Abstract
Vitamin A deficiency is a major public health problem in developing countries. Some studies also implicate a suboptimal vitamin A intake in certain parts of the population of the industrialized world. Provitamin A carotenoids such as β-carotene are the major source for retinoids (vitamin A and its derivatives) in the human diet. However, it is still controversial how much β-carotene intake is required and safe. An important contributor to this uncertainty is the lack of knowledge about the biochemical and molecular basis of β-carotene metabolism. Recently, key players of provitamin A metabolism have been molecularly identified and biochemically characterized. Studies in knockout mouse models showed that intestinal β-carotene absorption and conversion to retinoids is under negative feedback regulation that adapts this process to the actual requirement of vitamin A of the body. These studies also showed that in peripheral tissues a conversion of β-carotene occurs and affects retinoid-dependent physiologic processes. Moreover, these analyses provided a possible explanation for the adverse health effects of carotenoids by showing that a pathologic accumulation of these compounds can induce oxidative stress in mitochondria and cell signaling pathways related to disease. Genetic polymorphisms in identified genes exist in humans and also alter carotenoid homeostasis. Here, the advanced knowledge of β-carotene metabolism is reviewed, which provides a molecular framework for understanding the role of this important micronutrient in health and disease.
INTRODUCTION
Carotenoids are isoprenoid compounds (mostly C40) that contain up to 15 conjugated double bonds that are synthesized in plants, certain fungi, and bacteria. Among the 600 described carotenoids, ∼50 exist in the human diet, from which only ∼10 are present in significant amounts in human plasma. Carotenoids exert ≥2 important functions in human physiology. First, these compounds are generally proposed to act as antioxidants and blue light filters (1). The best-known example is in the macula lutea of the human retina where carotenoids such as zeaxanthin and lutein are sequestered in large quantities and specific patterns (2, 3). These so-called macula pigments may lessen chromatic aberration and protect the primate retina against light-induced oxidative stress (4). Second, so-called provitamin A carotenoids such β-carotene are the natural precursors for retinoids (5). To fulfill this function, provitamin A carotenoids must be converted by centric oxidative cleavage to all-trans-retinal (RAL)5. RAL can be then reduced to all-trans-retinol (ROL) and, on esterification, is stored in large quantities in stellate (also called ito cells) in the liver and in other tissues such as lung and fat (6). In the eye, retinoids can be converted to 11-cis-retinal, the visual chromophore, in a complex pathway known as the retinoid cycle (7). The chromophore then binds to a protein moiety (opsin) to establish functional visual pigments. Visual pigments are G-protein–coupled receptors that mediate phototransduction, the process by which light is translated into an electrical (nervous) signal (8). After bleaching, the visual pigments decay into the opsin and RAL, which must be recycled through this pathway to sustain vision (9). Retinoids are also oxidized to all-trans-retinoic acid (RA). RA is a hormone-like compound that binds to a certain class of nuclear receptors called the retinoic acid receptors (RARs) (10). RARs require the retinoid X receptors as obligate heterodimeric partners and, on ligand binding, influence gene expression in many physiologic processes.
In recent years, a large subset of molecular components of retinoid metabolism have been molecularly identified (6). Mutations in the corresponding genes can cause various diseases including blinding diseases such as retinitis pigmentosa and Stargardt disease (11). Moreover, mutations in these genes can cause Matthew-Wood syndrome. This fatal disease is associated with anophthalmia, pulmonary and cardiac malfunctions, and severe mental retardation (12). Furthermore, studies in knockout mouse models for these genes established the critical role of retinoids in embryonic development and later in the life cycle in physiologic processes as diverse as immunity and metabolic control (13–15).
Considering the critical role of retinoids throughout the mammalian life cycle, it is not surprising that dietary vitamin A deficiency (VAD) has utterly devastating consequences. This preventable ailment is associated with severe health problems that can cause blindness and contribute to increased mortality rates of children and pregnant women in Asia and Africa (16). Low vitamin A intake has also been described in Western societies (17). Intervention studies showed that β-carotene supplementation can reduce night blindness and mortality rates in pregnant women in populations at risk of VAD (18). Other strategies involve programs for the cultivation of indigenous β-carotene–rich fruits and vegetables as well as genetic engineering of the carotenoid pathway in major crops such as rice, potatoes, and corn (19–21) to increase β-carotene consumption and prevent vitamin A deficiency.
Despite the importance of β-carotene as major dietary vitamin A precursor, it is still controversial how much intake is required. One must attach some importance to this question because β-carotene is regarded as a “Janus-faced” micronutrient. On the one hand, epidemiologic studies associate high plasma concentrations of β-carotene with decreased risk of disease such as VAD, certain kinds of cancer, cardiovascular disease, and metabolic disease (22). On the other hand, clinical studies showed no positive outcomes of long-term β-carotene supplementation in healthy individuals (23). Surprisingly, β-carotene supplementation even has been associated with adverse health effects in people at risk of disease. Two large clinical trials in the 1990s showed that high-dose supplementation with provitamin A increases the risk of lung cancer and cardiovascular diseases in smokers (24, 25).
The mechanistic basis for the discrepancy of beneficial compared with adverse health effects of β-carotene remains to be defined. A major flaw in this endeavor is the limited knowledge on the molecular and biochemical basis of β-carotene metabolism. Without a proper molecular framework it is impossible to truly understand this process, including critical details about its regulation, its physiologic impact, and the role of genetics. In recent years, much progress has been made in identifying key players in β-carotene metabolism. Mutations in the corresponding genes impair carotenoid metabolism and induce various pathologies in animal models. Polymorphisms in these genes alter β-carotene metabolism in humans as well. This review summarizes the advanced knowledge on the molecular biology, biochemistry, and genetics of β-carotene metabolism.
MOLECULAR IDENTIFICATION AND BIOCHEMICAL CHARACTERIZATION OF KEY COMPONENTS OF CAROTENOID METABOLISM
Carotenoid metabolism can be portrayed in the following framework: These compounds must be absorbed from the intestine, transported in the circulation, and delivered to target tissues. In addition, mechanisms must exist to eliminate excess carotenoids to maintain a normal physiologic state. In the case of provitamin A, carotenoids must first be converted to retinoids, and these primary cleavage products must then be further metabolized for storage and/or production of biologically active retinoid derivatives.
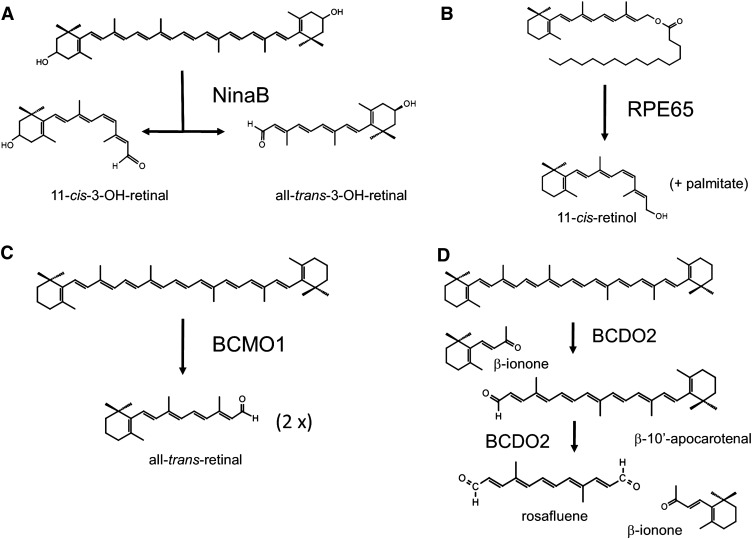
The combination of modern molecular biology and biochemistry as well as the use of a genetically well-defined model organism led to the identification of genes devoted to this process. In particular, the fruit fly, Drosophila melanogaster, proved to be a versatile model for these efforts. Like all animals that are endowed with the ability to detect light through visual pigments, Drosophila has evolved a pathway in which dietary carotenoids are metabolically converted to chromophore (11-cis-3-OH-retinal in the fly). In contrast to vertebrates, carotenoid function is restricted to vision in the fly, thus making this model suited for genetic dissection (26). In fact, molecular players in this pathway were identified in the fly (27). The importance of the different components of carotenoid/chromophore metabolism is strikingly shown by the blindness of respective mutants. In Drosophila, absorption of dietary carotenoids depends on the NinaD protein (28), which is expressed in the midgut (29). This transmembrane protein belongs to the gene family of so-called class B scavenger receptors and facilitates carotenoid uptake from micelles into cells (30). Absorbed carotenoids then must be transported in the hemolymph and are taken up into neuronal and glial cells of the optic lobes. The uptake of carotenoids into both cell types depends on an additional NinaD-related scavenger receptor named Santa Maria (29). Both of these cell types also express NinaB, which converts carotenoids into retinoids by symmetric oxidative cleavage at the 15,15′ position (29, 31, 32). Recent research showed that NinaB is a bifunctional enzyme that possesses not only a carotenoid-oxygenase but also intrinsic isomerase activity (33) (Figure 1). The reaction catalyzed by NinaB couples the energy delivering oxidative cleavage reaction with the energy consuming trans-to-cis isomerization reaction of double bonds in the carbon backbone of carotenoids. Accordingly, the cleavage of carotenoids such as zeaxanthin by NinaB results in the formation one molecule of 11-cis and one molecule of all-trans-3-hydroxy-retinal (33). The 11-cis-stereoisomer binds to opsin to generate functional visual pigments. The all-trans-stereoisomer cleavage product is converted in a light-dependent pathway to chromophore (33, 34). The latter pathway also contributes to the regeneration of chromophore (35).
FIGURE 1.
Transformations of carotenoids and apocarotenoids catalyzed by different metazoan carotenoid-oxygenases and all-trans-to-11-cis isomerases. A: Insect NinaB catalyzes a combined carotenoid cleavage and isomerase reaction. B, C: In mammals, carotenoid cleavage reaction and isomerase reaction are separated to 2 distinct proteins referred to as RPE65 and BCMO1. D: In mammals, a third family member is localized to mitochondria and catalyzes carotenoid breakdown by successively removing the ionone ring sites at position 9,10 and 9′,10′ in the carbon backbone of carotenoids. BCDO2, β,β-carotene-9′,10′-dioxygenase 2; BCMO1, β,β-carotene-15,15′-monooxygenase 1; RPE65, retinal pigment epithelium-specific 65-kDa protein.
Key components of carotenoid metabolism are evolutionarily well conserved but have adapted to the specific requirements of mammalian carotenoid/retinoid metabolism and functions. Studies in cell culture and in knockout mouse models showed that the NinaD-related scavenger receptor class B type 1 (SR-B1) mediates carotenoid absorption into cells (36, 37). SR-B1 also facilitates the uptake of other isoprenoid compounds, including non–provitamin A carotenoids, tocopherol, and cholesterol (38–40). In addition, a second NinaD-related scavenger receptor, CD36, has been implicated into the cellular uptake of carotenoids (41). For carotenoid conversion, 3 different NinaB homologs have been identified and their roles in cartenoid metabolism have been analyzed. The β,β-carotene-15,15′-monooxygenase (BCMO1) catalyzes the conversion of a limited number of provitamin A carotenoids to RAL (42–44) (Figure 1). Recombinant human BCMO1 catalyzes the cleavage of provitamin A carotenoid substrates with at least one nonsubstituted β-ionone ring, such as β-carotene, α-carotene, or β-cryptoxanthin, but fails to promote cleavage of non–provitamin A carotenoids such as lycopene or zeaxanthin (45). Studies in knockout mouse models showed that BCMO1 is the key enzyme for retinoid production (46). In humans, a heterozygotic mutation in BCMO1 was described with evidence of both elevated plasma β,β-carotene concentrations and low plasma retinol concentrations (47). In addition, common genetic polymorphisms exist in the BCMO1 gene that alter β-carotene metabolism in affected individuals (48, 49).
The second NinaB homolog, the retinal pigment epithelium-specific 65 kDa protein (RPE65), is not a carotenoid-oxygenase but the long-sought retinoid isomerase in the mammalian visual cycle. RPE65 was identified as an abundant protein in the retinal pigmented epithelium of the eyes (50). In initial studies, RPE65 was proposed to act as a retinoid binding protein (51, 52). However, 3 groups independently showed that RPE65 is an enzyme that catalyzes the conversion of retinyl esters (REs) to 11-cis-retinol (53–55) (Figure 1). In humans, mutations in RPE65 result in chromophore deficiency and thus blindness (56). RPE65-deficient mice accumulate REs in the RPE and lack the visual chromophore in photoreceptors (57, 58). Mice with mutations in RPE65 also have been used as an animal model to analyze the consequences of VAD for rod and cone photoreceptors of the retina (59, 60).
The third mammalian NinaB homolog is β-carotene-9′,10′-dioxygenase 2 (BCDO2). This gene has been cloned from human, mouse, zebrafish, and ferrets, and recombinant ferret and mouse BCDO2 has been biochemically characterized (61, 62). Initially, expression of BCDO2 in a β-carotene–accumulating Escherichia coli strain showed that it catalyzes the formation of β-10′-carotenal and β-ionone. Similar results were obtained with recombinant ferret BCDO2. In addition to β-carotene, BCDO2 also used the acyclic carotene lycopene as a substrate. Recombinant ferret BCDO2 cleaves 5-cis- and 13-cis-isomers of lycopene but not the all-trans-stereoisomer. Recent research showed that BCDO2 has much broader substrate specificity for cartenoids. In addition to carotenes, this enzyme also metabolizes xanthophylls such as zeaxanthin and lutein (63, 64). In this reaction, BCDO2 can remove both ionone ring sites of a carotenoid by oxidative cleavage at positions 9,10 and 9′,10′ of a carotenoid substrate (Figure 1). Mutations in BCDO2 have been shown to alter carotenoid homeostasis of tissues in chickens, sheep, and cows (65–68). Moreover, a BCDO2-deficient mouse model accumulates xanthophylls such as zeaxanthin and lutein in the form of their 3-oxo-derivatives (64). In accordance with the broad substrate specificity of this enzyme, lycopene metabolism also has been shown to be impaired in BCDO2-deficient mice (69). Thus, the third family member, BCDO2, plays a more general role in maintaining carotenoid homeostasis in tissues.
Taken together, genes encoding molecular players of carotenoid metabolism, including carotenoid-oxygenases, have been molecularly identified and biochemically characterized. Insect genomes encode a carotenoid-isomerooxygenase referred to as NinaB. In mammals, oxidative cleavage activity of β-carotene and all-trans-to-11-cis isomerization activity of the retinoid cleavage product are separated to 2 distinct proteins, BCMO1 and RPE65. A third mammalian member of this protein family, BCDO2, has broad substrate specificity and converts provitamin A and non–provitamin A carotenoids. The identification of these genes provided molecular tools to address important questions with regard to carotenoid function and homeostasis. It also allowed generation of knockout mouse models to study the pathophysiologic consequences of impairment in β-carotene metabolism.
REGULATION OF INTESTINAL beta-CAROTENE METABOLISM AND DISTRIBUTION OF RETINOID CLEAVAGE PRODUCTS TO TISSUES
The formal first step in vitamin A metabolism is the conversion of β-carotene to RAL. BCMO1 is expressed in enterocytes of the intestine where this conversion takes place (32, 70). The primary cleavage product is subsequently converted to ROL and RE. These REs, along with REs produced from preformed dietary vitamin A, are packaged into chylomicrons that are secreted into the blood (71). Approximately 66–75% of REs contained in chylomicrons are taken up by the liver and stored as esters in hepatic stellate cells. The remaining REs from chylomicrons are taken up by target tissues that include adipose tissue, heart, muscle, lungs, reproductive organs, and bone marrow. The clearance of chylomicron REs appears to involve the actions of lipoprotein lipase (72). From liver stores, vitamin A is secreted as ROL bound to serum retinol-binding protein (RBP) (73, 74). Retinol bound to RBP is distributed via the circulation (75). The uptake of RBP-bound ROL into specific target tissues, such as the eye, is mediated by the multidomain membrane protein stimulated by retinoic acid 6 (STRA6) (76). The transfer of retinol from RBP to the cytosol via STRA6 is bidirectional, which strongly suggests retinol channel/transporter and is driven by esterification of ROL to RE (77). STRA6 is expressed in several blood-organ barriers such as Sertoli cells, yolk sac, and chorioallantoic placenta, choroid plexi (78), and retinal pigmented epithelial cells (76, 78, 79).
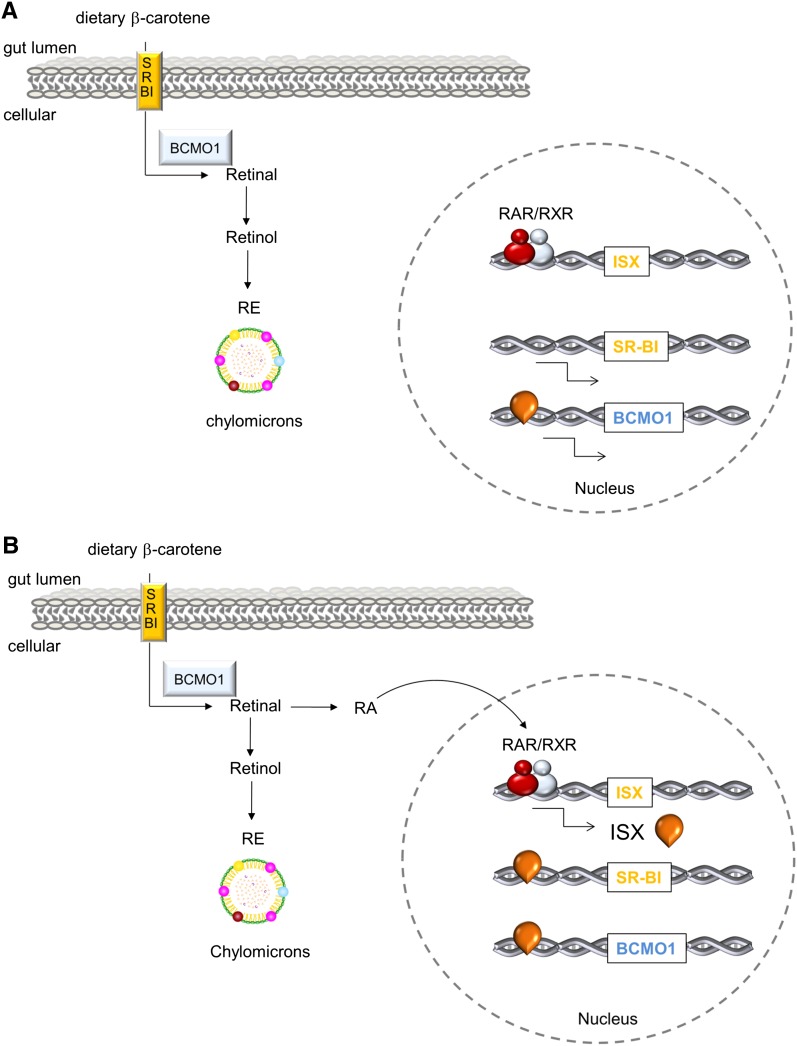
In contrast to preformed vitamin A, intestinal carotenoid uptake is saturable and protein mediated (28, 36). Evidence from cell culture studies and animal models suggests that intestinal β-carotene uptake and conversion is a regulated process involving the intestine-specific homeobox transcription factor (ISX) (80). In ISX-deficient mice, SR-B1 and BCMO1 expression is significantly enhanced and their expression extends to more distal parts of the intestine (80). Interestingly, ISX expression is reduced in VAD and SR-B1 and BCMO1 expression is increased (80), which implicates this transcription factor as a principal regulatory factor for intestinal carotenoid metabolism. The crosstalk between β-carotene/retinoid metabolism and ISX is mediated via a retinoic acid–responsive element in the ISX promoter to which RARs can bind (81). These findings suggest that RA produced from dietary vitamin A influences β-carotene bioavailability and conversion rates. Indeed, intestinal SR-B1 and BCMO1 expression is strongly increased in the intestine of mice with VAD (80, 81). Gavage of vitamin A–deficient mice with RA induced ISX expression, which in turn decreased SR-B1 and BCMO1 expression (81). Thus, ISX acts as a retinoic acid–sensitive “gatekeeper” for vitamin A production (for the current model; Figure 2). Negative feedback regulation of intestinal β-carotene absorption also has been shown in BCMO1-deficient mice. When fed a diet that provides β-carotene as the sole source for vitamin A, these mice accumulate large amounts of β-carotene because of genetic disruption of the vitamin A–forming enzyme. In the intestine, these mice showed highly elevated SR-B1 expression, whereas ISX expression was very low. When the diet was supplemented with a combination of β-carotene and vitamin A, ISX expression was induced and SR-B1 expression was reduced in the intestine (81). Consequently, much less β-carotene was absorbed and accumulated in this mutant mouse model fed diets supplemented with preformed vitamin A.
FIGURE 2.
Intestinal vitamin A production is under negative feedback regulation of RA. A: In vitamin A deficiency, SR-B1 (β-carotene absorption) and BCMO1 (β-carotene conversion) are highly expressed in the intestine. β-Carotene is efficiently taken up and converted to all-trans-retinal. The primary cleavage product then is converted to all-trans-retinol and esterified to REs that are incorporated into chylomicrons for transport. B: In vitamin A sufficiency, RA is produced that activates the expression of transcription factor ISX. In turn, ISX represses intestinal expression of SR-B1 and BCMO1, thus reducing β-carotene utilization for vitamin A production. BCMO1, β,β-carotene-15,15′-monooxygenase 1; ISX, intestine-specific homeobox; RA, all-trans-retinoic acid; RAR, retinoic acid receptor; RE, retinyl ester; RXR, retinoid X receptor; SR-B1, scavenger receptor class B type 1.
Studies in human colonic cell lines indicated that RA-dependent regulation of ISX expression is well conserved in humans (81). Therefore, this regulatory mechanism should be considered for recommendations for β-carotene intake. On the basis of the findings in mice, it might be predicted that there would be significant differences in β-carotene utilization in individuals with a high compared with a low vitamin A status. In VAD, enhanced and distally extended expression of SR-B1 and BCMO1 in the small intestine ensures that even small amounts of dietary β-carotene can be absorbed and used for vitamin A production. When sufficient dietary vitamin A is available, absorption and conversion of β-carotene is repressed at the level of SR-B1 and BCMO1, respectively. This negative feedback control also may explain why high-dose β-carotene supplementation does not result in hypervitaminosis A. In contrast, high-dose vitamin A supplementation can result in this pathology because of the lack of intestinal control mechanisms for its uptake.
BCMO1 AFFECTS RETINOID METABOLISM IN THE EMBRYO AND PERIPHERAL TISSUES OF ADULTS
In humans, substantial amounts of absorbed β-carotene are not cleaved in the intestine by BCMO1 (≤40% of dietary intake) (82) and, along with other lipids, become incorporated in chylomicrons and are found associated with circulating lipoproteins (83). Circulating carotenoids in association with lipoproteins can be then taken up by the lipoprotein-specific receptors. In mice, BCMO1 is expressed in both the intestine and liver but also in peripheral tissues, including the mammalian embryo (42, 43, 61). In humans, analyses of BCMO1 mRNA expression levels showed a comparable picture (84). Immunohistology showed that BCMO1 is expressed in the mucosal and glandular cells of stomach, small intestine, and colon; in liver; in cells comprising the exocrine glands in pancreas, glandular cells in prostate, endometrium, and mammary tissue; in kidney tubular cells; and in keratinocytes of skin squamous epithelium (85). In addition, steroidogenic cells in testis, ovary, and the adrenal gland, as well as skeletal muscle cells, express BCMO1 (85). Moreover, BCMO1 is abundantly expressed in the retinal pigmented epithelium of the eyes (84, 86). Together, these studies suggest that a local tissue-specific conversion of β-carotene can contribute to retinoid metabolism in peripheral tissues.
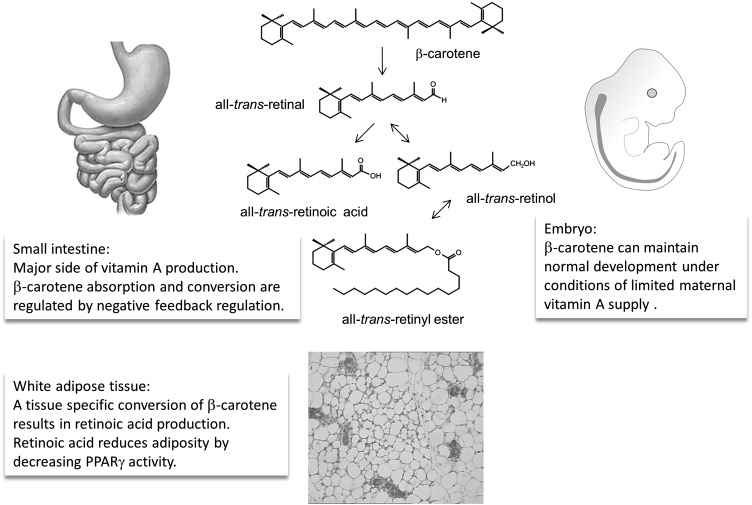
Studies in BCMO1 knockout mouse models provided evidence that such local conversion of β-carotene can influence retinoid-dependent processes in tissues. Kim et al (87) provided evidence that BCMO1 can maintain retinoid homeostasis in embryonic tissues of vitamin A–deficient mice. In an elegant genetic approach, they generated RBP BCMO1−/−−/− double-mutant female mice. In RBP deficiency, mice depend on a continuous dietary vitamin A supply (RE in chylomicrons) (88). These double-mutant mice were crossed with RBP−/− male mice so that dams were deficient both for RBP and BCMO1, whereas their offspring carried a functional BCMO1 allele. Pregnant dams were then subjected to dietary vitamin A restriction and received β-carotene or vehicle by intraperitoneal injection. The offspring developed severe embryonic malformations when supplemented with vehicle only. In contrast, β-carotene could maintain retinoid homeostasis and promoted normal development of the offspring (with a functional BCMO1 allele). Thus, β-carotene can serve as a retinoid precursor in embryonic tissue. A developmental role of BCMO1 also has been reported in lower vertebrates such as the zebrafish (89).
In addition, the study by Kim et al (87) provided evidence of crosstalk between β-carotene metabolism and retinoid homeostasis that involves the BCMO1 protein per se. These researchers reported that dietary vitamin A restriction induced more severe developmental malformations in RBP−/− BCMO1−/− double-mutant offspring than in RBP−/− single-mutant offspring. This observation was unexpected because diets essentially provided no β-carotene in this experiment. The authors found that embryonic retinoid metabolism was altered in BCMO1-deficient embryos even in the absence of β-carotene. Lecithin:retinol acyltransferase (LRAT) expression was significantly reduced compared with embryos carrying a wild-type BCMO1 allele. Accordingly, BCMO1-deficient embryos had lower concentrations of RE and RA, the developmentally active form of vitamin A. Thus, the authors established a critical role of BCMO1 protein for maintaining embryonic retinoid metabolism.
In adult mammals, BCMO1 gene transcription is under the control of peroxisome proliferator–activated receptor γ (PPARγ) (90), which is a key transcription factor of adipocyte differentiation and lipogenesis in mature adipocytes. The finding of responsiveness of BCMO1 to PPAR suggests a possible link between the regulation of fatty acid and β-carotene metabolism. Studies in BCMO1 knockout mice showed that this mutant mouse model develops dyslipidemia, is more susceptible to diet-induced obesity, and shows increased expression of PPARγ-induced genes in adipocytes, even when supplemented well with vitamin A (46). Furthermore, β-carotene supplementation reduced body adiposity in female wild-type (WT) mice, whereas this effect was absent in age- and sex-matched BCMO1−/− mice. Genome-wide microarray analysis of inguinal white adipose tissue showed a decrease in mRNA expression of PPARγ target genes. Consistently, the expression of this key transcription factor for lipogenesis decreased significantly at both the mRNA and protein levels (91).
To further establish the connection between PPARγ and BCMO1, the expression of this enzyme was investigated during PPARγ-dependent differentiation of NIH 3T3-L1 preadipocyte cells to mature adipocytes (92). During this process, BCMO1 is induced (5–7-fold) both at the mRNA and protein levels. Treatment of mature adipocytes with β-carotene resulted in reduced staining for lipids (Oil Red O stain) and triglyceride content, whereas microscopic inspection showed that lipid droplets were reduced in size and number. Interestingly, mRNA and protein expression levels of PPARγ and its downstream target fatty acid–binding protein 4/aP2 were also significantly reduced in β,β-carotene–treated mature adipocytes. No such observations were apparent in vitamin A–treated mature adipocytes.
To determine the biologically active retinoid derivative that mediates this effect, HPLC analysis for lipids was performed in β-carotene and ROL-treated mature adipocytes. β-Carotene metabolized to RA, whereas ROL metabolized to REs. In fact, endogenous RA derived from β-carotene cleavage decreased the expression of key lipogenic transcription factors, namely CCAAT/enhancer-binding protein α and PPARγ. It has been shown that the inhibitory effects of RA on adipogenesis are mediated by RARs (93). In addition, RA treatment of mice reduces fat depots and decreases PPARγ expression and other signaling pathways in fat (94, 95). In line with this concept, a 2.8-fold induction of Cyp26a1 at the transcriptional level (mRNA) was observed. Cyp26a1 is an RA hydroxylase, whose mRNA expression is induced by RA in a strictly RAR-dependent manner. Importantly, effects of β-carotene on adipocytes could be prevented by pretreatment of cells with LE540, a RAR pan-antagonist. In agreement with RA production, β-carotene effects were dependent on canonical RAR signaling.
The effect of β-carotene and ROL supplementation on the adipose tissue biology of mice were also analyzed in LRAT−/− mice (96). The examination of inguinal white adipose tissue from these mice showed no changes in the mRNA expression of either PPARγ or its downstream target aP2 as compared with LRAT−/− mice on vitamin A–deficient and vitamin A–sufficient diets, respectively. However, gavage of β-carotene but not ROL resulted in a 3-fold decrease in PPARγ and aP2 mRNA expression and a 2.1-fold increase in Cyp26a1 mRNA expression. Thus, studies in cell cultures and in mouse models provided evidence that β-carotene is a physiologic precursor for RA in adipose tissues and affects adipocyte biology.
Cumulatively, studies in knockout mice provide evidence that BCMO1 can influence tissue, specifically retinoid-dependent processes as diverse as embryonic development and metabolic control (Figure 3). Hence, the role of BCMO1 in the large panoply of retinoid-dependent biological processes deserves further research.
FIGURE 3.
Schematic overview of the role of BCMO1 and β-carotene in mammalian biology. BCMO1, β,β-carotene-15,15′-monooxygenase 1; PPARγ, peroxisome proliferator–activated receptor γ.
BCDO2, CAROTENOID HOMEOSTASIS, AND MITOCHONDRIA
The broad substrate specificity of the second mammalian carotenoid-oxygenase, BCDO2, implicates this enzyme in the metabolism of both provitamin A and non–provitamin A carotenoids. A critical role of BCDO2 for carotenoid metabolism was substantiated by findings in chickens. The yellow skin color of chickens (xanthophylls) is determined by a cis-acting and tissue-specific regulatory mutation that inhibits expression of BCDO2 in skin (68). In addition, it was shown that mutations in the BCDO2 gene cause the yellow fat phenotype (xanthophyll accumulation) of sheep (97). Moreover, the carotenoid content of cow milk and serum was shown to be significantly altered by a mutation that abolished BCDO2 function (66).
To further analyze the physiologic role for BCDO2, a knockout mouse model was established (97). Ablation of BCDO2 expression was confirmed by protein analysis of isolated hepatic mitochondria by using a BCDO2-specific antibody. BCDO2−/− mice developed normally and were fertile when fed a standard chow diet. Dietary supplementation of BCDO2−/− mice with either zeaxanthin or lutein showed impairment in carotenoid metabolism. HPLC analysis of lipid extracts of livers showed that these xanthophylls accumulated in the form of 3-oxo-metabolites (64). These compounds have been recently described as major xanthophyll derivatives in WT mice with very short half-life times (98). Notably, no accumulation of apocarotenoid cleavage products was found in WT mice, indicating that BCDO2-derived cleavage products are rapidly metabolized/degraded (64).
A consequence of carotenoid accumulation in the BCDO2−/− mice was apparent in liver histology. Histologic analysis showed that BCDO2+/− and BCDO2−/− mice developed liver steatosis with large lipid droplets in hepatocytes and a significantly increased triacylglycerol content. This phenotype was not evident in age-matched BCDO2−/− mice that were fed a chow diet, indicating that this pathology was induced by carotenoid accumulation. Carotenoids also accumulated in BCDO2+/− mice, which indicated that loss of a single allele can cause haplo-insufficiency and impairs carotenoid homeostasis. Furthermore, BCDO2 mRNA expression analysis by quantitative reverse transcriptase–polymerase chain reaction showed that BCDO2 expression was induced 7-fold in WT mice as compared with BCDO2+/− mice, thereby preventing accumulation of these carotenoids.
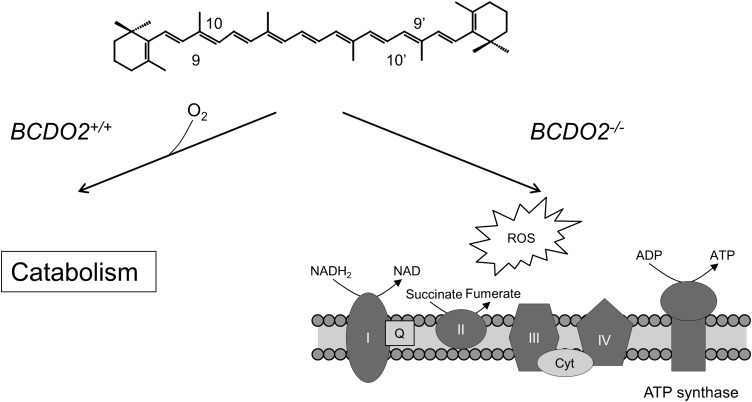
In agreement with the enzyme's intracellular localization, carotenoids accumulated in hepatic mitochondria of BCDO2−/− mice. Carotenoids are lipophilic molecules with an extended polyene chromophore that can act as an electron sink. In addition, these rigid lipids also may disturb membrane topology. Immunoblot analysis of manganese superoxide dismutase (MnSOD), an enzyme indicative of general mitochondrial stress and dysfunction, showed a 9-fold increase in hepatic mitochondria isolated from carotenoid-supplemented BCDO2−/− mice as compared with WT mice. Detailed analysis of the mitochondrial electron transport chain showed that ADP-dependent respiration, respiration with high ADP, and uncoupled respiration rates of mitochondria isolated from BCDO2−/− mice fed the carotenoid diets were all significantly reduced as compared with siblings on the similar but carotenoid-free diet.
Disturbances in the mitochondrial electron transport chain generally result in the production of excessive reactive oxygen species (ROS). Indeed, in vitro studies in human liver HepG2 cells showed that zeaxanthin and lutein or their 3-dehyro derivatives, the latter isolated from livers of BCDO2−/− mice, all could induce ROS production in a dose-dependent manner. In addition, treatment of these cells with β-carotene also evidently resulted in ROS. Conversely, overexpressing recombinant murine BCDO2 in HepG2 cells significantly reduced ROS production on β-carotene administration, further showing the importance of this carotenoid-oxygenase in preventing carotenoid-induced oxidative stress. Accordingly, determination of the mitochondrial membrane potential by using the well-established JC-1 dye technique yielded similar outcomes. Again, overexpressing recombinant murine BCDO2 in HepG2 cells significantly ameliorated membrane depolarization in carotenoid-treated cells.
In addition, various components of oxidative stress–regulated signaling pathways increased in hepatic and cardiac tissues of BCDO2−/− mice receiving carotenoid supplementation. The induction of these cell signaling pathways helps accommodate mice to carotenoid-induced oxidative stress. However, a constitutive activation of oxidative stress–related pathways also is associated with disease, including cardiovascular and neurodegenerative disease, type 2 diabetes, and cancer.
For BCDO2, a single base pair polymorphism in intron 2 has been identified and correlates with altered blood concentrations of IL-18, a proinflammatory cytokine associated with type 2 diabetes and cardiovascular disease (99). A relation between BCDO2 and cardiovascular disease has also been observed in mice (100). An induction of cell signaling pathways related to oxidative stress and cell proliferation has been previously described in rats and ferrets supplemented with supraphysiologic doses of β-carotene (101, 102). Large clinical trials showed that high-dose β-carotene supplementation can harm individuals at risk of disease related to oxidative stress, such as smokers and asbestos workers (for review, see references 103 and 104). Thus, studies in knockout mice implicate a polymorphism in the BCDO2 gene as a genetic risk factor for carotenoid-induced impairment and may provide a mechanistic explanation for the adverse health effects of high-dose supplementation with these compounds (Figure 4).
FIGURE 4.
BCDO2 is a mitochondrial protein that protects these organelles against oxidative stress. In wild-type mice (BCDO2+/+), carotenoids are catabolized by BCDO2, thus preventing mitochondrial accumulation of these compounds. In mutant mice (BCDO2−/−), carotenoids accumulate in mitochondria and impair respiration (complexes I to IV) and cause oxidative stress. BCDO2, β,β-carotene-9′,10′-dioxygenase 2; Cyt, cytochrome C; Q, ubiquinone; ROS, reactive oxygen species.
CONCLUSIONS
It is well established that β-carotene is a major source of vitamin A in the human diet. However, β-carotene metabolism was not well defined for many years because of a lack of knowledge about molecular factors devoted to this process. In recent years, genes encoding key factors for this metabolism have been identified, including carotenoid transporters and metabolizing enzymes. Studies in knockout mouse models unequivocally identified BCMO1 as the key enzyme for vitamin A production. Analysis in this mouse model also helped to unravel a diet-responsive regulatory network that controls intestinal vitamin A production. In this process, intestine-specific homeodomain transcription factor ISX acts as a retinoic acid–sensitive gatekeeper that controls intestinal β-carotene absorption and conversion to vitamin A. In addition, a large body of evidence has shown that a tissue-specific conversion of β-carotene via BCMO1 can influence vitamin A–dependent processes. Furthermore, β-carotene conversion to vitamin A can maintain normal development in the embryo under conditions of limited maternal vitamin A supply. A tissue-specific conversion of β-carotene to retinoids can also influence retinoid-dependent processes in adult mammals. In adipocytes, a reciprocal influence between the β-carotene metabolite retinoic acid and PPARγ activity has been shown. Importantly, preformed dietary vitamin A does not show this activity, which implicates a unique role of β-carotene for this process. Genetic studies in chickens, cows, and sheep showed that mutations in the BCDO2 gene can alter carotenoid homeostasis. A critical role of BCDO2 for this process has been confirmed in a mouse model. In accordance with the mitochondrial localization of this enzyme, carotenoids accumulated in these organelles and impaired respiration. This impairment caused oxidative stress and induced cell signaling pathways related to cell survival and proliferation. This action of carotenoids may explain the adverse health effects of these compounds reported in clinical studies and identifies BCDO2 as a key player for their prevention. It also shows that carotenoid homeostasis—such as for other isoprenoid lipids such as cholesterol—must be tightly regulated to avoid disease. Polymorphisms in the genes encoding key components of carotenoid metabolism exist in the human population. In the future, this genetic variability as well as regulatory aspects must be considered when effects of carotenoids are evaluated. However, we still are at the beginning in our of understanding of the complex role of β-carotene in health and disease. The advancing knowledge on β-carotene metabolism contributes to the understanding of the biochemical, physiologic, developmental, and medical roles of this important micronutrient.
Acknowledgments
I am grateful to Leslie Webster for critical comments and important suggestions on the manuscript. I apologize to all of the investigators whose research could not be appropriately cited because of space limitations. I extend a special thanks to my present and former coworkers for their great enthusiasm and research contributions.
The author declared no conflicts of interest.
Footnotes
Abbreviations used: BCDO2, β,β-carotene-9′,10′-dioxygenase 2; BCMO1, β,β-carotene-15,15′-monooxygenase 1; ISX, intestine-specific homeobox; LRAT, lecithin:retinol acyltransferase; PPARγ, peroxisome proliferator-activated receptor γ; RA, all-trans-retinoic acid; RAL, all-trans-retinal; RAR, retinoic acid receptor; RBP, retinol-binding protein; RE, retinyl ester; ROL, all-trans-retinol; ROS, reactive oxygen species; RPE65, retinal pigment epithelium-specific 65-kDa protein; SR-B1, scavenger receptor class B type 1; STRA6, stimulated by retinoic acid 6; VAD, vitamin A deficiency; WT, wild type.
REFERENCES
- 1.Demmig-Adams B, Adams WW., III Antioxidants in photosynthesis and human nutrition. Science 2002;298:2149–53 [DOI] [PubMed] [Google Scholar]
- 2.Bernstein PS, Yoshida MD, Katz NB, McClane RW, Gellermann W. Raman detection of macular carotenoid pigments in intact human retina. Invest Ophthalmol Vis Sci 1998;39:2003–11 [PubMed] [Google Scholar]
- 3.Barker FM, II, Snodderly DM, Johnson EJ, Schalch W, Koepcke W, Gerss J, Neuringer M. Nutritional manipulation of primate retinas, V: effects of lutein, zeaxanthin, and n-3 fatty acids on retinal sensitivity to blue-light-induced damage. Invest Ophthalmol Vis Sci 2011;52:3934–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krinsky NI, Landrum JT, Bone RA. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu Rev Nutr 2003;23:171–201 [DOI] [PubMed] [Google Scholar]
- 5.Moore T. Vitamin A and carotene: VI. The conversion of carotene to vitamin A in vivo. Biochem J 1930;24:692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Ambrosio DN, Clugston RD, Blaner WS, Vitamin A. Metabolism. An Update. 2011;3:63–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wald G. Molecular basis of visual excitation. Science 1968;162:230–9 [DOI] [PubMed] [Google Scholar]
- 8.Palczewski K. G protein-coupled receptor rhodopsin. Annu Rev Biochem 2006;75:743–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Lintig J, Kiser PD, Golczak M, Palczewski K. The biochemical and structural basis for trans-to-cis isomerization of retinoids in the chemistry of vision. Trends Biochem Sci 2010;35:400–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J 1996;10:940–54 [PubMed] [Google Scholar]
- 11.Palczewski K. Retinoids for treatment of retinal diseases. Trends Pharmacol Sci 2010;31:284–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blaner WS. STRA6, a cell-surface receptor for retinol-binding protein: the plot thickens. Cell Metab 2007;5:164–6 [DOI] [PubMed] [Google Scholar]
- 13.Mark M, Ghyselinck NB, Chambon P. Function of retinoid nuclear receptors: lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annu Rev Pharmacol Toxicol 2006;46:451–80 [DOI] [PubMed] [Google Scholar]
- 14.Ziouzenkova O, Plutzky J. Retinoid metabolism and nuclear receptor responses: new insights into coordinated regulation of the PPAR-RXR complex. FEBS Lett 2008;582:32–8 [DOI] [PubMed] [Google Scholar]
- 15.Agace WW. T-cell recruitment to the intestinal mucosa. Trends Immunol 2008;29:514–22 [DOI] [PubMed] [Google Scholar]
- 16.Underwood BA. Vitamin A deficiency disorders: international efforts to control a preventable “pox”. J Nutr 2004;134:231S–6S [DOI] [PubMed] [Google Scholar]
- 17.Feskanich D, Singh V, Willett WC, Colditz GA. Vitamin A intake and hip fractures among postmenopausal women. JAMA 2002;287:47–54 [DOI] [PubMed] [Google Scholar]
- 18.Christian P, West KP, Jr, Khatry SK, Katz J, LeClerq SC, Kimbrough-Pradhan E, Dali SM, Shrestha SR. Vitamin A or beta-carotene supplementation reduces symptoms of illness in pregnant and lactating Nepali women. J Nutr 2000;130:2675–82 [DOI] [PubMed] [Google Scholar]
- 19.Ye X, Al-Babili S, Kloti A, Zhang J, Lucca P, Beyer P, Potrykus I. Engineering the provitamin A (beta-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science 2000;287:303–5 [DOI] [PubMed] [Google Scholar]
- 20.Diretto G, Al-Babili S, Tavazza R, Papacchioli V, Beyer P, Giuliano G. Metabolic engineering of potato carotenoid content through tuber-specific overexpression of a bacterial mini-pathway. PLoS ONE 2007;2:e350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naqvi S, Zhu C, Farre G, Ramessar K, Bassie L, Breitenbach J, Perez Conesa D, Ros G, Sandmann G, Capell T, et al. Transgenic multivitamin corn through biofortification of endosperm with three vitamins representing three distinct metabolic pathways. Proc Natl Acad Sci USA 2009;106:7762–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krinsky NI, Johnson EJ. Carotenoid actions and their relation to health and disease. Mol Aspects Med 2005;26:459–516 [DOI] [PubMed] [Google Scholar]
- 23.Hennekens CH, Buring JE, Manson JE, Stampfer M, Rosner B, Cook NR, Belanger C, LaMotte F, Gaziano JM, Ridker PM, et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med 1996;334:1145–9 [DOI] [PubMed] [Google Scholar]
- 24.The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med 1994;330:1029–35 [DOI] [PubMed] [Google Scholar]
- 25.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Valanis B, Williams JH, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med 1996;334:1150–5 [DOI] [PubMed] [Google Scholar]
- 26.Stephenson RS, O'Tousa J, Scavarda NJ, Randall LL, Pak WL. Drosophila mutants with reduced rhodopsin content. Symp Soc Exp Biol 1983;36:477–501 [PubMed] [Google Scholar]
- 27.Wang T, Montell C. Phototransduction and retinal degeneration in Drosophila. Pflugers Arch 2007;454:821–47 [DOI] [PubMed] [Google Scholar]
- 28.Kiefer C, Sumser E, Wernet MF, Von Lintig J. A class B scavenger receptor mediates the cellular uptake of carotenoids in Drosophila. Proc Natl Acad Sci USA 2002;99:10581–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang T, Jiao Y, Montell C. Dissection of the pathway required for generation of vitamin A and for Drosophila phototransduction. J Cell Biol 2007;177:305–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voolstra O, Kiefer C, Hoehne M, Welsch R, Vogt K, von Lintig J. The Drosophila class B scavenger receptor NinaD-I is a cell surface receptor mediating carotenoid transport for visual chromophore synthesis. Biochemistry 2006;45:13429–37 [DOI] [PubMed] [Google Scholar]
- 31.von Lintig J, Dreher A, Kiefer C, Wernet MF, Vogt K. Analysis of the blind Drosophila mutant NinaB identifies the gene encoding the key enzyme for vitamin A formation in vivo. Proc Natl Acad Sci USA 2001;98:1130–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Lintig J, Vogt K. Filling the gap in vitamin A research: molecular identification of an enzyme cleaving beta-carotene to retinal. J Biol Chem 2000;275:11915–20 [DOI] [PubMed] [Google Scholar]
- 33.Oberhauser V, Voolstra O, Bangert A, von Lintig J, Vogt K. NinaB combines carotenoid oxygenase and retinoid isomerase activity in a single polypeptide. Proc Natl Acad Sci USA 2008;105:19000–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voolstra O, Oberhauser V, Sumser E, Meyer NE, Maguire ME, Huber A, von Lintig J. NinaB is essential for Drosophila vision but induces retinal degeneration in opsin-deficient photoreceptors. J Biol Chem 2010;285:2130–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X, Wang T, Jiao Y, von Lintig J, Montell C. Requirement for an enzymatic visual cycle in Drosophila. Curr Biol 2010;20:93–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Bennekum A, Werder M, Thuahnai ST, Han CH, Duong P, Williams DL, Wettstein P, Schulthess G, Phillips MC, Hauser H. Class B scavenger receptor-mediated intestinal absorption of dietary beta-carotene and cholesterol. Biochemistry 2005;44:4517–25 [DOI] [PubMed] [Google Scholar]
- 37.During A, Harrison EH. Mechanisms of provitamin A (carotenoid) and vitamin A (retinol) transport into and out of intestinal Caco-2 cells. J Lipid Res 2007;48:2283–94 [DOI] [PubMed] [Google Scholar]
- 38.Reboul E, Klein A, Bietrix F, Gleize B, Malezet-Desmoulins C, Schneider M, Margotat A, Lagrost L, Collet X, Borel P. Scavenger receptor class B type I (SR-BI) is involved in vitamin E transport across the enterocyte. J Biol Chem 2006;281:4739–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hauser H, Dyer JH, Nandy A, Vega MA, Werder M, Bieliauskaite E, Weber FE, Compassi S, Gemperli A, Boffelli D, et al. Identification of a receptor mediating absorption of dietary cholesterol in the intestine. Biochemistry 1998;37:17843–50 [DOI] [PubMed] [Google Scholar]
- 40.During A, Hussain MM, Morel DW, Harrison EH. Carotenoid uptake and secretion by CaCo-2 cells: beta-carotene isomer selectivity and carotenoid interactions. J Lipid Res 2002;43:1086–95 [DOI] [PubMed] [Google Scholar]
- 41.Moussa M, Gouranton E, Gleize B, Yazidi CE, Niot I, Besnard P, Borel P, Landrier JF. CD36 is involved in lycopene and lutein uptake by adipocytes and adipose tissue cultures. Mol Nutr Food Res 2011;55(4):578–84 [DOI] [PubMed] [Google Scholar]
- 42.Redmond TM, Gentleman S, Duncan T, Yu S, Wiggert B, Gantt E, Cunningham FX., Jr Identification, expression, and substrate specificity of a mammalian beta-carotene 15,15'-dioxygenase. J Biol Chem 2001;276:6560–5 [DOI] [PubMed] [Google Scholar]
- 43.Paik J, During A, Harrison EH, Mendelsohn CL, Lai K, Blaner WS. Expression and characterization of a murine enzyme able to cleave beta-carotene: the formation of retinoids. J Biol Chem 2001;276:32160–8 [DOI] [PubMed] [Google Scholar]
- 44.Wyss A, Wirtz G, Woggon W, Brugger R, Wyss M, Friedlein A, Bachmann H, Hunziker W. Cloning and expression of beta,beta-carotene 15,15'-dioxygenase. Biochem Biophys Res Commun 2000;271:334–6 [DOI] [PubMed] [Google Scholar]
- 45.Lindqvist A, Andersson S. Biochemical properties of purified recombinant human beta-carotene 15,15'-monooxygenase. J Biol Chem 2002;277:23942–8 [DOI] [PubMed] [Google Scholar]
- 46.Hessel S, Eichinger A, Isken A, Amengual J, Hunzelmann S, Hoeller U, Elste V, Hunziker W, Goralczyk R, Oberhauser V, et al. CMO1 deficiency abolishes vitamin A production from beta-carotene and alters lipid metabolism in mice. J Biol Chem 2007;282:33553–61 [DOI] [PubMed] [Google Scholar]
- 47.Lindqvist A, Sharvill J, Sharvill DE, Andersson S. Loss-of-function mutation in carotenoid 15,15'-monooxygenase identified in a patient with hypercarotenemia and hypovitaminosis A. J Nutr 2007;137:2346–50 [DOI] [PubMed] [Google Scholar]
- 48.Leung WC, Hessel S, Meplan C, Flint J, Oberhauser V, Tourniaire F, Hesketh JE, von Lintig J, Lietz G. Two common single nucleotide polymorphisms in the gene encoding β-carotene 15,15'-monoxygenase alter β-carotene metabolism in female volunteers. FASEB J 2009;23:1041–53 [DOI] [PubMed] [Google Scholar]
- 49.Ferrucci L, Perry JR, Matteini A, Perola M, Tanaka T, Silander K, Rice N, Melzer D, Murray A, Cluett C, et al. Common variation in the beta-carotene 15,15'-monooxygenase 1 gene affects circulating levels of carotenoids: a genome-wide association study. Am J Hum Genet 2009;84:123–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hamel CP, Tsilou E, Pfeffer BA, Hooks JJ, Detrick B, Redmond TM. Molecular cloning and expression of RPE65, a novel retinal pigment epithelium-specific microsomal protein that is post-transcriptionally regulated in vitro. J Biol Chem 1993;268:15751–7 [PubMed] [Google Scholar]
- 51.Mata NL, Moghrabi WN, Lee JS, Bui TV, Radu RA, Horwitz J, Travis GH. Rpe65 is a retinyl ester binding protein that presents insoluble substrate to the isomerase in retinal pigment epithelial cells. J Biol Chem 2004;279:635–43 [DOI] [PubMed] [Google Scholar]
- 52.Xue L, Gollapalli DR, Maiti P, Jahng WJ, Rando RR. A palmitoylation switch mechanism in the regulation of the visual cycle. Cell 2004;117:761–71 [DOI] [PubMed] [Google Scholar]
- 53.Jin M, Li S, Moghrabi WN, Sun H, Travis GH. Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium. Cell 2005;122:449–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moiseyev G, Chen Y, Takahashi Y, Wu BX, Ma JX. RPE65 is the isomerohydrolase in the retinoid visual cycle. Proc Natl Acad Sci USA 2005;102:12413–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Redmond TM, Poliakov E, Yu S, Tsai JY, Lu Z, Gentleman S. Mutation of key residues of RPE65 abolishes its enzymatic role as isomerohydrolase in the visual cycle. Proc Natl Acad Sci USA 2005;102:13658–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marlhens F, Bareil C, Griffoin JM, Zrenner E, Amalric P, Eliaou C, Liu SY, Harris E, Redmond TM, Arnaud B, et al. Mutations in RPE65 cause Leber's congenital amaurosis. Nat Genet 1997;17:139–41 [DOI] [PubMed] [Google Scholar]
- 57.Redmond TM, Yu S, Lee E, Bok D, Hamasaki D, Chen N, Goletz P, Ma JX, Crouch RK, Pfeifer K. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet 1998;20:344–51 [DOI] [PubMed] [Google Scholar]
- 58.Samardzija M, von Lintig J, Tanimoto N, Oberhauser V, Thiersch M, Reme CE, Seeliger M, Grimm C, Wenzel A. R91W mutation in Rpe65 leads to milder early-onset retinal dystrophy due to the generation of low levels of 11-cis-retinal. Hum Mol Genet 2008;17:281–92 [DOI] [PubMed] [Google Scholar]
- 59.Woodruff ML, Wang Z, Chung HY, Redmond TM, Fain GL, Lem J. Spontaneous activity of opsin apoprotein is a cause of Leber congenital amaurosis. Nat Genet 2003;35:158–64 [DOI] [PubMed] [Google Scholar]
- 60.Samardzija M, Tanimoto N, Kostic C, Beck S, Oberhauser V, Joly S, Thiersch M, Fahl E, Arsenijevic Y, von Lintig J, et al. In conditions of limited chromophore supply rods entrap 11-cis-retinal leading to loss of cone function and cell death. Hum Mol Genet 2009;18:1266–75 [DOI] [PubMed] [Google Scholar]
- 61.Kiefer C, Hessel S, Lampert JM, Vogt K, Lederer MO, Breithaupt DE, von Lintig J. Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J Biol Chem 2001;276:14110–6 [DOI] [PubMed] [Google Scholar]
- 62.Hu KQ, Liu C, Ernst H, Krinsky NI, Russell RM, Wang XD. The biochemical characterization of ferret carotene-9',10'-monooxygenase catalyzing cleavage of carotenoids in vitro and in vivo. J Biol Chem 2006;281:19327–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mein JR, Dolnikowski GG, Ernst H, Russell RM, Wang XD. Enzymatic formation of apo-carotenoids from the xanthophyll carotenoids lutein, zeaxanthin and beta-cryptoxanthin by ferret carotene-9',10'-monooxygenase. Arch Biochem Biophys 2011;506:109–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Amengual J, Lobo GP, Golczak M, Li HN, Klimova T, Hoppel CL, Wyss A, Palczewski K, von Lintig J. A mitochondrial enzyme degrades carotenoids and protects against oxidative stress. FASEB J 2011;25:948–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tian R, Pitchford WS, Morris CA, Cullen NG, Bottema CD. Genetic variation in the beta, beta-carotene-9',10'-dioxygenase gene and association with fat colour in bovine adipose tissue and milk. Anim Genet 2010;41:253–9 [DOI] [PubMed] [Google Scholar]
- 66.Berry SD, Davis SR, Beattie EM, Thomas NL, Burrett AK, Ward HE, Stanfield AM, Biswas M, Ankersmit-Udy AE, Oxley PE, et al. Mutation in bovine beta-carotene oxygenase 2 affects milk color. Genetics 2009;182:923–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Våge DI, Boman IA. A nonsense mutation in the beta-carotene oxygenase 2 (BCO2) gene is tightly associated with accumulation of carotenoids in adipose tissue in sheep (Ovis aries). BMC Genet 2010;11:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eriksson J, Larson G, Gunnarsson U, Bed'hom B, Tixier-Boichard M, Stromstedt L, Wright D, Jungerius A, Vereijken A, Randi E, et al. Identification of the yellow skin gene reveals a hybrid origin of the domestic chicken. PLoS Genet 2008;4:e1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ford NA, Clinton SK, von Lintig J, Wyss A, Erdman JW., Jr Loss of carotene-9',10'-monooxygenase expression increases serum and tissue lycopene concentrations in lycopene-fed mice. J Nutr 2010;140:2134–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.von Lintig J, Hessel S, Isken A, Kiefer C, Lampert JM, Voolstra O, Vogt K. Towards a better understanding of carotenoid metabolism in animals. Biochim Biophys Acta 2005;1740:122–31. [DOI] [PubMed]
- 71.Paik J, Vogel S, Quadro L, Piantedosi R, Gottesman M, Lai K, Hamberger L, Vieira Mde M, Blaner WS. Vitamin A: overlapping delivery pathways to tissues from the circulation. J Nutr 2004;134:276S–80S [DOI] [PubMed] [Google Scholar]
- 72.van Bennekum AM, Kako Y, Weinstock PH, Harrison EH, Deckelbaum RJ, Goldberg IJ, Blaner WS. Lipoprotein lipase expression level influences tissue clearance of chylomicron retinyl ester. J Lipid Res 1999;40:565–74 [PubMed] [Google Scholar]
- 73.Goodman DW, Huang HS, Shiratori T. Tissue distribution and metabolism of newly absorbed vitamin A in the rat. J Lipid Res 1965;6:390–6 [PubMed] [Google Scholar]
- 74.Packer L. Carotenoids and retinoids: molecular aspects and health issues. Champaign, IL: AOCS Press, 2005 [Google Scholar]
- 75.Soprano DR, Blaner WS. Plasma retinol binding orotein. In: Sporn MB, Roberts AB, Goodman DS, eds. The retinoids: biology, chemistry, and medicine 2nd ed New York, NY: Raven Press, 1994 [Google Scholar]
- 76.Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, Wiita P, Bok D, Sun H. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin a. Science 2007;315:820–5 [DOI] [PubMed] [Google Scholar]
- 77.Isken A, Golczak M, Oberhauser V, Hunzelmann S, Driever W, Imanishi Y, Palczewski K, von Lintig J. RBP4 disrupts vitamin A uptake homeostasis in a STRA6-deficient animal model for Matthew-Wood syndrome. Cell Metab 2008;7:258–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bouillet P, Sapin V, Chazaud C, Messaddeq N, Decimo D, Dolle P, Chambon P. Developmental expression pattern of Stra6, a retinoic acid-responsive gene encoding a new type of membrane protein. Mech Dev 1997;63:173–86 [DOI] [PubMed] [Google Scholar]
- 79.Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev 2002;23:201–29 [DOI] [PubMed] [Google Scholar]
- 80.Seino Y, Miki T, Kiyonari H, Abe T, Fujimoto W, Kimura K, Takeuchi A, Takahashi Y, Oiso Y, Iwanaga T, et al. Isx participates in the maintenance of vitamin A metabolism by regulation of beta-carotene 15,15'-monooxygenase (Bcmo1) expression. J Biol Chem 2008;283:4905–11 [DOI] [PubMed] [Google Scholar]
- 81.Lobo GP, Hessel S, Eichinger A, Noy N, Moise AR, Wyss A, Palczewski K, von Lintig J. ISX is a retinoic acid-sensitive gatekeeper that controls intestinal beta,beta-carotene absorption and vitamin A production. FASEB J 2010;24:1656–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Castenmiller JJ, West CE. Bioavailability and bioconversion of carotenoids. Annu Rev Nutr 1998;18:19–38 [DOI] [PubMed] [Google Scholar]
- 83.Johnson EJ, Russell RM. Distribution of orally administered beta-carotene among lipoproteins in healthy men. Am J Clin Nutr 1992;56:128–35 [DOI] [PubMed] [Google Scholar]
- 84.Yan W, Jang GF, Haeseleer F, Esumi N, Chang J, Kerrigan M, Campochiaro M, Campochiaro P, Palczewski K, Zack DJ. Cloning and characterization of a human beta,beta-carotene-15,15'-dioxygenase that is highly expressed in the retinal pigment epithelium. Genomics 2001;72:193–202 [DOI] [PubMed] [Google Scholar]
- 85.Lindqvist A, He YG, Andersson S. Cell type-specific expression of beta-carotene 9',10'-monooxygenase in human tissues. J Histochem Cytochem 2005;53:1403–12 [DOI] [PubMed] [Google Scholar]
- 86.Chichili GR, Nohr D, Schaffer M, von Lintig J, Biesalski HK. beta-Carotene conversion into vitamin A in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 2005;46:3562–9 [DOI] [PubMed] [Google Scholar]
- 87.Kim YK, Wassef L, Chung S, Jiang H, Wyss A, Blaner WS, Quadro L: β-Carotene and its cleavage enzyme β-carotene-15,15`-oxygenase (CMOI) affect retinoid metabolism in developing tissues. FASEB J 2011;25(5):1641–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Quadro L, Blaner WS, Salchow DJ, Vogel S, Piantedosi R, Gouras P, Freeman S, Cosma MP, Colantuoni V, Gottesman ME. Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein. EMBO J 1999;18:4633–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lampert JM, Holzschuh J, Hessel S, Driever W, Vogt K, Von Lintig J. Provitamin A conversion to retinal via the beta,beta-carotene-15,15'-oxygenase (bcox) is essential for pattern formation and differentiation during zebrafish embryogenesis. Development 2003;130:2173–86 [DOI] [PubMed] [Google Scholar]
- 90.Boulanger A, McLemore P, Copeland NG, Gilbert DJ, Jenkins NA, Yu SS, Gentleman S, Redmond TM. Identification of beta-carotene 15,15'-monooxygenase as a peroxisome proliferator-activated receptor target gene. FASEB J 2003;17:1304–6 [DOI] [PubMed] [Google Scholar]
- 91.Amengual J, Gouranton E, van Helden YG, Hessel S, Ribot J, Kramer E, Kiec-Wilk B, Razny U, Lietz G, Wyss A, et al. Beta-carotene reduces body adiposity of mice via BCMO1. PLoS ONE 2011;6:e20644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lobo GP, Amengual J, Li HN, Golczak M, Bonet ML, Palczewski K, von Lintig J. β,β-Carotene decreases peroxisome proliferator receptor γ activity and reduces lipid storage capacity of adipocytes in a β,β-carotene oxygenase 1-dependent manner. J Biol Chem 2010;285:27891–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schwarz EJ, Reginato MJ, Shao D, Krakow SL, Lazar MA. Retinoic acid blocks adipogenesis by inhibiting C/EBPbeta-mediated transcription. Mol Cell Biol 1997;17:1552–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Puigserver P, Vazquez F, Bonet ML, Pico C, Palou A. In vitro and in vivo induction of brown adipocyte uncoupling protein (thermogenin) by retinoic acid. Biochem J 1996;317:827–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Berry DC, Noy N. All-trans-retinoic acid represses obesity and insulin resistance by activating both peroxisome proliferation-activated receptor beta/delta and retinoic acid receptor. Mol Cell Biol 2009;29:3286–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.O'Byrne SM, Wongsiriroj N, Libien J, Vogel S, Goldberg IJ, Baehr W, Palczewski K, Blaner WS. Retinoid absorption and storage is impaired in mice lacking lecithin:retinol acyltransferase (LRAT). J Biol Chem 2005;280:35647–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Amengual J, Lobo GP, Golczak M, Li HN, Klimova T, Hoppel CL, Wyss A, Palczewski K, von Lintig J: A mitochondrial enzyme degrades carotenoids and protects against oxidative stress. FASEB J [DOI] [PMC free article] [PubMed]
- 98.Yonekura L, Kobayashi M, Terasaki M, Nagao A. Keto-carotenoids are the major metabolites of dietary lutein and fucoxanthin in mouse tissues. J Nutr 2010;140:1824–31 [DOI] [PubMed] [Google Scholar]
- 99.He M, Cornelis MC, Kraft P, van Dam RM, Sun Q, Laurie CC, Mirel DB, Chasman DI, Ridker PM, Hunter DJ, et al. Genome-wide association study identifies variants at the IL18-BCO2 locus associated with interleukin-18 levels. Arterioscler Thromb Vasc Biol 2010;30:885–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang SS, Schadt EE, Wang H, Wang X, Ingram-Drake L, Shi W, Drake TA, Lusis AJ. Identification of pathways for atherosclerosis in mice: integration of quantitative trait locus analysis and global gene expression data. Circ Res 2007;101:e11–30 [DOI] [PubMed] [Google Scholar]
- 101.Paolini M, Antelli A, Pozzetti L, Spetlova D, Perocco P, Valgimigli L, Pedulli GF, Cantelli-Forti G. Induction of cytochrome P450 enzymes and over-generation of oxygen radicals in beta-carotene supplemented rats. Carcinogenesis 2001;22:1483–95 [DOI] [PubMed] [Google Scholar]
- 102.Liu C, Wang XD, Bronson RT, Smith DE, Krinsky NI, Russell RM. Effects of physiological versus pharmacological beta-carotene supplementation on cell proliferation and histopathological changes in the lungs of cigarette smoke-exposed ferrets. Carcinogenesis 2000;21:2245–53 [DOI] [PubMed] [Google Scholar]
- 103.Gallicchio L, Boyd K, Matanoski G, Tao XG, Chen L, Lam TK, Shiels M, Hammond E, Robinson KA, Caulfield LE, et al. Carotenoids and the risk of developing lung cancer: a systematic review. Am J Clin Nutr 2008;88:372–83 [DOI] [PubMed] [Google Scholar]
- 104.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA 2007;297:842–57 [DOI] [PubMed] [Google Scholar]