Abstract
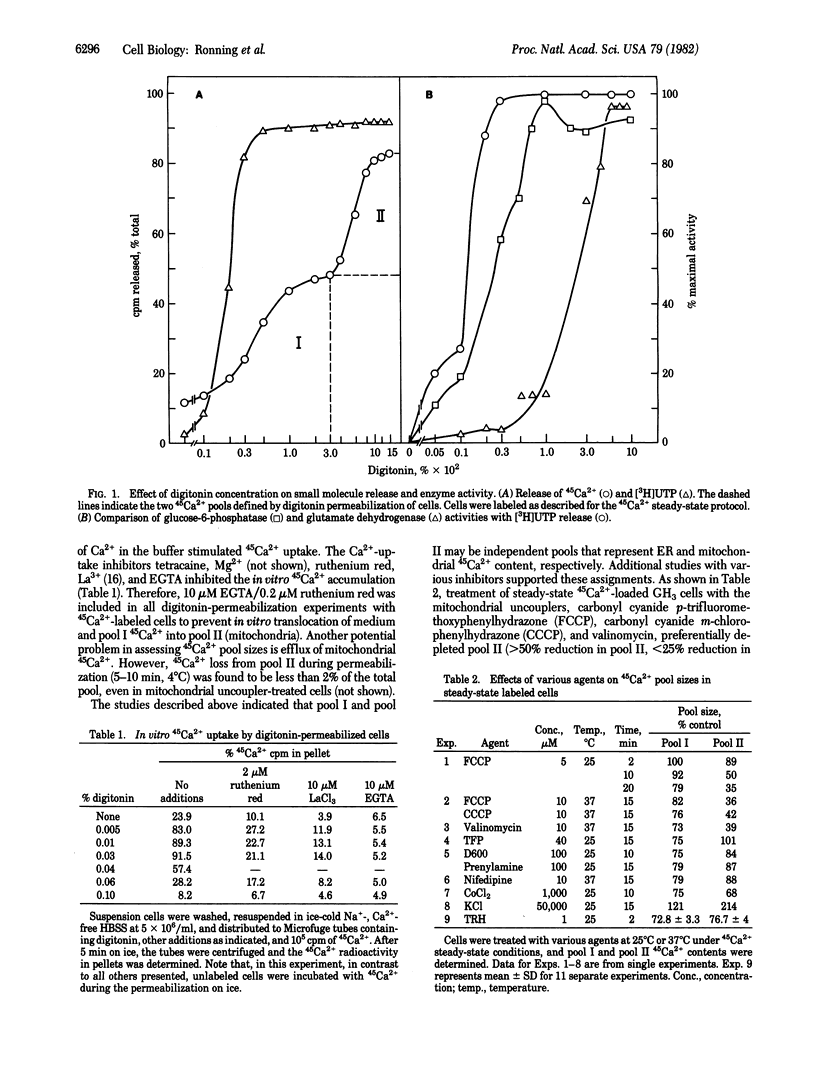
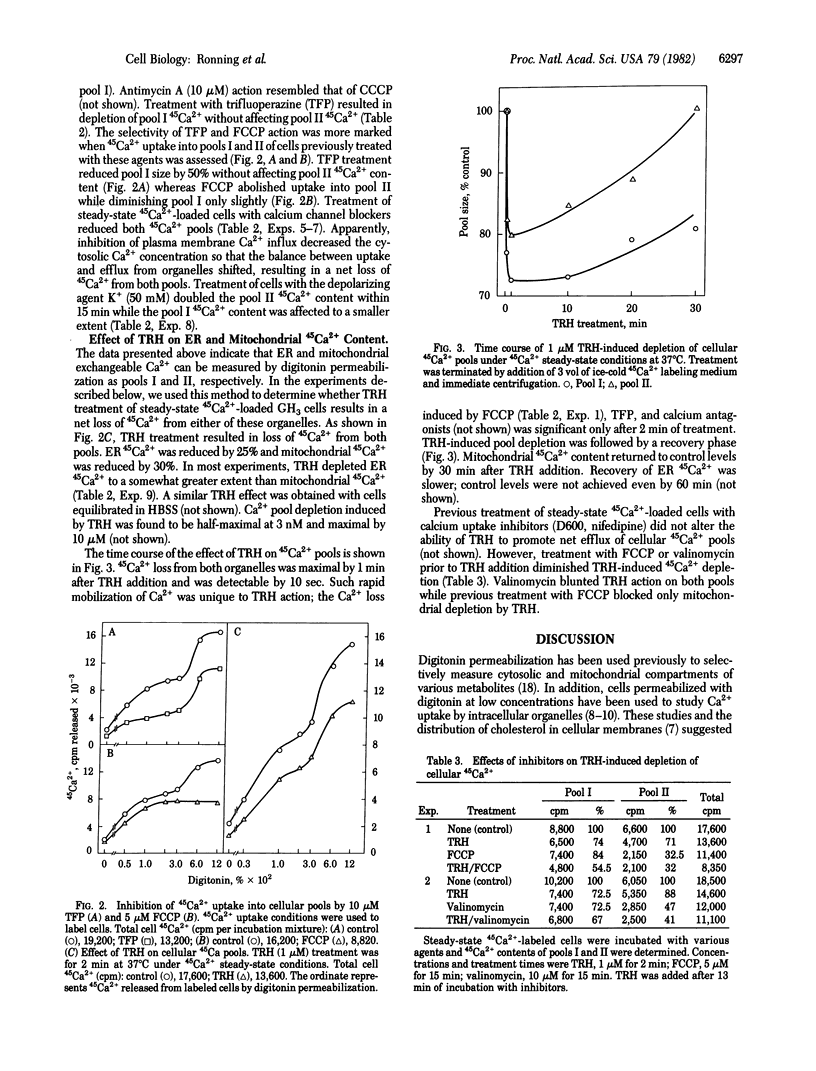
Treatment of 45Ca2+-loaded GH3 pituitary cells with various concentrations of digitonin revealed discrete pools (I and II) of cellular 45Ca2+ defined by differing detergent sensitivities. Markers for cytosol and intracellular organelles indicated that the two 45Ca2+ pools were correlated with the two major cellular Ca2+-sequestering organelles, endoplasmic reticulum (I) and mitochondria (II). Studies with various inhibitors were consistent with these assignments. Mitochondrial uncouplers preferentially depleted 45Ca2+ pool II while trifluoperazine selectively depleted 45Ca2+ pool I. Control experiments indicated that translocation of in situ organellar 45Ca2+ during and after permeabilization was negligible. We used the digitonin-permeabilization method to examine the effect of thyrotropin-releasing hormone (TRH) treatment on intracellular Ca2+ pools of GH3 pituitary cells. TRH was found to rapidly deplete both endoplasmic reticulum and mitochondrial exchangeable Ca2+ by 25-30%. The 45Ca2+ loss from both pools was maximal by 1 min after TRH addition and was followed by a recovery phase; mitochondrial 45Ca2+ content returned to control levels by 30 min. Previous treatment of cells with the mitochondrial uncoupler carbonyl cyanide p-trifluoromethoxy-phenylhydrazone blocked TRH-induced 45Ca2+ efflux from mitochondria, while previous treatment with valinomycin, an agent that depleted both 45Ca2+ pools, blocked any additional effect of TRH on these pools. We conclude that TRH rapidly promotes a net loss of exchangeable Ca2+ from GH3 cells as a result of hormone-induced mobilization of Ca2+ from endoplasmic reticulum and mitochondria.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker G. L., Fiskum G., Lehninger A. L. Regulation of free Ca2+ by liver mitochondria and endoplasmic reticulum. J Biol Chem. 1980 Oct 10;255(19):9009–9012. [PubMed] [Google Scholar]
- Borle A. B. Control, Modulation, and regulation of cell calcium. Rev Physiol Biochem Pharmacol. 1981;90:13–153. doi: 10.1007/BFb0034078. [DOI] [PubMed] [Google Scholar]
- Drust D. S., Martin T. F. Thyrotropin-releasing hormone rapidly and transiently stimulates cytosolic calcium-dependent protein phosphorylation in GH3 pituitary cells. J Biol Chem. 1982 Jul 10;257(13):7566–7573. [PubMed] [Google Scholar]
- Elias P. M., Goerke J., Friend D. S., Brown B. E. Freeze-fracture identification of sterol-digitonin complexes in cell and liposome membranes. J Cell Biol. 1978 Aug;78(2):577–596. doi: 10.1083/jcb.78.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershengorn M. C., Hoffstein S. T., Rebecchi M. J., Geras E., Rubin B. G. Thyrotropin-releasing hormone stimulation of prolactin release from clonal rat pituitary cells: evidence for action independent of extracellular calcium. J Clin Invest. 1981 Jun;67(6):1769–1776. doi: 10.1172/JCI110216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern J., Hinkle P. M. Direct visualization of receptors for thyrotropin-releasing hormone with a fluorescein-labeled analog. Proc Natl Acad Sci U S A. 1981 Jan;78(1):587–591. doi: 10.1073/pnas.78.1.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds T. R., Raess B. U., Vincenzi F. F. Plasma membrane Ca2+ transport: antagonism by several potential inhibitors. J Membr Biol. 1981 Jan 30;58(1):57–65. doi: 10.1007/BF01871034. [DOI] [PubMed] [Google Scholar]
- Katz S., Remtulla M. A. Phosphodiesterase protein activator stimulates calcium transport in cardiac microsomal preparations enriched in sarcoplasmic reticulum. Biochem Biophys Res Commun. 1978 Aug 29;83(4):1373–1379. doi: 10.1016/0006-291x(78)91373-6. [DOI] [PubMed] [Google Scholar]
- Markers for membranous cell components. The 6th International Subcellular Methology Form, Guildford/United Kingdom, 25-28 July 1978. Eur J Cell Biol. 1979 Dec;20(2):195–199. [PubMed] [Google Scholar]
- Martin T. F. Paradoxical effects of protein synthesis inhibitors on uridine uptake in cultured cells: possible role of uncharged tRNA in regulating metabolism. J Cell Physiol. 1980 Jun;103(3):489–502. doi: 10.1002/jcp.1041030314. [DOI] [PubMed] [Google Scholar]
- Meredith M. J., Reed D. J. Status of the mitochondrial pool of glutathione in the isolated hepatocyte. J Biol Chem. 1982 Apr 10;257(7):3747–3753. [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Moriarty C. M., Leuschen M. P. Role of calcium in acute stimulated release of prolactin from neoplastic GH3 cells. Am J Physiol. 1981 Jun;240(6):E705–E711. doi: 10.1152/ajpendo.1981.240.6.E705. [DOI] [PubMed] [Google Scholar]
- Murphy E., Coll K., Rich T. L., Williamson J. R. Hormonal effects on calcium homeostasis in isolated hepatocytes. J Biol Chem. 1980 Jul 25;255(14):6600–6608. [PubMed] [Google Scholar]
- Rebecchi M. J., Gerry R. H., Gershengorn M. C. Thyrotropin-releasing hormone causes loss of cellular calcium without calcium uptake by rat pituitary cells in culture. Studies using arsenazo III for direct measurement of calcium. J Biol Chem. 1982 Mar 25;257(6):2751–2753. [PubMed] [Google Scholar]
- Rebecchi M. J., Monaco M. E., Gershengorn M. C. Thyrotropin releasing hormone rapidly enhances [32P]orthophosphate incorporation into phosphatidic acid in cloned GH3 cells. Biochem Biophys Res Commun. 1981 Jul 16;101(1):124–130. doi: 10.1016/s0006-291x(81)80019-8. [DOI] [PubMed] [Google Scholar]
- Reed K. C., Bygrave F. L. The inhibition of mitochondrial calcium transport by lanthanides and ruthenium red. Biochem J. 1974 May;140(2):143–155. doi: 10.1042/bj1400143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel W., Roduit C., Zahnd G. Thyrotropin releasing hormone stimulates metabolism of phosphatidyl inositol in GH3 cells. A possible mechanism in stimulus-response coupling. FEBS Lett. 1981 Nov 2;134(1):47–49. doi: 10.1016/0014-5793(81)80547-9. [DOI] [PubMed] [Google Scholar]
- Shoshan V., MacLennan D. H., Wood D. S. A proton gradient controls a calcium-release channel in sarcoplasmic reticulum. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4828–4832. doi: 10.1073/pnas.78.8.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton C. A., Martin T. F. Thyrotropin-releasing hormone (TRH) selectively and rapidly stimulates phosphatidylinositol turnover in GH pituitary cells: a possible second step of TRH action. Endocrinology. 1982 Apr;110(4):1273–1280. doi: 10.1210/endo-110-4-1273. [DOI] [PubMed] [Google Scholar]
- Tan K. N., Tashjian A. H., Jr Receptor-mediated release of plasma membrane-associated calcium and stimulation of calcium uptake by thyrotropin-releasing hormone in pituitary cells in culture. J Biol Chem. 1981 Sep 10;256(17):8994–9002. [PubMed] [Google Scholar]
- Taraskevich P. S., Douglas W. W. Action potentials occur in cells of the normal anterior pituitary gland and are stimulated by the hypophysiotropic peptide thyrotropin-releasing hormone. Proc Natl Acad Sci U S A. 1977 Sep;74(9):4064–4067. doi: 10.1073/pnas.74.9.4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashjian A. H., Jr Clonal strains of hormone-producing pituitary cells. Methods Enzymol. 1979;58:527–535. doi: 10.1016/s0076-6879(79)58167-1. [DOI] [PubMed] [Google Scholar]
- Wakasugi H., Kimura T., Haase W., Kribben A., Kaufmann R., Schulz I. Calcium uptake into acini from rat pancreas: evidence for intracellular ATP-dependent calcium sequestration. J Membr Biol. 1982;65(3):205–220. doi: 10.1007/BF01869964. [DOI] [PubMed] [Google Scholar]


