Abstract
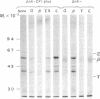
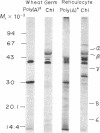
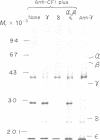
The chloroplast coupling factor 1 consists of five nonidentical subunits, three of which (α, β, and ε subunits) have been shown in several laboratories to be synthesized within chloroplasts. The site of synthesis of the remaining two (γ and δ subunits) was investigated by analyzing products directed by spinach leaf RNAs in wheat germ and reticulocyte translation systems in vitro. It was found that poly(A)+ RNA directs the synthesis of two distinct polypeptides, one of which is immunochemically related to the γ subunit but is 4,000 daltons larger. The other shares antigenic sites with the δ subunit but is 8,000 daltons larger. When wheat germ or reticulocyte translation systems were programmed with RNAs from purified chloroplasts, the only products related to CF1 that we could detect were a putative precursor of β, 2,000 daltons larger than the mature subunit, and some smaller polypeptides, which appear to be incomplete translation products of β. From these results it appears likely that the γ and δ subunits are synthesized in the cytoplasm as larger precursors and that β is synthesized within the chloroplast as a precursor.
Keywords: plastid genes, nuclear genes, protein precursor
Full text
PDF
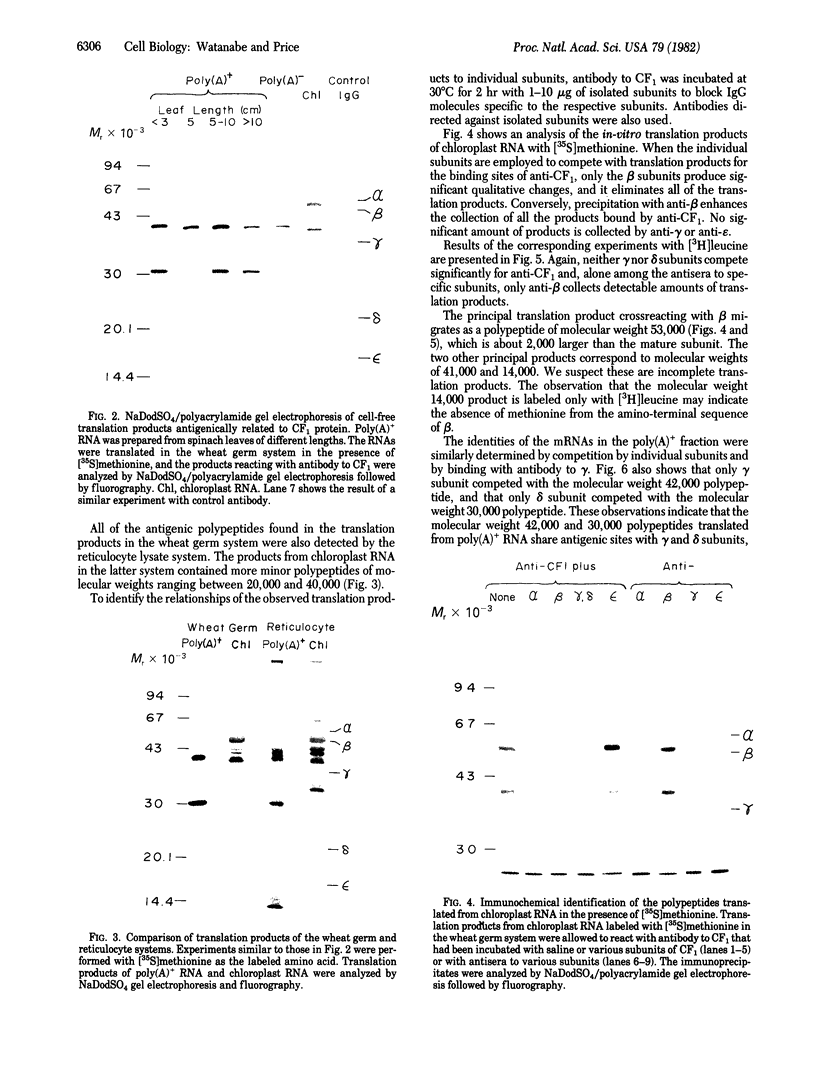
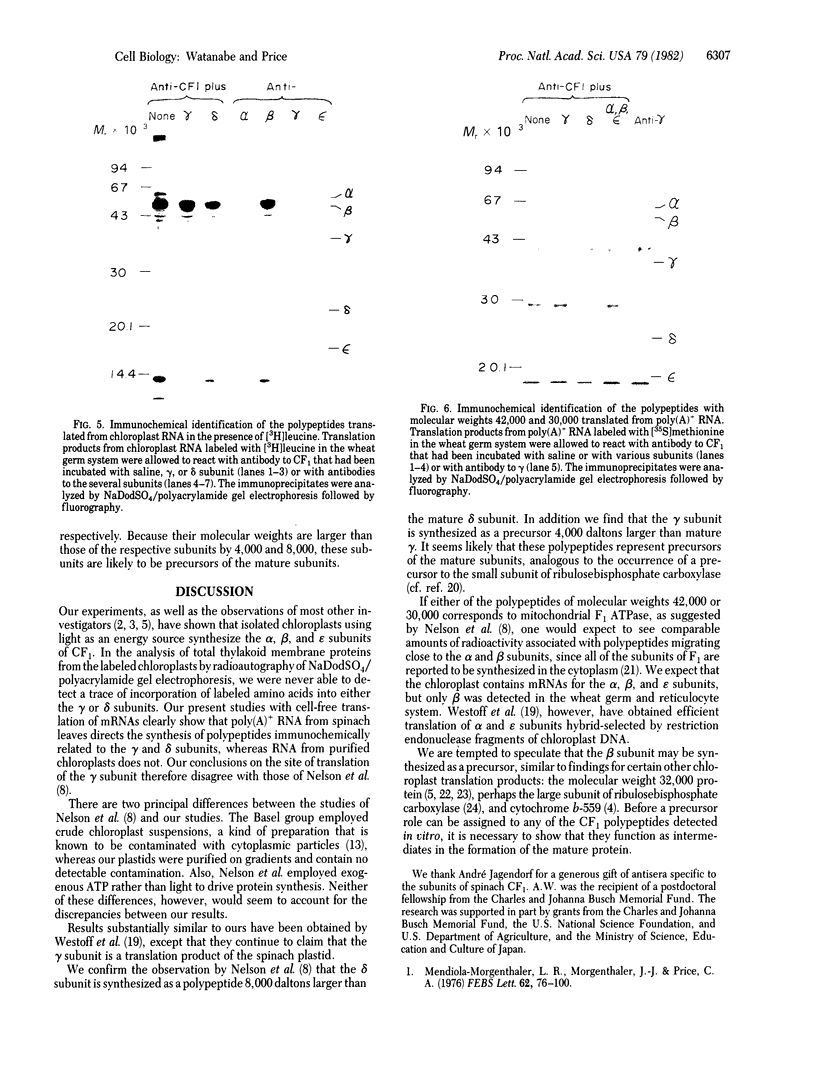

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolf M., Price C. A. Synthesis of poly(A)-containing RNA by isolated spinach chloroplasts. Biochemistry. 1979 May 1;18(9):1677–1680. doi: 10.1021/bi00576a007. [DOI] [PubMed] [Google Scholar]
- Binder A., Jagendorf A., Ngo E. Isolation and composition of the subunits of spinach chloroplast coupling factor protein. J Biol Chem. 1978 May 10;253(9):3094–3100. [PubMed] [Google Scholar]
- Brawerman G. The isolation of messenger RNA from mammalian cells. Methods Enzymol. 1974;30:605–612. doi: 10.1016/0076-6879(74)30058-4. [DOI] [PubMed] [Google Scholar]
- Driesel A. J., Speirs J., Bohnert H. J. Spinach chloroplast mRNA for a 32 000 dalton polypeptide: size and localization on the physical map of the chloroplast DNA. Biochim Biophys Acta. 1980 Dec 11;610(2):297–310. doi: 10.1016/0005-2787(80)90011-8. [DOI] [PubMed] [Google Scholar]
- Geetha V., Gnanam A. An in vitro protein-synthesizing system with isolated chloroplasts of Sorghum vulgare. An alternate assay system for exogenous template RNA. J Biol Chem. 1980 Jan 25;255(2):492–497. [PubMed] [Google Scholar]
- Grebanier A. E., Coen D. M., Rich A., Bogorad L. Membrane proteins synthesized but not processed by isolated maize chloroplasts. J Cell Biol. 1978 Sep;78(3):734–746. doi: 10.1083/jcb.78.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman A. R., Bartlett S. G., Schmidt G. W., Mullet J. E., Chua N. H. Optimal conditions for post-translational uptake of proteins by isolated chloroplasts. In vitro synthesis and transport of plastocyanin, ferredoxin-NADP+ oxidoreductase, and fructose-1,6-bisphosphatase. J Biol Chem. 1982 Feb 10;257(3):1558–1563. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maccecchini M. L., Rudin Y., Blobel G., Schatz G. Import of proteins into mitochondria: precursor forms of the extramitochondrially made F1-ATPase subunits in yeast. Proc Natl Acad Sci U S A. 1979 Jan;76(1):343–347. doi: 10.1073/pnas.76.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcu K., Dudock B. Characterization of a highly efficient protein synthesizing system derived from commercial wheat germ. Nucleic Acids Res. 1974 Nov;1(11):1385–1397. doi: 10.1093/nar/1.11.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendiola-Morgenthaler L. R., Morgenthaler J. J., Price C. A. Synthesis of coupling factor CF1 protein by isolated spinach chloroplasts. FEBS Lett. 1976 Feb 1;62(1):96–100. doi: 10.1016/0014-5793(76)80025-7. [DOI] [PubMed] [Google Scholar]
- Morgenthaler J. J., Marsden M. P., Price C. A. Factors affecting the separation of photosynthetically competent chloroplasts in gradients of silica sols. Arch Biochem Biophys. 1975 May;168(1):289–301. doi: 10.1016/0003-9861(75)90253-2. [DOI] [PubMed] [Google Scholar]
- Morgenthaler J. J., Price C. A. Photosynthetic activity of spinach chloroplasts after isopycnic centrifugation in gradients of silica. Plant Physiol. 1974 Oct;54(4):532–534. doi: 10.1104/pp.54.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson N., Nelson H., Schatz G. Biosynthesis and assembly of the proton-translocating adenosine triphosphatase complex from chloroplasts. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1361–1364. doi: 10.1073/pnas.77.3.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyton R. O., McKemmie E. A polyprotein precursor to all four cytoplasmically translated subunits of cytochrome c oxidase from Saccharomyces cerevisiae. J Biol Chem. 1979 Jul 25;254(14):6763–6771. [PubMed] [Google Scholar]
- Zielinski R. E., Price C. A. Synthesis of thylakoid membrane proteins by chloroplasts isolated from spinach. Cytochrome b559 and P700-chlorophyll a-protein. J Cell Biol. 1980 May;85(2):435–445. doi: 10.1083/jcb.85.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]