Abstract
Background
Red blood cell (RBC) preservation is essential to transfusion medicine. Many blood group reference laboratories need a method to preserve rare blood samples for serologic testing at a later date. This study offers a comparison of three common cryoprotective agents and protocols used today: bulk preservation with glycerol and droplet freezing with sucrose/dextrose (S+D) or polyvinyl pyrrolidone (PVP).
Study design and methods
Human blood from 14 volunteers was collected and frozen at set intervals over two weeks with PVP, S+D, or glycerol. The frozen RBCs were later thawed and the percentage of surviving RBCs was determined. Detailed protocols and an instructional video are supplied.
Results
Over a two week period, RBCs preserved with glycerol and thawed with a widely used protocol showed a recovery of 41 ± 16 % (mean ± standard deviation) while those thawed with a modified glycerol protocol showed a recovery of 76 ± 8 %. RBCs preserved by droplet freezing with S+D showed a recovery of 56 ± 11 % while those preserved by droplet freezing with PVP showed a recovery of 85 ± 6 %. Recovery values were similar with ethylenediaminetetraacetic acid (EDTA) or heparin anticoagulants, differing freezing rates, and varying droplet volumes.
Conclusion
Droplet freezing with PVP offered the greatest recovery. While bulk freezing with glycerol can be effective too, droplet freezing may be a more convenient method overall. It requires less effort to thaw, needs much less storage room, and allows blood group laboratories to be frugal with thawing rare samples.
Introduction
Red blood cell (RBC) preservation of small volumes (i.e. < 1 mL) is an important tool in modern transfusion medicine for serological testing. Many blood group reference laboratories need to have the ability to freeze, reconstitute, and then analyze rare RBCs that were collected months or years before. RBC preservation methods offer a reliable long-term supply of rare RBCs that have been collected once. Several cryopreservative methods are used for maintaining RBC ex vivo structure.
One common method is glycerol preservation.1,2 Glycerol is a permeant and relatively nontoxic cryoprotective agent (CPA) that protects RBCs from injury during slow cooling. It works by entering RBCs, shifting their osmotic balance, and stopping excessive volume reduction due to extracellular ice formation.3–7 By halting volume reduction, it is thought that RBCs avoid reaching a critically damaging electrolyte concentration.3–7 Glycerol also depresses the system’s freezing point, which may help mitigate the formation of extracellular ice.7–9 Although this method is effective, glycerol preservation is typically used with large volumes and deglyceralization after thawing is time consuming.10
Freezing RBCs with liquid nitrogen, notably by droplet freezing, was developed later and may be an underutilized alternative.11–13 Droplet freezing is a method that preserves RBC samples in small, individual droplets. Minute quantities of RBCs can then be recovered to obtain the minimal amount of sample needed to perform a serological test. This method uses non-permeable CPAs, like sucrose and dextrose (S+D) or polyvinyl pyrrolidone (PVP), to protect the RBCs. Non-permeable CPAs may work by dehydrating the RBCs and minimizing intracellular ice formation.5,14 They may also stabilize the RBC membranes and help macromolecules retain their native conformations.15–17
What is unknown about droplet freezing, however, is which CPA works best and whether droplet freezing can surpass the effectiveness of freezing with glycerol. We surveyed blood banks and blood group reference laboratories and determined that glycerol, S+D, and PVP were the most common CPAs used in small-volume blood storage. In this study we compared the recovery of RBCs frozen with glycerol and RBCs droplet frozen with S+D or PVP.
Materials and Methods
Survey methodology
Forty-seven invitations for an online survey (www.surveymonkey.com) were sent to contacts at reference laboratories through the International Society of Blood Transfusion (ISBT) Rare Donor Working Party, ISBT Red Cell Immunogenetics and Terminology Working Party, and Serum, Cells and Rare Fluids Exchange (SCARF) member lists. Twenty-five responses were received.
Red blood cell preparation and processing
Human blood (30 mL) was collected into ethylenediaminetetraacetic acid (EDTA) or heparin anticoagulant from 14 healthy volunteers after informed consent. This blood was then aliquoted (Vacutainer 5 mL Red Top (glass) tubes; Becton Dickinson, Franklin Lakes, NJ) and stored at 4°C. Over the next two weeks, aliquots from each donor were removed from storage and frozen on day 1, 4, 7, 10, and 14.
When the blood was removed from 4°C storage, it was centrifuged at 1000 × g for 5 min to isolate the RBCs. The packed RBCs were then repeatedly, for instance 3–5 times, washed with 0.9% saline and centrifuged at 1000 × g for 5 min until no hemolysis was present in the supernatant.
Bulk freezing with glycerol
(i) Freezing. For each sample, two volumes of glycerol freezing solution (Glycerolyte 57 Freezing Solution; Fenwal, Lake Zurich, IL) were used for every one volume of packed RBCs. First, 20% of the total glycerol freezing solution volume was added dropwise to the RBCs and allowed to incubate with mixing for 10 min. Second, the remaining glycerol freezing solution was added dropwise to the RBCs with gentle mixing. Third, the glycerol-RBC mixture was incubated for 10 min with gentle mixing. Finally, the RBCs were transferred directly to cryovials and placed at −80°C or the cryovials were cooled slowly at 1°C/min by incubating in a freezing tray at −80°C (Nalgene Cryo 1°C Freezing Container; Thermo Fisher Scientific, Pittsburg, PA). 1.8 mL of RBCs mixed with glycerol was in each cryovial. (ii) Thawing. A standard deglycerolization protocol18 and a modified protocol were used. Samples recovered with the standard deglycerolization procotol were first thawed at room temperature. Next, 1 mL of thawed RBCs was transferred to a 5 mL test tube (Becton Dickinson), 0.5 mL of 12% saline solution was added dropwise with gentle mixing, and this mixture was left standing for 3 minutes at room temperature. After 3 minutes, a 1.6% saline solution was added until the test tube was at half volume, and then 0.9% saline was added until the test tube was full. The samples were repeatedly centrifuged at 1000 × g for 1 minute and washed with 0.9% saline until the supernatants showed no signs of hemolysis.
For samples recovered with the modified procotol it was necessary to gradually lower the hypertonic intracellular environment with multiple wash steps. First, the cryovials were thawed at room termpature and the desired volume of RBCs was transferred to a 5 mL test tube (Becton Dickinson) and centrifuged at 1000 × g for 2 minutes. Second, the glycerol freezing solution was aspirated. Third, 0.16 mL of 12% saline for every 0.5 mL of RBCs was added dropwise and with gentle shaking over a period of 5 minutes. The RBCs were then left standing for 3 min at room temperature. Next, 0.5 mL of 0.2%/0.9% dextrose/saline was added slowly and dropwise with gentle shaking. The RBCs were then left standing for 2 minutes at room temperature. This process – adding 0.5 mL of 0.2%/0.9% dextrose/saline and letting the RBCs stand undisturbed at room temperature – was repeated until the total volume in the test tube was 4 mL.
At this point, the RBCs were centrifuged for 1000 × g for 1 min, and then 0.5 mL of supernatant was aspirated. The RBCs were then resuspended in the test tube by repeated inversion. Next, 0.5 mL of 0.2%/0.9% dextrose/saline was added slowly and dropwise with gentle shaking. The RBCs were then left standing for 2 minutes at room temperature. This process – centrifuging, removing the supernatant, resuspending the RBC pellet, adding a volume of 0.2%/0.9% dextrose/saline solution equal to what was aspirated, and then standing for 1 minute – was repeated with the following volumes: 1 mL, 1.5 mL, 2 mL, and 4 mL. Finally, the RBCs were centrifuged at 1000 × g for 1 min, and all of the supernatant was aspirated. The RBCs were then washed with 0.9% saline until no hemolysis was present.
Both protocols are available in detail in the supplement. The main difference between them is the rate that the RBCs are brought into an isotonic environment, which is greatly decreased in the modified protocol relative to the standard protocol.
Droplet freezing with PVP or sucrose/dextrose
(i) Freezing. For each sample, one volume of PVP freezing solution (27 mL of 30% bovine serum albumin [BSA] with 23 mL of 30% PVP in deionized water; Sigma-Aldrich, St. Louis, MO) or S+D freezing solution (15.4 g sucrose, 5.4 g dextrose, and 0.29 g NaCl in 100 mL of deionized water; Sigma-Aldrich) was added for every one volume of packed RBCs. The RBC-freezing solution mixture was allowed to incubate for 1 hour. Next, a fine tip transfer pipette (PCG Labs, Dahlonega, GA) was used to release individual droplets (about 20 µL) of RBCs into liquid nitrogen. This pipette was chosen based on preliminary tests with six pipette types yielding various droplet volumes. The pipette was held vertically about 15 cm above the liquid nitrogen, which was being stirred to produce a weak whirlpool. These steps help prevent the droplets from aggregating. The liquid nitrogen containing the frozen droplets was then poured over a metal sieve to isolate the droplets. To transfer the droplets to a cryovial for storage, the cryovial cap was first punctured 2 or 3 times to prevent the vial from bursting due to pressure. This cryovial and a funnel were then chilled in liquid nitrogen to prevent the droplets from sticking and thawing on contact. The funnel was used to channel the droplets into the chilled cryovial, which was then placed in −180°C storage. (ii) Thawing. To thaw the droplets, approximately 4–6 droplets (about 80–120 µL) were placed into 1.5 mL of 0.9% saline at 37°C with gentle shaking. After the droplets were completely suspended in warm saline, the total volume was diluted to 2 mL for sampling and then washed until no hemolysis. The freezing and thawing methods are explained in detail in the supplement and shown in the supplementary video.
Percent recovery calculation
The recovery was evaluated using hemoglobin (Hb) measurements before (total Hb) and after (RBC Hb) the washing procedure. Hb was determined with the cyanmethemoglobin method.19 Samples were incubated in 1.5 mL of Drabkin’s reagent (Sigma-Aldrich) for 15 min before absorbance measurement at 540 nm (DU530 spectrophotometer; Beckman, Brea, CA). Absolute Hb values in mg/mL were calculated using a standard curve. The method was validated against the routine Hb measurements in the NIH Clinical Center Department of Laboratory Medicine.
Specific volumes were sampled from the RBC solutions to keep the Hb values within the standard curve range. These specific volumes were also dependent on the solution volume during the thawing procedure so that we sampled 10 µL of 1.5 mL for the standard glycerol, 25 µL of 4.0 mL for the modified glycerol, and 120 µL of 2 mL for both droplet freezing protocols. The volumes sampled for total Hb and RBC Hb were controlled to be identical. Because the sample for total Hb was up to 6% of the solution’s total volume, we took this loss of volume and RBCs into account in our calculation so that
| (1) |
Statistical analysis
Data are shown as mean ± standard deviation (SD). Data analysis was done with Excel (Microsoft, Redmond, WA) and MedCalc (MedCalc Software, Mariakerke, Belgium).
Results
Laboratory survey
Various RBC preservation additives were used in 24 reference laboratories for either bulk or droplet freezing (Table 1). One laboratory replied and indicated that no RBC freezing is done due to costs, complexity, and lack of adequate instructions. We concluded from this non-representative set of replies that glycerol and S+D are often used, while use of PVP may be uncommon.
Table 1.
Survey of red blood cell preservation additives in 24 reference laboratories.
| Cryoprotective agent (CPA) | Usage* |
|---|---|
| Glycerol, various concentrations and freezing speeds | 12 |
| Sucrose, alone or with dextrose, albumin or plasma | 10 |
| Polyvinyl pyrrolidone (PVP), alone | 2 |
| Other† | 5 |
Multiple answers were possible.
Including a glycerol/gelatin mix (Glycigel),27 the MAPI system (CryoBioSystem, Paris, France) and direct storage in liquid nitrogen.
Comparison of freezing methods
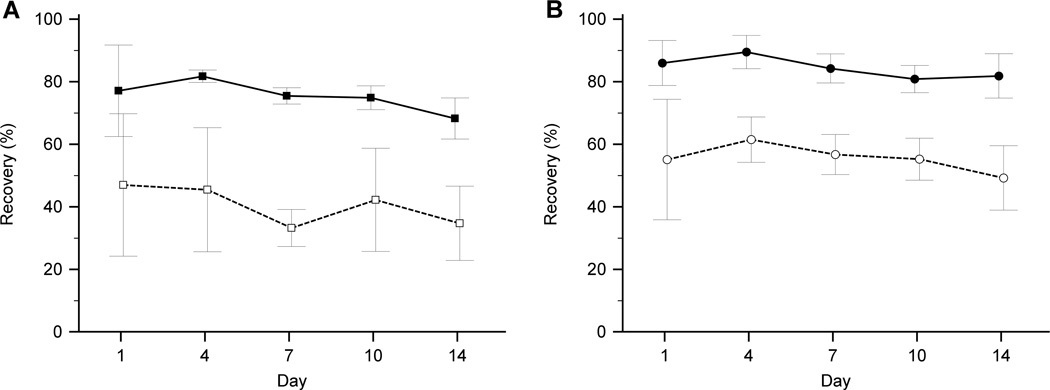
RBCs from 14 healthy volunteers were collected and preserved with glycerol, S+D, or PVP at set intervals over period of two weeks. Across all intervals, the average recovery of RBCs preserved by bulk freezing with glycerol and thawed with the standard protocol was 41 ± 16 % (n=4) and with the modified protocol was 76 ± 8 % (n=8) (Fig. 1A).
Figure 1.
Recovery of RBCs preserved by various methods over a period of two weeks (mean ± SD). (A) Bulk glycerol freezing with standard thawing protocol (n=4; □) and modified thawing protocol (n=8; ■). (B) Droplet freezing with PVP (n=8; ●) or S+D (n=8; ○).
Across all intervals, the average recovery of RBCs preserved by droplet freezing with S+D was 56 ± 11 % (n=8) and with PVP was 85 ± 6 % (n=8) (Fig. 1B).
Lack of impact by freezing rate, anticoagulants, and droplet volume
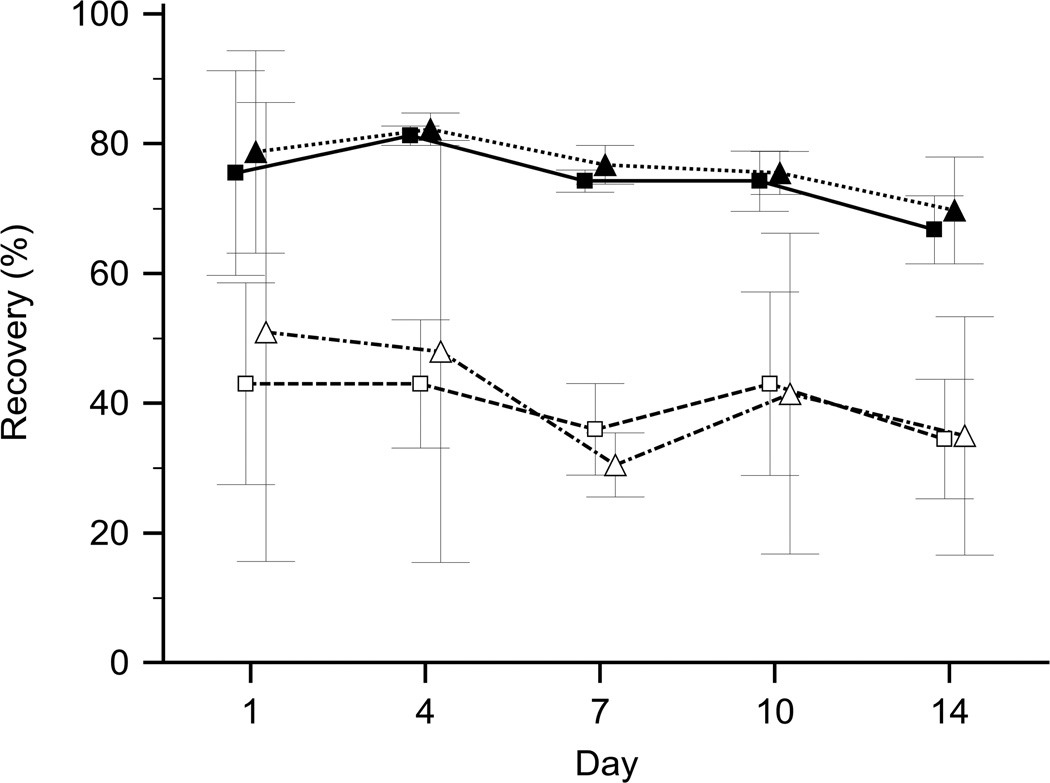
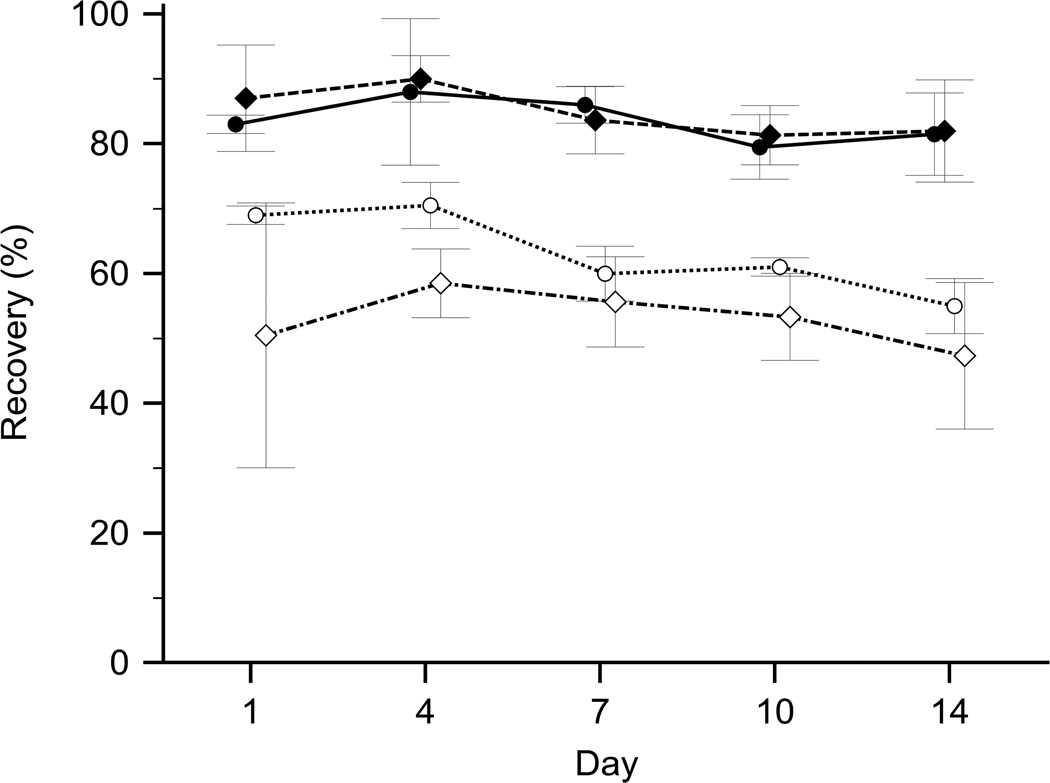
Differences in freezing rate, i.e. slow or fast freezing, had minimal impact on the recovery of RBCs (Fig. 2). EDTA and heparin as anticoagulants differed little in regard to the recovery of RBCs (Fig. 3). Preliminary tests with six different pipette types creating droplet volumes between 15 µL and 50 µL revealed no correlation between droplet volumes and recovery rates, which showed little variation (SD 3.9% and 4.3% for S+D and PVP, respectively). A fine tip transfer pipette, which yielded an average droplet volume of approximately 20 µL, was selected for this study due to its practicality.
Figure 2.
Effect of controlled freezing rate on bulk glycerol frozen samples. Recovery of RBCs preserved by various glycerol freezing and thawing methods over a period of two weeks (mean ± SD). Controlled freezing rate (n=2; Δ) compared to regular bulk glycerol freezing (n=2; □) with standard thawing protocol, and controlled freezing rate (n=4; ▲) compared to regular bulk glycerol freezing (n=4; ■) with the modified thawing protocol.
Figure 3.
Effects of the anticoagulant on droplet freezing with S+D or PVP. Recovery of preserved RBCs over a period of two weeks (mean ± SD). Droplet freezing of PVP-treated RBCs obtained from blood anticoagulated with EDTA (n=2; ●) compared to heparin (n=6; ♦), and droplet freezing of S+D-treated RBCs obtained from blood anticoagulated with EDTA (n=2; ○) compared to heparin (n=6; ◊).
Discussion
The aim of this study was to compare which RBC preservation methods offer good approaches to preserving blood for serologic tests. We propose that determining the best method involves weighing two factors: first, the effectiveness of the CPA, and second, the convenience of the protocol.
A CPA is effective insofar as it prevents hemolysis during freezing and thawing. One of the oldest CPAs is glycerol, a simple compound that remains widely used for freezing whole blood units. AABB regulation 5.7.5.4 stipulates that the mean recovery of a glycerol-preserved unit intended for transfusion should be at least 80%20, but no minimum recovery has been identified for RBCs intended only for serologic testing. The standard protocol for thawing small volumes of RBCs preserved with glycerol,18 which is simpler and much faster than the protocol for whole units, was unable to provide such a recovery (Fig. 1A). This may be attributed to the fact that the small volume protocol was designed to be convenient for serologic testing. A yield of 55% or less can easily accommodate multiple serologic tests. Nevertheless, a modified protocol enabled a recovery of 80% (Fig. 1A). We found that the freezing rate had little impact on the recovery (Fig. 2); however a slow transition from a hypertonic to an isotonic environment during thawing was essential. Unfortunately, the modified protocol takes approximately twice as long as the standard protocol. And because the standard protocol already yields enough RBCs for serologic tests, the modified protocol may not offer much advantage in many laboratories.
Droplet freezing requires less thawing time and produces impressive recoveries. Our survey indicated that S+D was the most common CPA used for droplet freezing (Table 1), but S+D only achieved a recovery of 55% (Fig. 1B). PVP offers the same benefits of S+D and better recoveries. PVP is a water-soluble polymer that was initially used as a blood plasma substitute.21 We used 30% BSA to create the PVP freezing solution in our experiments. For routine application, laboratories are using 22% BSA. Hence, BSA concentrations in this range are suitable and can be chosen based on practicability. There are no published recovery rates of human RBCs preserved by droplet freezing with PVP. Our results are comparable with previously reported recovery rates of trypsin-treated RBCs protected with dextran (82%) or hydroxyethyl starch (40%).22 Droplet freezing with PVP is more effective than the standard bulk glycerol preservation. EDTA and heparin as anticoagulants were tested, but they had little impact on the recovery of S+D or PVP droplets (Fig. 3).
Overall, droplet freezing may be more convenient than bulk glycerol preservation. We propose that droplet freezing holds primarily two advantages over glycerol preservation: one advantage is thawing ease. Droplet freezing only requires warm saline to thaw. The CPAs are non-permeable, and therefore the RBCs require no additional wash step. Another advantage is prudence. Droplet freezing allows one to thaw the very minimum amount of rare sample needed for a serologic test, which is often a critical consideration for very rare RBC samples.
Our study has several limitations because we focused on simulating the conditions a technologist in a blood group reference laboratory would often encounter. Therefore we opted to produce the droplets with a commonly used fine tip transfer pipette rather than a standardized 100 µL pipette, which may produce even more consistent droplet volumes. We also recognize that liquid nitrogen may not be available in all labs because it is expensive and requires training to handle. In this study we focused on quantifying RBC recovery by measuring ratios of Hb concentration rather than the serologic properties of the reconstituted RBCs. Nevertheless, RBCs have been shown to maintain their antigen pattern and usability for serologic testing even after freezing in liquid nitrogen.23–25 Finally, our comparison of short-term freezing cannot address any potential differences among the three methods after long-term freezing, which may often exceed 10 years.
It should be noted that thawed RBCs are generally a poor source of DNA and RNA. Washing reduces leukocytes and reticulocytes. Mature RBCs contain spurious RNA and no genomic DNA. Special procedures for leukoreduced blood are required to extract the minimal amounts of residual DNA and RNA.26
In conclusion, the recovery of frozen RBCs treated with glycerol, S+D, or PVP was measured and the effectiveness of the CPAs was determined. Droplet freezing with PVP was the most effective method. Glycerol was effective too, but it may be less convenient than droplet freezing, a method that offers many advantages over bulk glycerol preservation.
Supplementary Material
Acknowledgments
We thank Sherry L. Sheldon for sample retrieval and technical support, Supatta M. Lucas for demonstrating the droplet freezing technique for an instructional video presentation, and the NIH Events Management Video Department for producing and editing the video. WAF thanks Joyce Poole, Bristol/UK for introduction to droplet freezing with PVP in April 2001, and Andreas Sputtek, Hamburg/Germany and Hein Hustinx, Bern/Switzerland for helpful discussions in May 2003. We thank Marianne Lotsch, Ulm/Germany for helpful discussions in May 2011. We also thank the following laboratory representatives for participating in our survey: Amy Gerlach, USA; Anne Long, Canada; Hein Hustinx, Switzerland; Karen Kirkley, USA; Kathleen Larimore, USA; Kent Eliason, USA; Mark Lambert, Ireland; Rita Fontão-Wendel, Brazil; Sanmukh Joshi, Sultanate of Oman; Vered Yahalom, Israel; Shannon Long, USA; Sue Frandson, USA; Yew-Wah Liew, Australia; and 12 anonymous responders.
Footnotes
Conflict of interest disclosure: None.
Statement of Disclaimer: The views expressed do not necessarily represent the view of the National Institutes of Health, the Department of Health and Human Services, or the U.S. Federal Government.
Authorship contribution: Experiment design: PS, MJH, KMB, WAF. Protocol development: PS, MJH. Experiment execution: PS, MJH, AHLS. Manuscript preparation: PS, MJH, WAF. Video production: PS, MJH, JYL, WAF. Preliminary tests: JYL. Survey: JYL, KMB, WAF.
Supporting Information
Additional supporting information may be found in the online version of this article:
Protocol for red blood cell preservation by bulk glycerol freezing.
Protocol for red blood cell preservation by droplet freezing with polyvinyl pyrrolidone or sucrose/dextrose. Instructional video on droplet freezing with polyvinyl pyrrolidone or sucrose/dextrose.
References
- 1.Polge C, Smith AU, Parkes AS. Revival of spermatozoa after vitrification and dehydration at low temperatures. Nature. 1949;164:666. doi: 10.1038/164666a0. [DOI] [PubMed] [Google Scholar]
- 2.Smith AU. Prevention of haemolysis during freezing and thawing of red blood-cells. Lancet. 1950;2:910–911. doi: 10.1016/s0140-6736(50)91861-7. [DOI] [PubMed] [Google Scholar]
- 3.Lovelock JE. The mechanism of the protective action of glycerol against haemolysis by freezing and thawing. Biochim Biophys Acta. 1953;11:28–36. doi: 10.1016/0006-3002(53)90005-5. [DOI] [PubMed] [Google Scholar]
- 4.Lovelock JE. The protective action of neutral solutes against haemolysis by freezing and thawing. Biochem J. 1954;56:265–270. doi: 10.1042/bj0560265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meryman HT. Cryoprotective agents. Cryobiology. 1971;8:173–183. doi: 10.1016/0011-2240(71)90024-1. [DOI] [PubMed] [Google Scholar]
- 6.McGann LE. Differing actions of penetrating and nonpenetrating cryoprotective agents. Cryobiology. 1978;15:382–390. doi: 10.1016/0011-2240(78)90056-1. [DOI] [PubMed] [Google Scholar]
- 7.Scott KL, Lecak J, Acker JP. Biopreservation of red blood cells: past, present, and future. Transfus Med Rev. 2005;19:127–142. doi: 10.1016/j.tmrv.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Mazur P. Cryobiology: the freezing of biological systems. Science. 1970;168:939–949. doi: 10.1126/science.168.3934.939. [DOI] [PubMed] [Google Scholar]
- 9.Meryman HT. Freezing injury and its prevention in living cells. Annu Rev Biophys Bioeng. 1974;3:341–363. doi: 10.1146/annurev.bb.03.060174.002013. [DOI] [PubMed] [Google Scholar]
- 10.Meryman HT, Hornblower M. A simplified procedure for deglycerolizing red blood cells frozen in a high glycerol concentration. Transfusion. 1977;17:438–442. doi: 10.1046/j.1537-2995.1977.17578014580.x. [DOI] [PubMed] [Google Scholar]
- 11.Huntsman RG, Hurn BA, Lehmann H. Storage of red cells for blood-grouping after freezing in liquid nitrogen. Br Med J. 1960;2:118. doi: 10.1136/bmj.2.5192.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bronson WR, McGinniss MH. The preservation of human red blood cell agglutinogens in liquid nitrogen: study of a technic suitable for routine blood banking. Blood. 1962;20:478–484. [PubMed] [Google Scholar]
- 13.Reid ME, Ellisor SS. A rapid and simple method for freezing small volumes of erythrocytes in liquid nitrogen. Transfusion. 1974;14:75–76. doi: 10.1111/j.1537-2995.1974.tb04490.x. [DOI] [PubMed] [Google Scholar]
- 14.Mazur P. Freezing of living cells: mechanisms and implications. Am J Physiol. 1984;247:C125–C142. doi: 10.1152/ajpcell.1984.247.3.C125. [DOI] [PubMed] [Google Scholar]
- 15.Rapatz G, Sullivan JJ, Luyet B. Preservation of erythrocytes in blood containing various cryoprotective agents, frozen at various rates and brought to a given final temperature. Cryobiology. 1968;5:18–25. doi: 10.1016/s0011-2240(68)80139-7. [DOI] [PubMed] [Google Scholar]
- 16.Anchordoguy TJ, Rudolph AS, Carpenter JF, Crowe JH. Modes of interaction of cryoprotectants with membrane phospholipids during freezing. Cryobiology. 1987;24:324–331. doi: 10.1016/0011-2240(87)90036-8. [DOI] [PubMed] [Google Scholar]
- 17.Crowe JH, Crowe LM, Carpenter JF, Rudolph AS, Wistrom CA, Spargo BJ, Anchordoguy TJ. Interactions of sugars with membranes. Biochim Biophys Acta. 1988;947:367–384. doi: 10.1016/0304-4157(88)90015-9. [DOI] [PubMed] [Google Scholar]
- 18.American Red Cross. Immunohematology Methods. Method #125. 1 ed. Rockville, MD: 1993. [Google Scholar]
- 19.Drabkin DL, Austin JH. Spectrophotometric studies. II. Preparations from washed blood cells; nitric oxide hemoglobin and sulfhemoglobin. J Biol Chem. 1935;112:51–65. [Google Scholar]
- 20.AABB. Standards for Blood Banks and Transfusion Services. 26 ed. Bethesda, MD: 2009. [Google Scholar]
- 21.Reppe W. Polyvinylpyrrolidon. Angew Chem. 1953;65:577–578. [Google Scholar]
- 22.Pegg DE, Diaper MP, Scholey SE, Coombs RR. Droplet freezing of antibody-linked indicator red cells of sheep, ox, and human origin. Cryobiology. 1982;19:573–584. doi: 10.1016/0011-2240(82)90187-0. [DOI] [PubMed] [Google Scholar]
- 23.Rowe AW, Borel H, Allen FH., Jr Droplet-frozen sensitized red cells for Gm typing. Vox Sang. 1972;22:188–191. doi: 10.1111/j.1423-0410.1972.tb05125.x. [DOI] [PubMed] [Google Scholar]
- 24.Pegg DE, Diaper MP, Scholey SE, Coombs RR. Cryopreservation of antibody-coupled red cells for use in immunoassays. J Immunol Methods. 1982;54:331–341. doi: 10.1016/0022-1759(82)90317-9. [DOI] [PubMed] [Google Scholar]
- 25.Henkelman S, Lagerberg JW, Graaff R, Rakhorst G, van OW. The effects of cryopreservation on red blood cell rheologic properties. Transfusion. 2010;50:2393–2401. doi: 10.1111/j.1537-2995.2010.02730.x. [DOI] [PubMed] [Google Scholar]
- 26.Klapper E, Zhang Y, Figueroa P, Ness P, Stubbs J, Abumuhor I, Bailey J, Epperson L, Tauscher C, Enriquez E, et al. Toward extended phenotype matching: a new operational paradigm for the transfusion service. Transfusion. 2010;50:536–546. doi: 10.1111/j.1537-2995.2009.02462.x. [DOI] [PubMed] [Google Scholar]
- 27.Klein HG, Anstee DJ. Mollison’s Blood Transfusion in Clinical Medicine. 11 ed. Oxford, UK: Blackwell Publishing; 2005. p. 857. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.