Abstract
β-Carotene is the major dietary source of provitamin A. Central cleavage of β-carotene yields 2 molecules of retinal followed by further oxidation to retinoic acid. Eccentric cleavage of β-carotene occurs at double bonds other than the central double bond, and the products of these reactions are β-apocarotenals and β-apocarotenones. We reviewed recent developments in 3 areas: 1): the enzymatic production of β-apocarotenoids in higher animals; 2) the occurrence of β-apocarotenoids in foods and animal tissues; and 3) the biological activity of β-apocarotenoids, particularly on retinoid receptors. HPLC–mass spectrometry techniques were developed to quantify these compounds in mouse serum and tissues and in foods. β-Apo-10′- and -12′-carotenals were detected in mouse serum and liver. β-Apo-8′-, β-apo-10′-, β-apo-12′-, and β-apo-14′-carotenals and β-apo-13-carotenone were detected in orange-fleshed melons. Transactivation assays were performed to see whether apocarotenoids activate or antagonize retinoid X receptor (RXR) α. Reporter gene constructs and retinoid receptor (RXRα) were transfected into cells, which were used to perform quantitative assays for the activation of this ligand-dependent transcription factor. None of the β-apocarotenoids significantly activated RXRα. However, β-apo-13-carotenone antagonized the 9-cis-retinoic acid activation of RXRα. Competitive radioligand binding assays showed that this antagonist competes directly with the agonist for binding to purified receptor, a finding confirmed by molecular modeling studies. These findings suggest that a possible biological function of β-apocarotenoids is their ability to interfere with nuclear receptor signaling. Recent work showed that β-apo-13-carotenone is also a high-affinity antagonist of all 3 retinoic acid receptors (RARα, RARβ, and RARγ).
ENZYMATIC PRODUCTION OF β-APOCAROTENALS IN HIGHER ANIMALS
The first in vitro studies in 1965 by Olson and Hayaishi (1) and Goodman and Huang (2) showed enzymatic production of retinal (vitamin A) from the central cleavage of β-carotene. However, early researchers also found indirect evidence for the formation of eccentric cleavage products, β-apocarotenoids, in studies in which radioactive β-carotene was fed to rats (3, 4). Doubt was cast as to whether the observed β-apocarotenoids were formed enzymatically, because it had been observed that the incubation of β-carotene under conditions similar to those used in the early in vitro studies of Goodman and Olson but without enzyme led to the formation of small but significant amounts of the eccentric cleavage products (5). Later in vitro work by Tang et al (6) and Wang et al (7) in the early 1990s showed that β-apocarotenoids can be produced by intestine homogenates, although the same group later reported that retinal was the sole product in the presence of α-tocopherol (8). They further concluded that the random cleavage products observed must be due to enzyme-related radicals, and α-tocopherol served as an antioxidant to reduce these random cleavage reactions.
An obvious problem in these efforts to resolve the central compared with eccentric cleavage debate was that these studies were performed with tissue homogenates. The intestine has peroxidases that can potentially oxidize β-carotene [as reviewed by Hansen and Maret (5)] and contribute to random cleavage. In fact, none of the studies performed with tissue homogenates yielded the theoretical 2:1 retinal:β-carotene ratio, with the closest being 1.88:1 (9).
An important development in the study of carotenoid metabolism was the identification and characterization of the enzyme responsible for central cleavage of β-carotene in independent studies by von Lintig and Vogt (10) and Wyss (11) in 2000. This allowed for studies with purified enzyme [currently called β-carotene 15,15′-oxygenase (BCO1)5; 12] from various animal sources, and these studies generally agreed that the enzyme primarily cleaves at the center of the β-carotene molecule (10, 13–18). However, substrate specificity studies using purified chicken BCO1 showed that the enzyme can also cleave β-apo-8′-carotenal and β-apo-4′-carotenal, although at lower rates (∼10%) than β-carotene (18). Thus, although central cleavage appears to be the primary mode of action of BCO1, it is still plausible that it can generate relatively small amounts of eccentric cleavage products, but these are cleaved to generate retinal.
A second carotenoid cleavage enzyme that cleaves β-carotene eccentrically (specifically at the 9′,10′ bond) was identified in 2001 (19). This enzyme [called β-carotene 9′,10′-oxygenase (BCO2)] was found to cleave at the 9′,10′ bond with a Vmax of 32.2 ± 2.9 pmol β-apo-10′-carotenal · mg protein−1 · h−1 and a Km of 3.5 ± 1.1 μmol/L β-carotene (20). For comparison, the Vmax for recombinant BCO1 ranges from 2.16 to 420 nmol retinal · mg protein−1 · h−1 and the Km from 6 to 31 μmol/L β-carotene (human and mouse data) (12). In addition to β-carotene, lycopene was also a substrate for BCO2 (19, 20) [lycopene was not shown to be a substrate for BCO1, as reviewed by Lietz et al (12)].
In mice, BCO1 and BCO2 are both expressed in the small intestine, liver, kidney, and testis (19). The presence of both enzymes in the intestine and the relatively lower activity of BCO2 with β-carotene may explain the detection of small amounts of β-apocarotenoids observed by early researchers in vivo and with tissue homogenates.
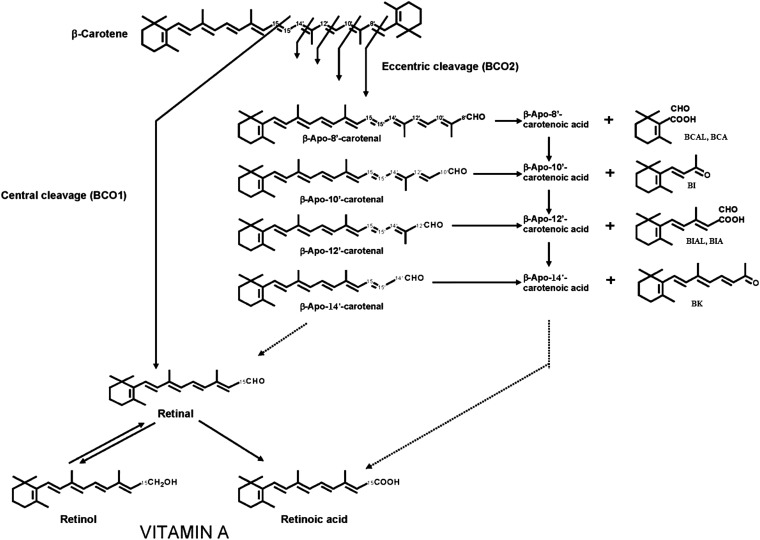
In summary, it is clear that BCO1 and BCO2 can catalyze the formation of certain β-apocarotenoids but that other enzymes such as peroxidases and lipoxygenases may also catalyze the production of these molecules. Finally, it is probable that nonenzymatic processes can also cause the oxidative cleavage of the double bonds of β-carotene. Much remains to be understood about the details of the enzymatic formation of β-apocarotenoids from ingested β-carotene in humans and animals. The pathways of central and eccentric cleavage of β-carotene as they occur in vertebrates are shown in Figure 1.
FIGURE 1.
Pathways of central and eccentric cleavage of β-carotene in vertebrates. Eccentric cleavage at the 9,10 double bond is catalyzed by BCO2 to yield β-apo-10′-carotenal and β-ionone. Other eccentric cleavages may be enzyme-catalyzed or may occur chemically (see text). The carotenals can presumably be oxidized to the corresponding carboxylic acids, but this has not yet been definitively shown. β-Apo-13-carotenone was shown to be an RXRα antagonist. BCA, β-cyclogeranic acid; BCAL, β-cyclocitral; BCO1, β-carotene 15,15′-oxygenase; BCO2, β-carotene 9′,10′-oxygenase 2; BI, β-ionone; BIA, β-ionylideneacetic acid; BIAL, β-ionylidene acetaldehyde; BK, β-apo-13-carotenone; RXRα, retinoid X receptor α.
OCCURRENCE OF APOCAROTENOIDS IN FOODS AND ANIMAL TISSUES
In plants, the oxidative cleavage of carotenoids leads to the production of apocarotenoids and is catalyzed by a family of carotenoid cleavage dioxygenases (21). Many of these apocarotenoids have been indentified in plants, including short-chain products such as β-ionone and long-chain products (21–24). Recently, we described the detection and quantification of all of the long-chain β-apocarotenoids, including β-apo-8′-carotenal, β-apo-10′-carotenal, β-apo-12′-carotenal, β-apo-14′-carotenal, and β-apo-13-carotenone in melons (25). Kopec et al (26) also described the detection of lycopenals in a variety of fruit and vegetables. They found apolycopenal amounts to be present in lycopene-containing foods at ∼0.1% of the amount of lycopene, whereas β-apocarotenals account for ∼1.5% of the amount of β-carotene in β-carotene–containing foods. Thus, long-chain β-apocarotenoids are present in small but significant amounts in β-carotene–containing foods.
There is limited evidence for the presence of apocarotenoids in vivo, but recently Shmarakov et al (27) described a Bcmo1-deficient mouse that may be useful in assessing β-carotene absorption and eccentric cleavage in the mouse model. The Bcmo1-deficient mice were fed a diet rich in β-carotene and, as expected, there was significantly more β-carotene in both the serum and liver when compared with wild-type mice fed the same diet. They were also able to detect β-apo-12′-carotenal and β-apo-10′-carotenal in both the Bcmo1-deficient and wild-type β-carotene–fed mice. There was a consistent trend for β-apo-12′-carotenal and β-apo-10′-carotenal amounts to be higher in serum and livers of Bcmo1-deficient mice than in wild-type mice, but this was only significant (P < 0.05) for hepatic apo-12′-carotenal amounts. They also analyzed the β-carotene feed and found β-apo-8′-carotenal, β-apo-10′-carotenal, β-apo-12′-carotenal, and β-apo-14′-carotenal in the feed itself and in the β-carotene beadlets used in formulating the diet. This may indicate a preferential uptake of β-apo-12′-carotenal and β-apo-10′-carotenal or the eccentric cleavage of β-carotene by BCO2 in vivo.
Eccentric cleavage of β-carotene to apocarotenoids has been shown in various in vitro models (7, 19, 20, 28), but evidence is limited in humans. Ho et al (29) detected the presence of β-apo-8′-carotenal in human plasma after an isotopically labeled dose of 14C-β-carotene. The β-apo-8′-carotenal did not appear in the plasma until 3 d after the dose and the authors suggested that this is evidence that the β-apocarotenals are formed in the peripheral tissues from dietary β-carotene. They also detected a second peak on day 3 that may have been β-apo-8′-carotenyl esters. Confirmation of eccentric cleavage in humans is needed as well as the identification of other apocarotenoids in vivo. Thus, β-apocarotenoids are present in the diet and may be absorbed intact as well as formed in vivo from the eccentric cleavage of β-carotene.
BIOLOGICAL FUNCTIONS OF APOCAROTENOIDS IN MAMMALS
It was proposed that β-apocarotenals formed by eccentric cleavage could be converted to corresponding β-apocarotenoic acids and subsequently to all-trans-retinoic acid, similar to β-oxidation of fatty acids (30, 31). However, more direct evidence of the specific enzymes involved in the oxidation of β-apocarotenals to β-apocarotenoic acids is needed. Given the large number of aldehyde dehydrogenases in mammals and their ubiquitous distribution in tissues, it is likely that most β-apocarotenoids can be converted to the corresponding carboxylic acids.
The interaction between benzo[a]pyrene (an important carcinogen found in cigarette smoke) and β-carotene or β-apo-14′-carotenoic acid to transactivate retinoic acid receptor (RAR) β in normal human bronchial epithelial cells was examined by Prakash et al (32). They found that the downregulation of RARβ by smoke-borne carcinogens was reversed by β-carotene or β-apo-14′-carotenoic acid in normal human bronchial epithelial cells. Another β-carotene metabolite, β-apo-13-carotenone, was used in the same study and was not found to transactivate the RARβ promoter.
β-Carotene metabolites such as β-apo-14′-carotenoic acid was found to stimulate the differentiation of U937 leukemia cells (33), whereas β-apo-12′-carotenoic acid was observed to be a biologically active compound that was capable of inhibiting the proliferation of HL-60 cells (34). Also, both of these β-apocarotenoic acids were found to be effective in inhibiting the growth of breast cancer cells (35). More recently, β-apo-14′-carotenal was shown to inhibit peroxisome proliferator-activated receptor signaling and to promote inflammation in both cell cultures and mice (36, 37) via a mechanism involving the retinoid X receptor (RXR).
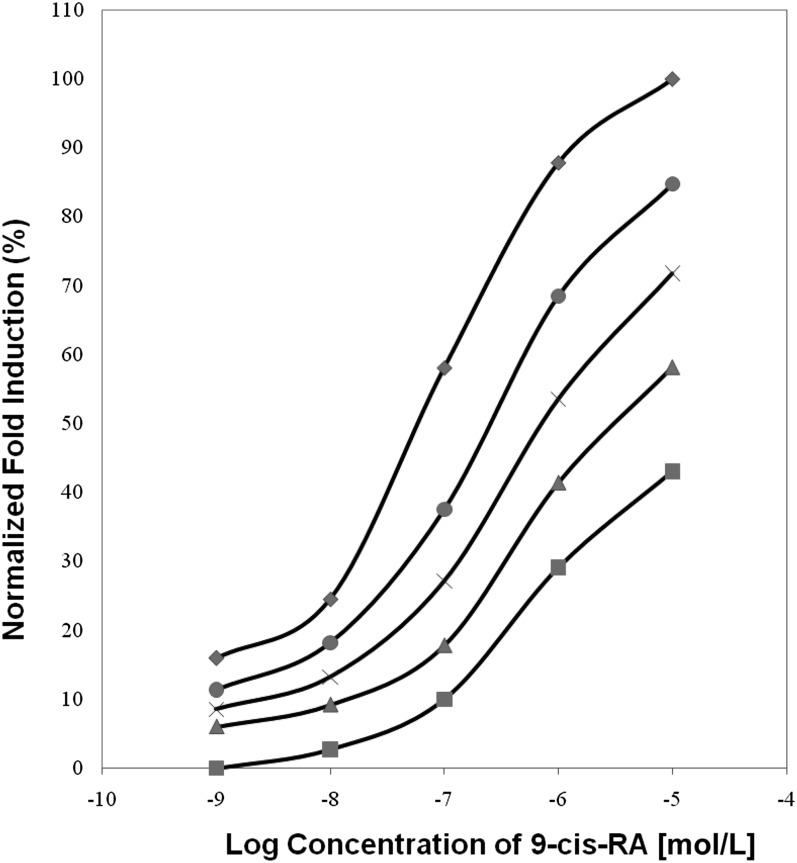
We recently investigated the effects of all possible eccentric cleavage products of β-carotene—ie, β-apocarotenoids as both the aldehydes (or ketones) and the carboxylic acids—on RXRα signaling (38). Transactivation assays were performed to test whether β-apocarotenoids activate or antagonize RXRα. Reporter gene constructs and RXRα were transfected into cells and used to perform these assays. None of the β-apocarotenoids tested activated RXRα. Among the compounds tested, β-apo-13-carotenone was found to antagonize the activation of RXRα by 9-cis-retinoic acid and was effective at concentrations as low as 1 nmol/L (Figure 2). Molecular modeling studies showed that β-apo-13-carotenone binds to RXRα as an antagonist. Assays of direct binding showed that β-apo-13-carotenone binds to RXRα with the same affinity as the known agonist 9-cis-retinoic acid (39). Recent work from our laboratory has shown that β-apo-13-carotenone also functions as an antagonist of all 3 RAR isotypes (RARα, RARβ, and RARγ), and it binds to these with the same affinity as the agonist all-trans retinoic acid (39). β-Apo-14′-carotenal and β-apo-14′-carotenoic acid also bind to RARs but with ∼5-fold lower affinity. All 3 compounds were found to inhibit retinoic acid–induced expression of genes for RARβ and CYP26A1 in human hepatoma cells in culture.
FIGURE 2.
Inhibition of 9-cis-RA–induced transactivation of RXRα in cells transfected with vectors for RXRα and an RXRE-luciferase reporter by β-apo-13-carotenone. The dose-response curve of 9-cis-RA alone (◆). Increasing concentrations of β-apo-13-carotenone were as follows: 10−9 mol/L (•), 10−8 mol/L (×), 10−7 mol/L (▴), and 10−6 mol/L (▪). The fold induction of 10−5 mol/L cis-RA was set to 100%, and the other experimental points were calculated relative to this. Adapted from reference 38. RA, retinoic acid; RXRα, retinoid X receptor α; RXRE, retinoid X response element.
In summary, the available evidence suggests that β-apocarotenoids exert powerful biological effects on mammalian cells. The underlying mechanism or mechanisms involved in these effects may be a disruption of normal signaling through multiple ligand-activated nuclear receptors.
Acknowledgments
We thank our colleagues and collaborators at Ohio State University (Robert Curley, Steven Schwartz, Ken Riedl, Rachel Kopec, and Vanessa Reed) and William Blaner of Columbia University for their contributions to the work summarized here.
The authors’ responsibilities were as follows—EHH: reviewed the literature and wrote the manuscript; and CdS, AE, and MKF: compiled literature in various areas and wrote initial drafts of parts of the manuscript. None of the authors had any conflicts of interest to report.
Footnotes
Abbreviations used: BCO1, β-carotene 15,15′-oxygenase; BCO2, β-carotene 9′,10′-oxygenase; RAR, retinoic acid receptor; RXR, retinoid X receptor.
REFERENCES
- 1.Olson JA, Hayaishi O. The enzymatic cleavage of beta-carotene into vitamin A by soluble enzymes of rat liver and intestine. Proc Natl Acad Sci USA 1965;54:1364–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodman DS, Huang HS. Biosynthesis of vitamin A with rat intestinal enzymes. Science 1965;149:879–80 [DOI] [PubMed] [Google Scholar]
- 3.Olson JA. The absorption of beta-carotene and its conversion into vitamin A. Am J Clin Nutr 1961;9:1–12 [DOI] [PubMed] [Google Scholar]
- 4.Sharma RV, Mathur S, Dmitrovskii A, Das R, Ganguly J. Studies on the metabolism of β-carotene and apo-β-carotenoids in rats and chickens. Biochim Biophys Acta 1976;486:183–94 [DOI] [PubMed] [Google Scholar]
- 5.Hansen S, Maret W. Retinal is not formed in vitro by enzymic central cleavage of beta-carotene. Biochemistry 1988;27:200–6 [DOI] [PubMed] [Google Scholar]
- 6.Tang GW, Wang XD, Russell RM, Krinsky NI. Characterization of beta-apo-13-carotenone and beta-apo-14'-carotenal as enzymic products of the excentric cleavage of beta-carotene. Biochemistry 1991;30:9829–34 [DOI] [PubMed] [Google Scholar]
- 7.Wang XD, Tang GW, Fox JG, Krinsky NI, Russell RM. Enzymatic conversion of beta-carotene into beta-apo-carotenals and retinoids by human, monkey, ferret, and rat tissues. Arch Biochem Biophys 1991;285:8–16 [DOI] [PubMed] [Google Scholar]
- 8.Yeum KJ, dos Anjos Ferreira AL, Smith D, Krinsky NI, Russell RM. The effect of alpha-tocopherol on the oxidative cleavage of beta-carotene. Free Radic Biol Med 2000;29:105–14 [DOI] [PubMed] [Google Scholar]
- 9.Nagao A, During A, Hoshino C, Terao J, Olson JA. Stoichiometric conversion of all trans-beta-carotene to retinal by pig intestinal extract. Arch Biochem Biophys 1996;328:57–63 [DOI] [PubMed] [Google Scholar]
- 10.von Lintig J, Vogt K. Filling the gap in vitamin A research. Molecular identification of an enzyme cleaving beta-carotene to retinal. J Biol Chem 2000;275:11915–20 [DOI] [PubMed] [Google Scholar]
- 11.Wyss A. Cloning and expression of β,β-carotene 15,15′-dioxygenase. Biochem Biophys Res Commun 2000;271:334–6 [DOI] [PubMed] [Google Scholar]
- 12.Lietz G, Lange J, Rimbach G. Molecular and dietary regulation of beta,beta-carotene 15,15'-monooxygenase 1 (BCMO1). Arch Biochem Biophys 2010;52:8–16 [DOI] [PubMed] [Google Scholar]
- 13.Redmond TM, Gentleman S, Duncan T, Yu S, Wiggert B, Gantt E, Cunningham FX. Identification, expression, and substrate specificity of a mammalian beta-carotene 15,15'-dioxygenase. J Biol Chem 2001;276:6560–5 [DOI] [PubMed] [Google Scholar]
- 14.Paik J, During A, Harrison EH, Mendelsohn CL, Lai K, Blaner WS. Expression and characterization of a murine enzyme able to cleave beta-carotene: the formation of retinoids. J Biol Chem 2001;276:32160–8 [DOI] [PubMed] [Google Scholar]
- 15.Leuenberger MG, Engeloch-Jarret C, Woggon W. The reaction mechanism of the enzyme-catalyzed central cleavage of beta-carotene to retinal. Angew Chem Int Ed Engl 2001;40:2613–7 [DOI] [PubMed] [Google Scholar]
- 16.Lindqvist A, Andersson S. Biochemical properties of purified recombinant human beta-carotene 15,15'-monooxygenase. J Biol Chem 2002;277:23942–8 [DOI] [PubMed] [Google Scholar]
- 17.Park CS, Lee SW, Kim YS, Kim EJ, Sin HS, Oh DK, Kim SW, Um SJ. Utilization of the recombinant human beta-carotene-15,15'-monooxygenase gene in Escherichia coli and mammalian cells. Biotechnol Lett 2008;30:735–41 [DOI] [PubMed] [Google Scholar]
- 18.Kim YS, Oh D. Substrate specificity of a recombinant chicken beta-carotene 15,15'-monooxygenase that converts beta-carotene into retinal. Biotechnol Lett 2009;31:403–8 [DOI] [PubMed] [Google Scholar]
- 19.Kiefer C, Hessel S, Lampert JM, Vogt K, Lederer MO, Breithaupt DE, von Lintig J. Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J Biol Chem 2001;276:14110–6 [DOI] [PubMed] [Google Scholar]
- 20.Hu KQ, Liu C, Ernst H, Krinsky NI, Russell RM, Wang WD. The biochemical characterization of ferret carotene-9',10'-monooxygenase catalyzing cleavage of carotenoids in vitro and in vivo. J Biol Chem 2006;281:19327–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Auldridge ME, McCarty DR, Klee HJ. Plant carotenoid cleavage oxygenases and their apocarotenoid products. Curr Opin Plant Biol 2006;9:315–21 [DOI] [PubMed] [Google Scholar]
- 22.Singh H, Cama HR. Enzymatic cleavage of carotenoids. Biochim Biophys Acta 1974;370:49–61 [DOI] [PubMed] [Google Scholar]
- 23.Winterstein A, Hegedus B. On the occurrence of retinene in nature. Hoppe Seylers Z Physiol Chem 1960;321:97–106 [DOI] [PubMed] [Google Scholar]
- 24.Bauernfeind JC, Adams CR, Marusich WL. Carotenes and other vitamin A precursors in animal feed. : Bauernfeind JC. ed. Carotenoids as colorants and vitamin A precursors New York, NY: Academic Press, 1981:563–743 [Google Scholar]
- 25.Fleshman MK, Lester GE, Riedl KM, Kopec RE, Narayanasamy S, Curley RW, Schwartz SJ, Harrison EH. Carotene and novel apocarotenoid concentrations in orange-fleshed Cucmis melo melons: determinations of β-carotene bioaccessibility and bioavailability. J Agric Food Chem 2011;59:4448–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kopec RE, Riedl KM, Harrison EH, Curley RW, Jr, Hruszkewycz DP, Clinton SK, Schwartz SJ. Identification and quantification of apo-lycopenals in fruits, vegetables, and human plasma. J Agric Food Chem 2010;58:3290–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shmarakov I, Fleshman MK, D'Ambrosio DN, Piantedosi R, Riedl KM, Schwartz SJ, Curley RW, von Lintig J, Rubin LP, Harrison EH, et al. Hepatic stellate cells are an important cellular site for beta-carotene conversion to retinoid. Arch Biochem Biophys 2010;504:3–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barua AB, Olson JA. Beta-carotene is converted primarily to retinoids in rats in vivo. J Nutr 2000;130:1996–2001 [DOI] [PubMed] [Google Scholar]
- 29.Ho CC, de Moura FF, Kim SH, Clifford AJ. Excentral cleavage of beta-carotene in vivo in a healthy man. Am J Clin Nutr 2007;85:770–7 [DOI] [PubMed] [Google Scholar]
- 30.Liu C, Wang X-D, Russell RM. Biosynthesis of retinoic acid from β-apo-14′-carotenal in ferret in vivo. J Nutr Biochem 1997;8:652–7 [Google Scholar]
- 31.Wang X-D, Russell RM, Liu C, Stickel F, Smith DE, Krinsky NI. β-Oxidation in rabbit liver in vitro and in the perfused ferret liver contributes to retinoic acid biosynthesis from β-apocarotenoic acids. J Biol Chem 1996;271:26490–8 [PubMed] [Google Scholar]
- 32.Prakash P, Liu C, Hu KQ, Krinsky NI, Russell RM, Wang XD. Beta-carotene and beta-apo-14'-carotenoic acid prevent the reduction of retinoic acid receptor beta in benzo[a]pyrene-treated normal human bronchial epithelial cells. J Nutr 2004;134:667–73 [DOI] [PubMed] [Google Scholar]
- 33.Winum JY, Kamal M, Defacque H, Commes T, Chavis C, Lucas M, Marti J, Montero JL. Synthesis and biological activities of higher homologues of retinoic acid. Farmaco 1997;52:39–42 [PubMed] [Google Scholar]
- 34.Suzuki T, Matsui M, Murayama A. Biological activity of (all-E)-beta-apo-12'-carotenoic acid and the geometrical isomers on human acute promyelocytic leukemia cell line HL-60. J Nutr Sci Vitaminol (Tokyo) 1995;41:575–85 [DOI] [PubMed] [Google Scholar]
- 35.Tibaduiza EC, Fleet JC, Russell RM, Krinsky NI. Excentric cleavage products of β-carotene inhibit estrogen receptor positive and negative breast tumor cell growth in vitro and inhibit activator protein-1–mediated transcriptional activation. J Nutr 2002;132:1368–75 [DOI] [PubMed] [Google Scholar]
- 36.Ziouzenkova O, Orasanu G, Sukhova G, Lau E, Bergen JP, Tang G, Krinsky NI, Dolnikowski GG, Plutzky J. Asymmetric cleavage of beta-carotene yields a transcriptional repressor of retinoid X receptor and peroxisome proliferator-activated receptor responses. Mol Endocrinol 2007;21:77–88 [DOI] [PubMed] [Google Scholar]
- 37.Ziouzenkova O, Plutzky J. Retinoid metabolism and nuclear receptor responses: new insights into coordinated regulation of the PPAR-RXR complex. FEBS Lett 2008;582:32–8 [DOI] [PubMed] [Google Scholar]
- 38.Eroglu A, Hruszkewycz DP, Curley RW, Harrison EH. The eccentric cleavage product of β-carotene, β-apo-13-carotenone, functions as an antagonist of RXRα. Arch Biochem Biophys 2010;504:11–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eroglu A, Hruszkewycz DP, dela Sena C, Narayanasamy S, Riedl KM, Kopec RE, Schwartz SJ, Curley RW, Harrison EH. Naturally-occurring eccentric cleavage products of provitamin A β-carotene function as antagonists of retinoic acid receptors. J Biol Chem 2012;287:15886–95 [DOI] [PMC free article] [PubMed] [Google Scholar]