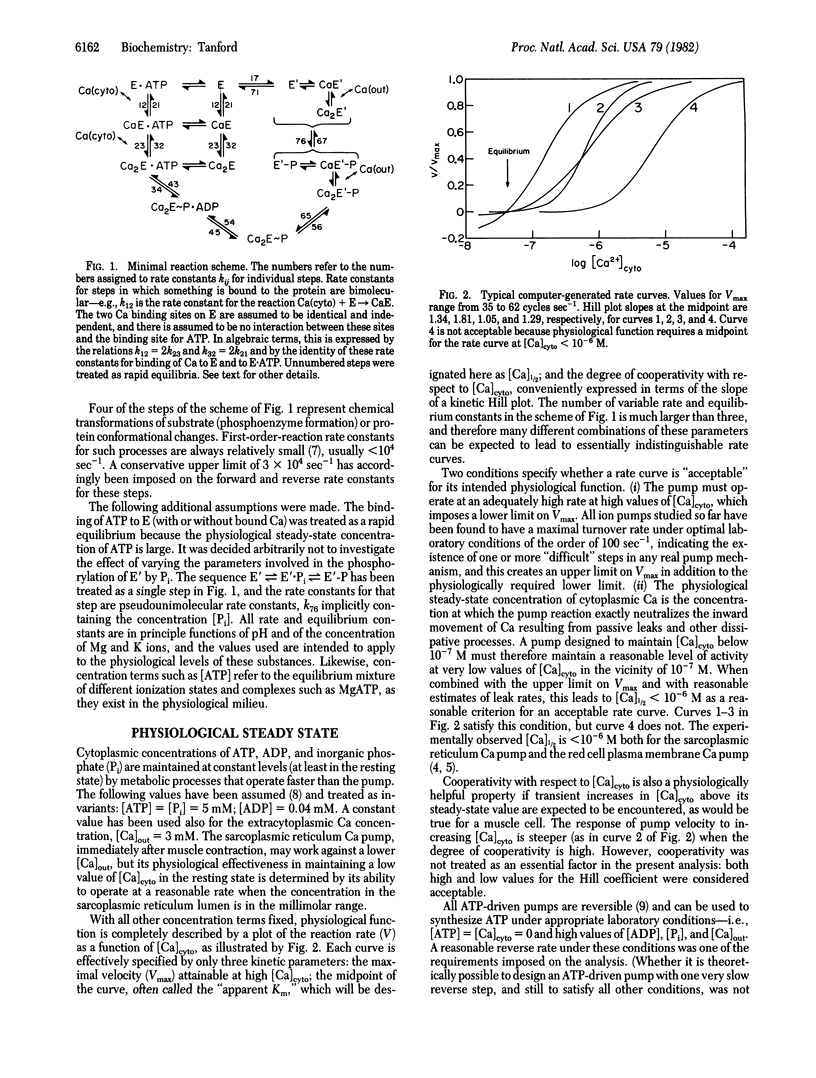
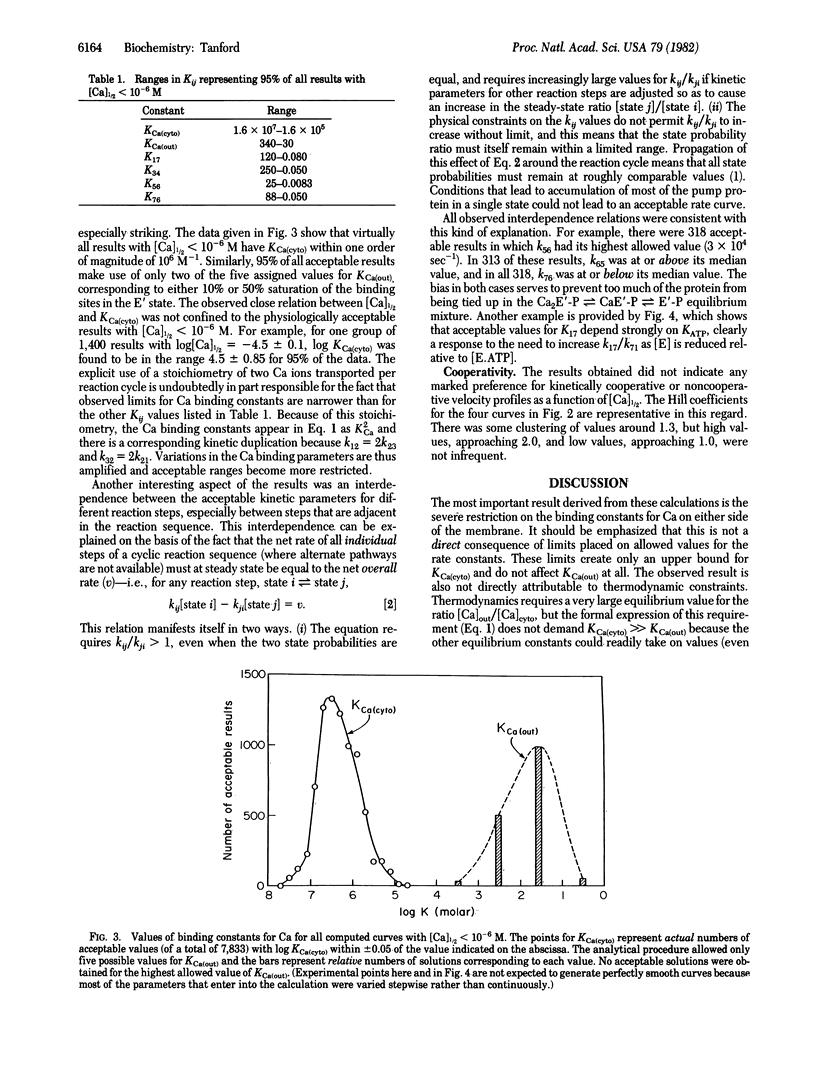
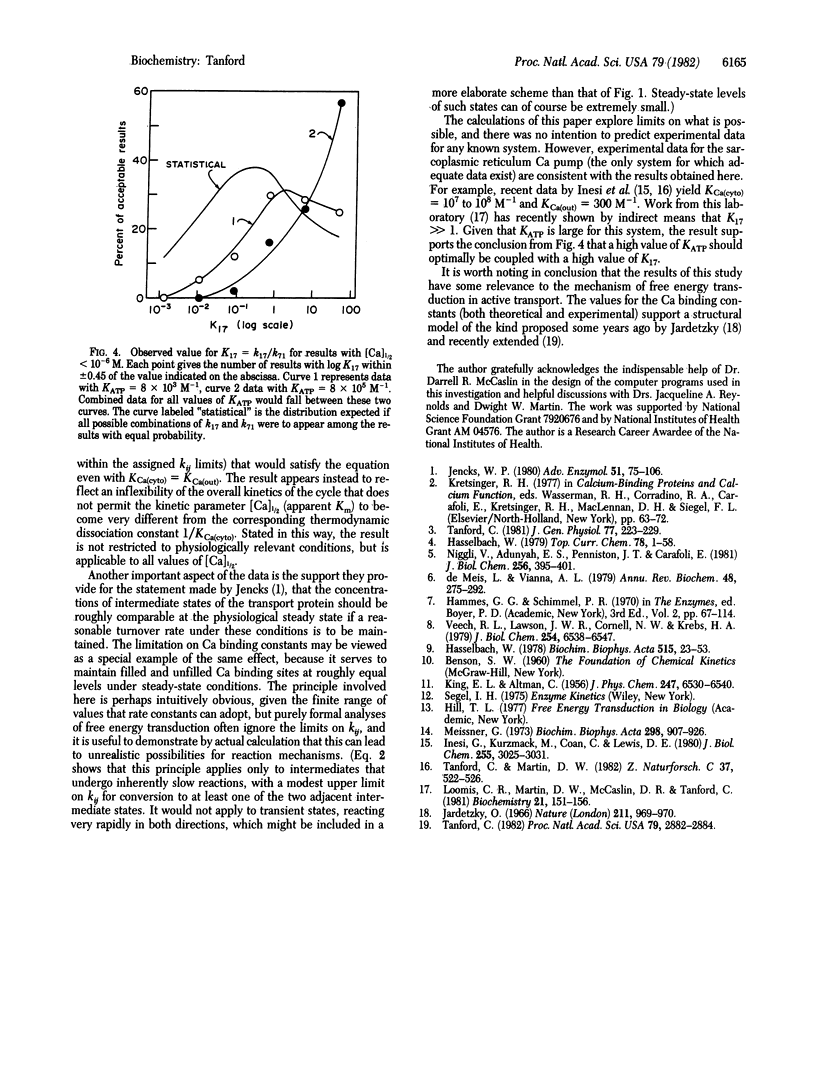
Abstract
A numerical analysis was carried out to explore limitations on kinetic and thermodynamic parameters for an ATP-driven Ca pump. A conventional pump reaction cycle was employed, with a transport stoichiometry of two Ca ions per cycle. Rigid requirements were imposed to represent the needs of physiological function, defined as the ability to maintain the cytoplasmic Ca concentration below 10(-7) M against a 3 mM concentration on the opposite side of the membrane. Realistic physical limits were placed on the magnitudes of rate constants for individual reaction steps. Reversibility under laboratory conditions was assumed. The results show that these requirements can be satisfied simultaneously only if the equilibrium constant for binding Ca from the cytoplasmic (uptake) side of the membrane is much larger than the binding constant on the discharge side. More generally, the results demonstrate that limitations on rate constants make it possible for the pump to maintain an adequate rate only if steady-state levels of kinetically important (slowly reacting) reaction intermediates do not become too disparate. Experimental data for the sarcoplasmic reticulum calcium pump support these theoretical conclusions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hasselbach W. The reversibility of the sarcoplasmic calcium pump. Biochim Biophys Acta. 1978 Apr 10;515(1):23–53. doi: 10.1016/0304-4157(78)90007-2. [DOI] [PubMed] [Google Scholar]
- Hasselbach W. The sarcoplasmic calcium pump. A model of energy transduction in biological membranes. Top Curr Chem. 1979;78:1–56. [PubMed] [Google Scholar]
- Inesi G., Kurzmack M., Coan C., Lewis D. E. Cooperative calcium binding and ATPase activation in sarcoplasmic reticulum vesicles. J Biol Chem. 1980 Apr 10;255(7):3025–3031. [PubMed] [Google Scholar]
- Jardetzky O. Simple allosteric model for membrane pumps. Nature. 1966 Aug 27;211(5052):969–970. doi: 10.1038/211969a0. [DOI] [PubMed] [Google Scholar]
- Jencks W. P. The utilization of binding energy in coupled vectorial processes. Adv Enzymol Relat Areas Mol Biol. 1980;51:75–106. doi: 10.1002/9780470122969.ch2. [DOI] [PubMed] [Google Scholar]
- Loomis C. R., Martin D. W., McCaslin D. R., Tanford C. Phosphorylation of calcium adenosinetriphosphatase by inorganic phosphate: reversible inhibition at high magnesium ion concentrations. Biochemistry. 1982 Jan 5;21(1):151–156. doi: 10.1021/bi00530a026. [DOI] [PubMed] [Google Scholar]
- Meissner G. ATP and Ca2+ binding by the Ca2+ pump protein of sarcoplasmic reticulum. Biochim Biophys Acta. 1973 Apr 16;298(4):906–926. doi: 10.1016/0005-2736(73)90395-7. [DOI] [PubMed] [Google Scholar]
- Niggli V., Adunyah E. S., Penniston J. T., Carafoli E. Purified (Ca2+-Mg2+)-ATPase of the erythrocyte membrane. Reconstitution and effect of calmodulin and phospholipids. J Biol Chem. 1981 Jan 10;256(1):395–401. [PubMed] [Google Scholar]
- Tanford C. Equilibrium state of ATP-driven ion pumps in relation to physiological ion concentration gradients. J Gen Physiol. 1981 Feb;77(2):223–229. doi: 10.1085/jgp.77.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanford C., Martin D. W. Equilibrium constants for some steps of the reaction cycle of the sarcoplasmic reticulum calcium pump. Z Naturforsch C. 1982 May-Jun;37(5-6):522–526. doi: 10.1515/znc-1982-5-626. [DOI] [PubMed] [Google Scholar]
- Tanford C. Simple model for the chemical potential change of a transported ion in active transport. Proc Natl Acad Sci U S A. 1982 May;79(9):2882–2884. doi: 10.1073/pnas.79.9.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veech R. L., Lawson J. W., Cornell N. W., Krebs H. A. Cytosolic phosphorylation potential. J Biol Chem. 1979 Jul 25;254(14):6538–6547. [PubMed] [Google Scholar]
- de Meis L., Vianna A. L. Energy interconversion by the Ca2+-dependent ATPase of the sarcoplasmic reticulum. Annu Rev Biochem. 1979;48:275–292. doi: 10.1146/annurev.bi.48.070179.001423. [DOI] [PubMed] [Google Scholar]


