Abstract
Mammalian selenoproteins have diverse functions, cellular locations, and evolutionary histories, but all use the amino acid selenocysteine (Sec), often present in the enzyme’s active site. Only about half of mammalian selenoproteins have been functionally characterized, with most being oxidoreductases. The cellular role of selenoprotein T (SelT), manifesting a CxxU motif in a thioredoxin-like fold and localized to Golgi and the endoplasmic reticulum, is not known. To examine its biological function, we knocked down SelT expression in mouse fibroblast cells and found that SelT deficiency alters cell adhesion and enhances the expression of several oxidoreductase genes, while decreasing the expression of genes involved in cell structure organization, suggesting the involvement of SelT in redox regulation and cell anchorage. Furthermore, we found that the loss of SelT elevates expression of another selenoprotein, selenoprotein W (SepW1). SelT and SepW1 belong to the same protein family, suggesting that SepW1 may functionally compensate for SelT.
Keywords: carbonyl reductase 3, oxidoreductase, selenoprotein T, selenoprotein T deficiency, selenoprotein W
Introduction
Selenium is a dietary trace element that is essential for human health and is an important component of various metabolic pathways. Many trace elements are used as cofactors of proteins, but selenium, in addition to serving as a cofactor in some prokaryotic enzymes (Gladyshev et al. 1994), is incorporated into proteins such as selenocysteine (Sec), the 21st naturally-occurring amino acid (Hatfield and Gladyshev 2002). Proteins containing Sec residues are known as selenoproteins, with Sec most often present at the active sites of these proteins (Brown and Arthur 2001). Evolutionary histories, enzymatic activities, tissue expression profiles, and the location of the Sec residue in selenoproteins vary significantly (Kryukov et al. 2003), as reflected in their functional diversity in physiological processes, such as redox regulation (Behne and Kyriakopoulos 2001), thyroid hormone metabolism, and immune response (Beckett and Arthur 2005). Selenium is thought to exert most of its beneficial roles in health through the action of selenoproteins (Hatfield and Gladyshev 2002). However, among the selenoproteins identified, only about half have been functionally characterized, most of which are involved in redox reactions. The biological functions of many others remain unknown.
Selenoprotein T (SelT) is a selenium-containing protein whose cellular function has not been characterized. It was originally identified through in silico studies, cloned and expressed in a mammalian cell line, and confirmed as a selenoprotein (Kryukov et al. 1999). SelT possess a CxxU motif in a thioredoxin-like fold and, based on these characteristics, was assigned to a new protein family, Rdx, which is a thioredoxin-like family (Dikiy et al. 2007). Intracellular localization studies in mouse cell lines suggested that SelT mainly occurs in Golgi and possibly the endoplasmic reticulum (Dikiy et al. 2007); in rat cell lines, it was found to be localized in the endoplasmic reticulum (Grumolato et al. 2008). The latter study suggested a role for SelT in Ca2+ homeostasis through a redox mechanism and involvement in neuroendocrine secretion (Grumolato et al. 2008). Selt mRNA has also been shown to be elevated in the brains of hypoxia-induced mice, though the reason for this increase is not known (Ikematsu et al. 2007).
To assess the biological function of SelT, its expression was knocked down in mouse fibroblast cells. The resulting data indicate that adhesion ability of stable SelT knockdown cells has decreased. Microarray analyses showed that SelT removal elevated the expression of several oxidoreductase genes, while decreasing the expression of several extracellular matrix genes involved in cell structure organization. The elevated oxidoreductase genes might compensate for the loss of SelT activity, whereas a change in cell adhesion implicates a role in cell anchorage. Interestingly, the loss of SelT increased the expression of selenoprotein W (SepW1), another member of the Rdx family, which might functionally compensate for SelT.
Materials and methods
Materials
Selenium (75Se, specific activity 1000 Ci·mmol−1) in the form of selenious acid was procured from the Research Reactor Facility (University of Missouri, Columbia, Mo.), and neutralized with sodium hydroxide prior to use. Oligonucleotides were procured from Sigma-Genosys (St. Louis, Mo.). Polyacrylamide gels, polyvinylidene fluoride (PVDF) membranes, fetal bovine serum (FBS), Dulbecco’s modified Eagle’s medium (DMEM), antibiotic–antimycotic solution, Lipofectamine™ 2000, TRIzol reagent, and Hygromycin B were from Invitrogen (Carlsbad, Calif.); BCA protein assay reagent, SuperSignal West Dura substrate, HRP-conjugated secondary antibodies were from Thermo Fisher Scientific Inc. (Waltham, Mass.); and Cy3 and Cy5 mono-reactive dyes were from GE Healthcare (Chalfont St. Giles, UK). SepW1 antibodies were from Rockland Immunochemicals Inc. (Gilbertsville, Penn.) and β-actin antibodies were from Abcam (Cambridge, Mass.). The Fairplay® II microarray labeling kit was obtained from Stratagene (La Jolla, Calif.) and the CellTiter 96 AQueous One Solution cell proliferation assay kit was obtained from Promega (Madison, Wis.). The iScript™ cDNA synthesis kit and SYBR green supermix were from Bio Rad Laboratories (Hercules, Calif.). The SelT and glutathione peroxidase 4 (Gpx4) antibodies were generated in our laboratories. All other reagents were of the highest grade available.
Generation of Selt siRNA constructs
The pU6-m3 vector (Xu et al. 2007) was used for cloning siSelT targeting sequences and to transfect NIH 3T3 cells as the control. The siRNA Target Finder (Ambion Inc.) was used to select 5 candidate target regions to knockdown the expression of Selt (GenBank accession No. NM_001040396), which spanned nucleotides 205–224, 296–316, 562–580, 716–736, and 971–989, respectively. Oligonucleotides containing respective sense and antisense sequences were annealed and cloned into the BamHI–HindIII restriction sites of pU6-m3. The resulting constructs were designated siSelT-1 through siSelT-5 and verified by sequencing. These constructs and the control plasmid were separately transfected into NIH 3T3 cells and transfected cells were stabilized with 150 μg·mL−1 hygromycin B. Selt mRNA levels in the knockdown cells were determined by quantitative real-time polymerase chain reaction (qPCR) analysis.
Culture of mammalian cells, transfection, cell growth and cell adhesion assays
NIH 3T3 cells were grown in DMEM supplemented with 10% FBS and antibiotic–antimycotic solution. Transfections with Lipofectamine™ 2000 were carried out according to the manufacturer’s protocol. The growth media was supplemented with 100 nmol·L−1 sodium selenite and growth rates of cells were assessed by seeding 1 × 105 cells per well in a 6-well tissue culture plate, harvesting after 24, 48, and 72 h with trypsin-EDTA, and counting using the trypan blue extrusion method. The cell adhesion assay was carried out as described (Brake et al. 1990) by seeding 3 × 104 cells onto each well of a 96-well tissue culture plate in triplicate.
Sensitivity of SelT knockdown cells to oxidative stress
The sensitivity of control and SelT knockdown cells to oxidative stress was analyzed by seeding 5 × 103 cells into each well of 96-well plates and growing overnight in DMEM supplemented with 10% FBS. Cells were washed twice with PBS and incubated in serum- and phenol-red-free medium containing hydrogen peroxide for 1 h at 37 °C. Twenty microlitres of CellTiter 96 AQueous One Solution reagent was added to each well and incubated for an additional 4 h. The absorbance was recorded at 450 nm and cell viability was expressed as a percentage of untreated controls.
Quantitative real-time PCR
Relative gene expression was verified by qPCR as described (Sengupta et al. 2008), using primer sequences listed in Table 1. Total RNA (1–2 μg) from each sample was reverse transcribed to cDNA using the iScript™ cDNA Synthesis Kit, according to the manufacturer’s protocol and used for qPCR in combination with respective primers and SYBR green supermix. Reactions were carried out in triplicate and primer specificity was confirmed by melting curve analysis.
Table 1.
Primers used in qPCR assays.
| Gene | Forward sequence (5′→3′) | Reverse sequence (5′→3′) |
|---|---|---|
| Actr6 | TTGTCGACAGTGGCTATTCC | GCAGCTGCCTGTAGGATATG |
| Aldh3a1 | GAAGATGCTAAGCAGTCCCA | GGTGGGAGCTATGTATCGTG |
| Cbr3 | GGACAGGATTCTGCTCAATG | CGTGAGGTTCAGTGGCAT |
| Cyp7a1 | TACGACCGGTACCTTGATGA | TGCTTGATTTCTTGGACAGC |
| Fbn1 | AATGAACGGTGGTTGTGAGA | ACTGGCCACCATCACAGATA |
| Gpx1 | CAGGAGAATGGCAAGAATGA | GAAGGTAAAGAGCGGGTGAG |
| Gpx4 | GCAGGAGCCAGGAAGTAATC | GGCTGGACTTTCATCCATTT |
| Gstm1 | CCAAACACACAGGTCAGTCC | CGTCACCCATGGTGTATCTC |
| Gusb | AACCTCTGGTGGCCTTACCT | CTTGCTCTTTGTGACAGCCA |
| Hmox1 | GCCACCAAGGAGGTACACAT | GCTTGTTGCGCTCTATCTCC |
| Igfbp7 | GCTGGAGAGTATGAGTGCCA | TACAGAGGCTCATGGATTCG |
| Memo1 | ATGTGGAAGACATCCCATTG | CTCCAGCTGCGTAACTGACT |
| Rarres2 | GAGCCTACAGGTGGCTCTG | TTGGTCTGCTGGAGCTTAAA |
| Selh | CTACCTGTGCAAGTGAACCC | TGAGGCTCAGGAAATTTGAG |
| Seli | CCGTGTTTGCTCTTCACTTT | GCTGACATGTGATATTGGCA |
| Selk | TGGTGGATGAGGAAGGTAAA | ACAGAGCAATCCTTGTTTGG |
| Selm | GATTGGAACCGTCTTCGAG | GTGCTTCATCACCAGGTTGT |
| Selo | ATCGACTATGGACCCTTTGG | TTTCTGCAGATTCCACTTGC |
| Sels | CTTTGCGAGGAGGTGGTTAT | CCTTGCTAATGTCAGAGCGA |
| Selt | TGGTCTAAGCTGGAATCTGG | TTTCGGTGCTGATAGGTAGG |
| Sep15 | TGGAACACAGACAGTGTGGA | TGACCAATGTAAGCATGCAA |
| Sephs2 | GATAGTGCCGTGGTAGGAGA | CTCTGGAAACCACCATCTTG |
| Sepn1 | CCCTAGGTAGCCTTCTGTGC | ACAGGCTGAAGGGTAGCAGT |
| Sepp1 | ATCTTGGCAGCAGTAAGCCT | TCACTTGCTGTGGTGTCTCA |
| Sepw1 | TAGAGGCAGGGTCCTGAAAG | AATCCATCTCTGGCCTGACT |
| Sepx1 | TCCAGTCACTCGAAGTACGC | CTTGCCACAGGACACCTTTA |
| Sod1 | AACCATCCACTTCGAGCAGA | ATGAGGTCCTGCACTGGTACA |
| Thbs1 | CACAAGGGCTCAGGGATACT | GAAATCTTTCCAGCCGATGT |
| Txnrd1 | CTACAGACCATTGCCTTGCT | ACCTCCTACCCACAAGATCC |
| Txnrd2 | TCACTGGAATTGGACTGGAT | ACACAGCCTTTCAGGAACTG |
| Txnrd3 | CTGGAATATGGCTGTTGTGG | AGGTGTTGTTGTCTCTGCCA |
Protein extracts preparation, Western blot analysis and selenoprotein labeling
Protein extracts were prepared from cells using lysis buffer (20 mmol·L−1 Tris–HCl, 150 mmol·L−1 NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 10 mmol·L−1 NaF, 5 mmol·L−1 EDTA, and protease inhibitor cocktail) and protein concentration was measured using BCA protein assay reagent. Extracts were electrophoresed on 4%–12% polyacrylamide gels, transferred to PVDF membranes, and immunodetected with antibodies against SelT (1:800 dilution), β-actin (1:1000 dilution), SepW1 (1:1000 dilution), and Gpx4 (1:1000 dilution). Following incubation with primary antibodies, the membranes were processed as described (Sengupta et al. 2008). To label selenoproteins with 75Se and visualize the labeling, control and SelT knockdown cells were seeded onto a 6 well plate (3 × 105 cells per well) and processed as described earlier (Xu et al. 2007).
Microarray analysis
Mouse oligonucleotide glass arrays containing 70mer oligonucleotides were procured from the NCI Microarray Facility (Frederick, Md.). Each slide in these oligonucleotide arrays has 48 blocks containing 28 rows and 28 columns each, with 36960 oligonucleotide spots at a spacing of 155 μm. Probe preparation, hybridization, data processing, and analysis for microarray were done as described by Sengupta et al. (2008). Briefly, total RNA (10–15 μg) isolated from control and SelT knockdown cells was reverse transcribed to cDNA and labeled with monofunctional dye (Cy3 or Cy5) by the Fairplay® II microarray labeling kit (Stratagene) according to the manufacturer’s protocol. The Cy3- and Cy5-labeled cDNAs were combined and hybridized to mouse oligonucleotide arrays (designated Mm-MEEBO-v1.3px) then scanned using a Genepix® 4000B scanner and analyzed with GenePix Pro 3.0 software (Axon Instruments). Data were imported into the microarray database (mAdb) and analyzed as described by Sengupta et al. (2008).
Results
Knockdown of SelT in NIH 3T3 cells
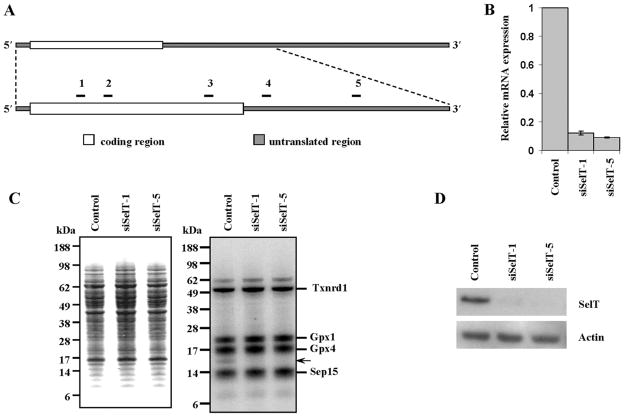
Five target regions were selected to knockdown Selt mRNA wherein 3 were in the coding region and 2 were in the 3′-untranslated region (3′-UTR) (Fig. 1A). NIH 3T3 cells were stably transfected with the corresponding siSelT constructs and vector. Selt mRNA levels were depleted approximately 90% in cells transfected with siSelT-1 or siSelT-5 constructs (Fig. 1B). Selt mRNA was depleted 24% in cells transfected with the siSelT-2 construct and remained unchanged in cells transfected with the other constructs (data not shown). As the siSelT-1 and siSelT-5 transfected cells manifested virtually a total loss of Selt mRNA, these two cell lines were used for further analysis. Vector-transfected NIH 3T3 cells were designated as control, while cells lacking SelT were designated as SelT knockdown. Control and SelT knockdown cells were labeled with 75Se, protein extracts electrophoresed, and the expression of labeled selenoproteins examined by autoradiography (Fig. 1C). Relative intensities of various 75Se-labeled bands appeared unchanged, whereas SelT was diminished dramatically in the 2 knockdown cell lines (Fig. 1C). The loss of SelT was further confirmed in the knockdown cells by Western blot analysis (Fig. 1D).
Fig. 1.
Knocking down SelT in murine fibroblast cells. (A) Schematic representation of siSelT target regions (not to scale) for SelT knockdown in NIH 3T3 cells. The position of each selected target site is given in Materials and methods and the two most efficient siSelT sites used in subsequent studies are discussed in the text. (B) The relative Selt mRNA levels in NIH 3T3 cells stably transfected with siSelT-1, siSelT-5, or vector and grown in the presence of 100 nmol·L−1 sodium selenite, were analyzed by qPCR and normalized to Gusb. (C) Protein extracts were prepared from 75Se-labeled cells, electrophoresed, the gels stained with Coomassie blue (left panel), and labeled selenoproteins analyzed by autoradiography (right panel). Selenoproteins identified previously are shown on the right of the 75Se-labeled panel (Kryukov et al. 1999), the band corresponding to SelT is indicated with an arrow, and the corresponding molecular masses of proteins are shown on both panels. (D) Western blot analysis of cell lysates with SelT antibodies, with β-actin as a loading control.
Effect of SelT knockdown on cell adhesion, growth, and oxidative stress
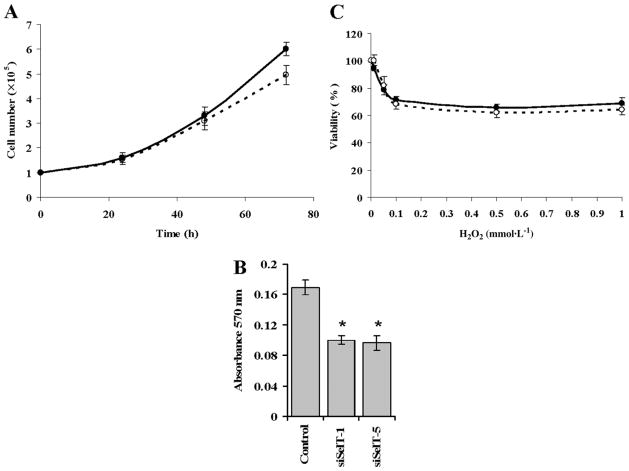
Several characteristics of the SelT knockdown and control cells were examined. Both cell lines grew in monolayer, without visible differences in morphology2 with the growth rate of SelT knockdown cells being slightly slower than in control cells, wherein the reduction was about 17% after 72 h (Fig. 2A). However, upon treatment with trypsin–EDTA, the SelT knockdown cells detached more rapidly from plates as compared with the control cells2. To determine if the loss of SelT alters cell adhesion, tissue culture microtiter plates were seeded with 3 × 104 cells for the adhesion assay, as described by Brake et al. (1990). The SelT knockdown cells showed ~40% less adhesion as compared with control cells (Fig. 2B). Among the functionally characterized selenoproteins, most have been shown to be involved in redox reactions. We therefore examined the sensitivity of the SelT knockdown cells to hydrogen peroxide treatment. There was virtually no difference between SelT knockdown and control cells with respect to their response to exposure to hydrogen peroxide (Fig. 2C).
Fig. 2.
Adhesion, growth rate, and sensitivity to oxidative stress of NIH 3T3 cells deficient in SelT. (A) Control and the 2 SelT knockdown cells were seeded at a density of 1 × 105 cells per well in a 6-well culture dish and cell numbers counted at 24, 48, and 72 h intervals to assess growth rates. (B) The adherence of control and SelT knockdown cells plated in a 96-well tissue culture plate was measured by optical density reading (570 nm) as described in Materials and methods. (* p ≤ 0.05). (C) Sensitivity of cells to oxidative stress following treatment with hydrogen peroxide was examined as described in Materials and methods. The growth rates and oxidative stress sensitivity of control cells are shown by filled circles and solid lines (—●—), whereas those of SelT knockdown cells are shown by open circles with dashed lines (–○–). The data plotted for the knockdown cells are the average of siSelT-1 and siSelT-5 cells.
Effect of SelT knockdown on selenoprotein expression
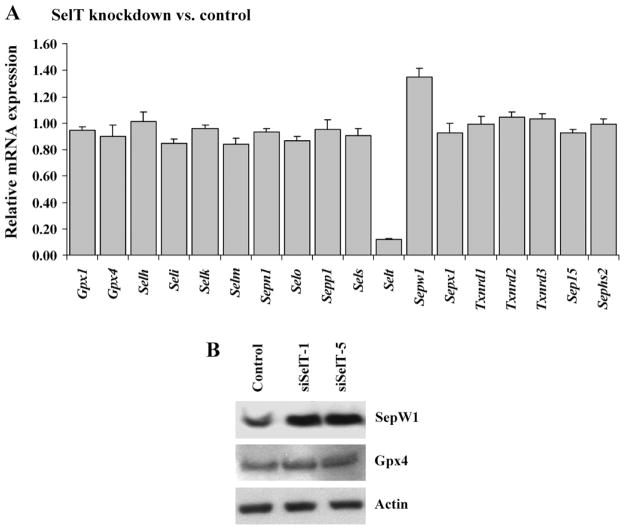
Metabolic 75Se-labeling of cells showed that the levels of major selenoproteins, Txnrd1, Gpx1, Gpx4, and Sep15, were not affected by the loss of SelT expression (Fig. 1C). The relative expression levels of all mouse selenoprotein transcripts were determined in SelT knockdown and control cell lines by qPCR (Fig. 3A). Transcripts that were below detection limits under these experimental conditions are not shown. The expression of various mRNAs was normalized to the expression of Gusb and expression levels of SelT knockdown cells were compared with control cells. The data indicated that although the transcript levels of most selenoproteins analyzed were virtually unchanged, the levels of selenoprotein W (Sepw1) showed an increase of about 1.4 fold (Fig. 3A). As the results were averaged for siSelT-1 and siSelT-5 constructs compared with the control, they provide strong evidence that the increase in Sepw1 is not an effect of off-targeting. These data were corroborated by Western blot analysis wherein the level of SepW1 was found to be higher in SelT knockdown cells compared with control cells (Fig. 3B). The levels of Gpx4 were very similar in the two cells lines, as was the level of β-actin, which was used as a loading control.
Fig. 3.
Selenoprotein W expression in SelT knockdown cells. (A) Relative expression levels of selenoprotein genes were examined by qPCR analysis. The 2 SelT knockdown lines were compared with control cells and data are means ± SE of the comparison. (B) Protein lysates of SelT knockdown and control cells were analyzed by Western blot assays with SepW1 and Gpx4 antibodies, with β-actin as the loading control.
Gene expression profile of SelT knockdown NIH 3T3 cells
The gene expression profile of SelT knockdown cells was examined by mouse oligonucleotide arrays containing approximately 29 000 genes. From the arrays, transcripts expressed in at least 50% of the samples and showing an increase or decrease of ≥ 2-fold (vs. control) and a p value ≤ 0.01 were filtered. Following filtering, the genes were grouped according to their functions and sorted as upregulated (Table 2) or downregulated (Table 3) using GENECODIS (Carmona-Saez et al. 2007), DAVID (Dennis et al. 2003), or mAdb software.
Table 2.
Genes elevated in SelT knockdown cells.
| Symbol | UniGene | Gene description | Fold change | p value |
|---|---|---|---|---|
| Redox regulation | ||||
| Aldh3a1 | Mm.4257 | Aldehyde dehydrogenase family 3, subfamily A1 | 2.110 | 0.00002 |
| Cbr3 | Mm.4512 | Carbonyl reductase 3 | 6.806 | 0.00489 |
| Cyp7a1 | Mm.57029 | Cytochrome P450, family 7, subfamily a, polypeptide 1 | 2.817 | 0.00024 |
| Dio2 | Mm.21389 | Deiodinase, iodothyronine, type II | 2.088 | 0.00024 |
| Gstm1 | Mm.37199 | Glutathione S-transferase, mu 1 | 2.639 | 0.00003 |
| Hmox1 | Mm.276389 | Heme oxygenase (decycling) 1 | 2.236 | 0.00004 |
| Sepw1 | Mm.42829 | Selenoprotein W, muscle 1 | 2.278 | 0.00028 |
| Sod1 | Mm.276325 | Superoxide dismutase 1 | 2.272 | 0.00009 |
| Cell differentiation | ||||
| Aff4 | Mm.395281 | AF4/FMR2 family, member 4 | 2.512 | 0.00772 |
| Ggnbp1 | Mm.440437 | Gametogenetin binding protein 1 | 2.405 | 0.00015 |
| Igf1 | Mm.268521 | Insulin-like growth factor 1 | 2.075 | 0.00004 |
| Il11ra1 | Mm.193451 | Interleukin 11 receptor, α chain 1 | 2.419 | 0.00090 |
| Runx1t1 | Mm.4909 | Runt-related transcription factor 1; translocated to, 1 | 2.224 | 0.00190 |
| Cell adhesion | ||||
| Jup | Mm.299774 | Junction plakoglobin | 2.460 | 0.00004 |
| Kif17 | Mm.271936 | Kinesin family member 17 | 2.601 | 0.00010 |
| Rapgef6 | Mm.254404 | Rap guanine nucleotide exchange factor (GEF) 6 | 2.476 | 0.00012 |
| Tctex1d1 | Mm.111346 | Tctex1 domain containing 1 | 2.450 | 0.00058 |
| Smarcd2 | Mm.21772 | SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily d, member 2 | 2.380 | 0.00004 |
| Organ development | ||||
| Cul7 | Mm.329078 | Cullin 7 | 2.285 | 0.00128 |
| Foxk1 | Mm.24214 | Forkhead box K1 | 2.142 | 0.00017 |
| Hmgn1 | Mm.2756 | High mobility group nucleosomal binding domain 1 | 2.602 | 0.00001 |
| Osr2 | Mm.46336 | Odd-skipped related 2 | 2.351 | 0.00112 |
| Rpl24 | Mm.458082 | Ribosomal protein L24 | 2.807 | 0.00190 |
| Protein metabolism | ||||
| Camk2b | Mm.439733 | Calcium/calmodulin-dependent protein kinase II, β | 2.405 | 0.00007 |
| Pigp | Mm.472732 | Phosphatidylinositol glycan anchor biosynthesis, class P | 2.212 | 0.00000 |
| Rps2 | Mm.328846 | Ribosomal protein S2 | 2.575 | 0.00573 |
| Rps6kb2 | Mm.271937 | Ribosomal protein S6 kinase, polypeptide 2 | 2.414 | 0.00039 |
| Rps7 | Mm.279839 | Ribosomal protein S7 | 2.402 | 0.00484 |
| Usp37 | Mm.66568 | Ubiquitin specific peptidase 37 | 2.923 | 0.00239 |
Table 3.
Genes decreased in SelT knockdown cells.
| Symbol | UniGene | Gene description | Fold change | p value |
|---|---|---|---|---|
| Cell structure organization | ||||
| Actr6 | Mm.335292 | ARP6 actin-related protein 6 homolog | −2.584 | 0.00054 |
| Fbn1 | Mm.271644 | Fibrillin 1 | −2.770 | 0.00005 |
| Igfbp7 | Mm.233470 | Insulin-like growth factor binding protein 7 | −2.634 | 0.00037 |
| Memo1 | Mm.311874 | Mediator of cell motility 1 | −2.866 | 0.00034 |
| Nupr1 | Mm.18742 | Nuclear protein 1 | −2.164 | 0.00000 |
| Rarres2 | Mm.28231 | Retinoic acid receptor responder 2 | −2.796 | 0.00029 |
| Skil | Mm.15406 | SKI-like | −2.147 | 0.00006 |
| Thbs1 | Mm.4159 | Thrombospondin 1 | −2.813 | 0.00028 |
| Regulation of transcription | ||||
| Bptf | Mm.343986 | Bromodomain PHD finger transcription factor | −2.478 | 0.00134 |
| Cnot2 | Mm.351553 | CCR4-NOT transcription complex, subunit 2 | −2.605 | 0.00081 |
| Mysm1 | Mm.208868 | Myb-like, SWIRM and MPN domains 1 | −2.265 | 0.00002 |
| Tbp | Mm.244820 | TATA box binding protein | −2.965 | 0.00181 |
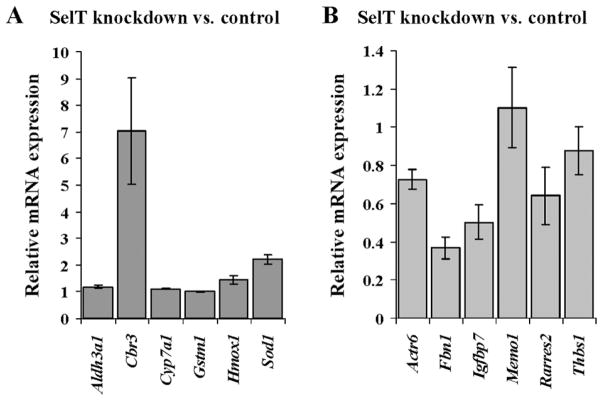
Genes elevated in SelT knockdown cells had roles in cell differentiation, cell adhesion, redox regulation, organ development, and protein metabolism (Table 2), whereas those genes with decreased expression were involved in cell structure organization and regulation of transcription (Table 3). Several genes significantly upregulated in SelT knockdown cells such as carbonyl reductase 3 (Cbr3), cytochrome P450 family 7 subfamily a polypeptide 1 (Cyp7a1), glutathione S-transferase mu 1 (Gstm1), and superoxide dismutase 1 (Sod1), are involved in redox control. The levels of the up-regulated genes were verified by qPCR (Fig. 4A) and it was noted that the expression of Cbr3 was consistent with the microarray data, whereas Sod1 showed a 2-fold increase. However, immunodetection of Sod1 did not show a significant change (data not shown). Among the downregulated genes, many localized to the extracellular region and had a role in cell structural integrity. We validated these genes by qPCR and found that although the levels of fibrillin 1 (Fbn1) and insulin-like growth factor binding protein 7 (Igfbp7) were substantially reduced as noted in the microarray analysis, the other downregulated genes involved in cell structure organization (e.g., Actr6, Rarres2, Thbs1) did not decrease as much as was seen in the microarray analysis (Fig. 4B).
Fig. 4.
Examination of genes altered in SelT knockdown cells. Relative expression levels of genes altered in SelT knockdown cells by microarray in Tables 2 and 3 were analyzed by qPCR. (A) Expression level of genes involved in redox regulation and elevated in SelT knockdown cells. (B) Expression levels of genes decreased in SelT knockdown cells and involved in cell structural organization. Data are a comparison of the 2 SelT knockdown lines and control cells wherein the quantity of RNA for each gene was normalized to Gusb. Data shown are means ± SE and the average of 3 analyses for SelT knockdown cells.
Discussion
To elucidate the function of the selenoprotein SelT, we used RNAi technology to knockdown its expression in NIH 3T3 cells. Two stably transfected cell lines, wherein SelT expression was efficiently knocked down by about 90%, grew in monolayer without any visible morphological or growth changes as compared with control cells transfected with vector. However, the knockdown cells detached more rapidly from culture plates when treated with trypsin. The cell adhesion assay supports this observation, as the SelT knockdown cells show less adhesion in comparison with their control counterparts under identical conditions. Trypsin detaches cells by breaking their adhesion sites and making them spherical in shape, but a contact between the rounded cells and the culture plate is maintained by some retraction fibres (Revel et al. 1974). The SelT knockdown cells round up and detach faster than control cells, presumably due to the lack of retraction fibres. Interestingly, many downregulated genes observed by microarray analysis in SelT knockdown cells localized to the extracellular region and associated with maintaining cell integrity. Fibrillin 1 (Fbn1), a glycoprotein, which is an important constituent of the structural microfibrils (Sakai et al. 1986), has diminished expression in SelT knockdown cells and may be responsible for the decreased adhesion of these cells.
Examination of other selenoprotein transcripts in SelT knockdown cells revealed an increase in the levels of Sepw1, which was confirmed by Western blot assays. Though the subcellular and tissue localization profiles of SepW1 and SelT are different, both are members of the Rdx protein family characterized by a thioredoxin-like fold and a conserved CxxU/C motif (Dikiy et al. 2007). The presence of the CxxU/C motif in representatives of the Rdx family suggests a role in redox regulation. SepW1 possesses antioxidant properties (Jeong et al. 2002) and we speculate that its increase in SelT knockdown cells might be a functional compensation by SelT deficient cells. The redox function of SelT is further suggested by the fact that gene expression analysis of SelT knockdown cells by microarray show a highly upregulated level of Cbr3 gene product. Consistent with the microarray data, a significant 7-fold increase in mRNA expression was observed for Cbr3 by qPCR analysis. Cbr3, a monomeric protein (Watanabe et al. 1998), is a member of the short-chain dehydrogenase/reductase (SDR) family of proteins, consisting of enzymes with NAD(P)H-dependent oxidoreductase activity (Hoffmann and Maser 2007), but not much is known about the murine protein. Interestingly, loss of thioredoxin reductase in yeast also led to an increase in the levels of Cbr3 (Bondareva et al. 2007) and reports have indicated that human carbonyl reductases reduce lipid carbonyl groups, generated from lipid peroxidation during oxidative stress to protect cells from oxidative damage (Doorn et al. 2004). The similarity in elevation of Cbr3 in mammalian cells lacking SelT and yeast lacking thioredoxin reductase might reflect a protective effect against oxidative damage, but such a proposition warrants further investigation.
In summary, the present study shows that loss of SelT in murine fibroblast cells alters adhesion of the resultant cells and treatment with trypsin hastens detachment of these cells from support used in growing the cells. SelT-deficient cells also manifest elevated levels of two genes involved in redox regulation, namely Cbr3 and Sepw1, whose expression levels may functionally compensate for the lack of SelT, supporting the proposal that this protein, like many other selenoproteins, serves an oxidoreductase role. Therefore, the current study extends the functional role of SelT to redox regulation and cell adhesion.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of NIH, NCI, CCR, to D.L.H. and by NIH grants to V.N.G.
Footnotes
Supplementary data for this article are available on the journal Web site (http://bcb.nrc.ca) or may be purchased from the Depository of Unpublished Data, Document Delivery, CISTI, National Research Council Canada, Building M-55, 1200 Montreal Road, Ottawa, ON K1A 0R6, Canada. DUD 5294. For more information on obtaining material refer to http://cisti-icist.nrc-cnrc.gc.ca/eng/ibp/cisti/collection/unpublished-data.html.
Contributor Information
Aniruddha Sengupta, Molecular Biology of Selenium Section, Laboratory of Cancer Prevention, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892, USA.
Bradley A. Carlson, Molecular Biology of Selenium Section, Laboratory of Cancer Prevention, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892, USA
Vyacheslav M. Labunskyy, Department of Biochemistry and Redox Biology Center, University of Nebraska, Lincoln, NE 68588, USA
Vadim N. Gladyshev, Department of Biochemistry and Redox Biology Center, University of Nebraska, Lincoln, NE 68588, USA
Dolph L. Hatfield, Molecular Biology of Selenium Section, Laboratory of Cancer Prevention, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892, USA
References
- Beckett GJ, Arthur JR. Selenium and endocrine systems. J Endocrinol. 2005;184(3):455–465. doi: 10.1677/joe.1.05971. [DOI] [PubMed] [Google Scholar]
- Behne D, Kyriakopoulos A. Mammalian selenium-containing proteins. Annu Rev Nutr. 2001;21(1):453–473. doi: 10.1146/annurev.nutr.21.1.453. [DOI] [PubMed] [Google Scholar]
- Bondareva AA, Capecchi MR, Iverson SV, Li Y, Lopez NI, Lucas O, et al. Effects of thioredoxin reductase-1 deletion on embryogenesis and transcriptome. Free Radic Biol Med. 2007;43(6):911–923. doi: 10.1016/j.freeradbiomed.2007.05. 026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brake DA, Debouck C, Biesecker G. Identification of an Arg-Gly-Asp (RGD) cell adhesion site in human immunodeficiency virus type 1 transactivation protein, tat. J Cell Biol. 1990;111(3):1275–1281. doi: 10.1083/jcb.111.3.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KM, Arthur JR. Selenium, selenoproteins and human health: a review. Public Health Nutr. 2001;4(2B):593–599. doi: 10.1079/PHN2001143. [DOI] [PubMed] [Google Scholar]
- Carmona-Saez P, Chagoyen M, Tirado F, Carazo JM, Pascual-Montano A. GENECODIS: a web-based tool for finding significant concurrent annotations in gene lists. Genome Biol. 2007;8(1):R3. doi: 10.1186/gb-2007-8-1-r3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis G, Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003;4(5):3. doi: 10.1186/gb-2003-4-5-p3. [DOI] [PubMed] [Google Scholar]
- Dikiy A, Novoselov SV, Fomenko DE, Sengupta A, Carlson BA, Cerny RL, et al. SelT, SelW, SelH, and Rdx12: genomics and molecular insights into the functions of selenoproteins of a novel thioredoxin-like family. Biochemistry. 2007;46(23):6871–6882. doi: 10.1021/bi602462q. [DOI] [PubMed] [Google Scholar]
- Doorn JA, Maser E, Blum A, Claffey DJ, Petersen DR. Human carbonyl reductase catalyzes reduction of 4-oxonon-2-enal. Biochemistry. 2004;43(41):13106–13114. doi: 10.1021/bi049136q. [DOI] [PubMed] [Google Scholar]
- Gladyshev VN, Khangulov SV, Stadtman TC. Nicotinic acid hydroxylase from Clostridium barkeri: electron paramagnetic resonance studies show that selenium is coordinated with molybdenum in the catalytically active selenium-dependent enzyme. Proc Natl Acad Sci USA. 1994;91(1):232–236. doi: 10.1073/pnas.91.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumolato L, Ghzili H, Montero-Hadjadje M, Gasman S, Lesage J, Tanguy Y, et al. Selenoprotein T is a PA-CAP-regulated gene involved in intracellular Ca2+ mobilization and neuroendocrine secretion. FASEB J. 2008;22(6):1756–1768. doi: 10.1096/fj.06-075820. [DOI] [PubMed] [Google Scholar]
- Hatfield DL, Gladyshev VN. How selenium has altered our understanding of the genetic code. Mol Cell Biol. 2002;22(11):3565–3576. doi: 10.1128/MCB.22.11.3565-3576.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann F, Maser E. Carbonyl reductases and pluripotent hydroxysteroid dehydrogenases of the short-chain dehydrogenase/reductase superfamily. Drug Metab Rev. 2007;39(1):87–144. doi: 10.1080/03602530600969440. [DOI] [PubMed] [Google Scholar]
- Ikematsu K, Tsuda R, Tsuruya S, Nakasono I. Identification of novel genes expressed in hypoxic brain condition by fluorescence differential display. Forensic Sci Int. 2007;169(2–3):168–172. doi: 10.1016/j.forsciint.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Jeong D, Kim TS, Chung YW, Lee BJ, Kim IY. Selenoprotein W is a glutathione-dependent antioxidant in vivo. FEBS Lett. 2002;517(1–3):225–228. doi: 10.1016/S0014-5793(02) 02628-5. [DOI] [PubMed] [Google Scholar]
- Kryukov GV, Kryukov VM, Gladyshev VN. New mammalian selenocysteine-containing proteins identified with an algorithm that searches for selenocysteine insertion sequence elements. J Biol Chem. 1999;274(48):33888–33897. doi: 10.1074/jbc.274.48.33888. [DOI] [PubMed] [Google Scholar]
- Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigó R, Gladyshev VN. Characterization of mammalian selenoproteomes. Science. 2003;300(5624):1439–1443. doi: 10.1126/science.1083516. [DOI] [PubMed] [Google Scholar]
- Revel JP, Hoch P, Ho D. Adhesion of culture cells to their substratum. Exp Cell Res. 1974;84(1):207–218. doi: 10.1016/0014-4827(74)90398-X. [DOI] [PubMed] [Google Scholar]
- Sakai LY, Keene DR, Engvall E. Fibrillin, a new 350-kD glycoprotein, is a component of extracellular microfibrils. J Cell Biol. 1986;103(6 Pt 1):2499–2509. doi: 10.1083/jcb.103.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta A, Carlson BA, Weaver JA, Novoselov SV, Fomenko DE, Gladyshev VN, Hatfield DL. A functional link between housekeeping selenoproteins and phase II enzymes. Biochem J. 2008;413(1):151–161. doi: 10.1042/BJ20080277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Sugawara C, Ono A, Fukuzumi Y, Itakura S, Yamazaki M, et al. Mapping of a novel human carbonyl reductase, CBR3, and ribosomal pseudogenes to human chromosome 21q22.2. Genomics. 1998;52(1):95–100. doi: 10.1006/geno.1998.5380. [DOI] [PubMed] [Google Scholar]
- Xu XM, Carlson BA, Irons R, Mix H, Zhong N, Gladyshev VN, Hatfield DL. Selenophosphate synthetase 2 is essential for selenoprotein biosynthesis. Biochem J. 2007;404(1):115–120. doi: 10.1042/BJ20070165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.