Abstract
Background: Palmitoleic acid has been shown to regulate adipokine expression and systemic metabolic homeostasis in animal studies. However, its association with human metabolic diseases remains controversial.
Objective: We aimed to investigate associations of erythrocyte palmitoleic acid with adipokines, inflammatory markers, and metabolic syndrome (MetS) in a Chinese population.
Design: Erythrocyte fatty acids were measured in a population-based sample of 3107 men and women aged 50–70 y, for whom plasma glucose, insulin, lipid profile, adiponectin, retinol binding protein 4 (RBP-4), plasminogen activator inhibitor type 1, and high-sensitivity C-reactive protein (hsCRP) were measured. MetS was defined according to the updated National Cholesterol Education Program Adult Treatment Panel III criteria for Asian Americans.
Results: The mean (±SD) erythrocyte palmitoleic acid value was 0.41 ± 0.20% of total fatty acids. Palmitoleic acid was positively correlated with RBP-4 (r = 0.14, P < 0.001) and inversely correlated with adiponectin (r = −0.15, P < 0.001). After multivariable adjustment, palmitoleic acid was strongly associated with MetS and its components. ORs (95% CIs) for comparisons of extreme quartiles of palmitoleic acid were 3.50 (2.66, 4.59) for MetS, 7.88 (5.90, 10.52) for hypertriglyceridemia, 2.13 (1.66, 2.72) for reduced HDL cholesterol, 1.99 (1.60, 2.48) for central obesity, and 1.86 (1.41, 2.44) for elevated blood pressure (all P < 0.001). Further control for adipokines and hsCRP abolished the association of palmitoleic acid with central obesity but not with other MetS components.
Conclusion: Erythrocyte palmitoleic acid is associated with an adverse profile of adipokines and inflammatory markers and an increased risk of MetS in this Chinese population.
INTRODUCTION
Palmitoleic acid (16:1ω-7, 16:1n−7) is an MUFA that mainly originates from de novo lipogenesis in humans. Lipogenesis is mediated by stearoyl-CoA desaturase (SCD)4, a key enzyme involved in the biosynthesis of MUFAs from SFAs (1). In the human body, palmitoleic acid of dietary origin is negligible, because most is oxidized shortly after absorption (2, 3). In a recent study using a mouse model with genetic deficiency of fatty acid binding protein, palmitoleic acid from adipose tissue was found to promote insulin sensitivity in muscles and to suppress not only hepatosteatosis but also the expression of monocyte chemoattractant protein-1 and tumor necrosis factor-α in adipose tissue (4). Subsequent animal studies also corroborate the favorable effects of palmitoleic acid on insulin action and lipid profile (5–8).
In contrast, results from human studies regarding the associations between this fatty acid and metabolic disorders remain controversial. Some investigations generated evidence consistent with the aforementioned animal experiments (9–11), whereas most other studies reached contradictory findings (11–23). To date, several studies with small sample sizes have sought to explicitly evaluate the association between this fatty acid and metabolic syndrome (MetS) (14–16), although none has been conducted among Chinese populations, who have an increased prevalence of MetS because of recent economic development and related changes in diet (24). Moreover, the role of adipokines and inflammatory markers in the association between palmitoleic acid and MetS is largely unknown. In animal experiments, palmitoleic acid reduced the expression of adipokines and inflammatory markers (4, 25), both of which are closely associated with the pathogenesis of metabolic disorders (26).
Therefore, we examined the association between erythrocyte palmitoleic acid and MetS risk among >3000 middle-aged and older Chinese men and women. With respect to adipokines and inflammatory markers, we focused on adiponectin, retinol-binding protein 4 (RBP-4), plasminogen activator inhibitor type 1 (PAI-1), and C-reactive protein, all of which are established or proposed risk factors for metabolic disorders (27, 28). Of note, our study used palmitoleic acid in erythrocytes to reflect its concentration in the human body. Erythrocytes are more accessible than adipose tissue, and, at the same time, the fatty acid concentrations in erythrocytes persist longer than those in plasma or its subfractions (29). In addition, because fatty acids in various blood compartments and tissues are highly correlated (20), palmitoleic acid in erythrocytes should reflect its concentration in tissues at the population level.
SUBJECTS AND METHODS
Study population
This study used a population-based sample from the Nutrition and Health of Aging Population in China study, which investigated associations of environmental and genetic factors, as well as their interactions, with aging-related chronic diseases. From March to June 2005, 3289 participants aged 50–70 y were recruited from Beijing and Shanghai if they had been residents in the respective cities for >20 y. Details of the study were described previously (30). The study protocol was approved by the Institutional Review Board of the Institute for Nutritional Sciences, and written informed consent was obtained from all participants. Participants were excluded if one or both of the following criteria were met: an insufficient volume of erythrocytes for fatty acid measurement (n = 31) or implausibly high (>4000 kcal/d for men or >3500 kcal/d for women) or low (<800 kcal/d for men or <500 kcal/d for women) energy intake. After the exclusions, 3107 participants remained in the current analysis.
Data collection
During a home interview, data on demographic variables, health status, health behavior, and physical activities were obtained by trained staff using a standardized questionnaire. Educational levels were grouped according to self-reported years in school (0–6, 7–9, ≥10 y). Smoking status was defined as current, former, or never. Alcohol drinking was categorized as yes or no. Total physical activity was categorized as low, moderate, or high based on the Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire for the short-form questionnaire (31, 32). Definitions of family history of chronic diseases and self-reported chronic diseases (including coronary heart disease, stroke, hypertension, and diabetes) were described elsewhere (30, 33). Dietary data were collected by using a 74-item food-frequency questionnaire (34). Dietary variables were adjusted for total energy intake by using the residual method (35). After they fasted overnight, participants underwent a physical examination. Body weight, height, waist circumference, and blood pressure were measured by trained medical workers following a standard protocol (30). BMI was calculated as weight in kilograms divided by height squared in meters.
Laboratory measurements
Venous fasting blood samples were collected by using EDTA as the anticoagulant. Blood samples were centrifuged at 3000 rpm for 15 min; portioned into aliquots of plasma, buffy coat, and erythrocytes; and then stored at −80°C before analysis. Plasma fasting glucose, total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides were measured by using commercial reagents (Wako Pure Chemical Industries) on an automatic analyzer (Hitachi 7080) (30). Fasting insulin was measured by radioimmunoassay (Linco Research) (36). HOMA-IR was calculated as insulin (μU/mL) × glucose (mmol/L)/24. Plasma high-sensitivity C-reactive protein (hsCRP) concentrations were measured with a particle-enhanced immunoturbidimetric assay (Ultrasensitive CRP kit, Orion Diagnostica) (30). Plasma adiponectin and PAI-1 were measured with commercial ELISA kits, and plasma RBP-4 was measured by a sandwich ELISA kit developed in-house (30, 36). All samples were consecutively analyzed in a random sequence. Intraassay and interassay CVs for these analyses were <8% and <21%, respectively (30, 36).
Erythrocyte fatty acid measurement
Erythrocyte fatty acids were extracted by hexane and isopropanol and then incubated with a mixture of methanol and sulfuric acid to produce fatty acid methyl esters (FAMEs). FAMEs were separated by gas chromatography (Agilent 6890 GC; SP-2560 capillary column: 100 m × 0.25 mm internal diameter × 0.2 μm film; Supelco; nitrogen was used as the carrier gas). Individual FAMEs were identified by positive chemical ionization with the use of methane as the reagent gas (Agilent 5975B). Prepared samples were measured under identical conditions by the same technicians in a random sequence. The relative amount of each fatty acid was quantified, by dividing the area under each peak by summed areas of all fatty acids, except the internal standard. The CV for palmitoleic acid was 9.8%.
Definition of MetS
MetS was defined according to the updated National Cholesterol Education Program Adult Treatment Panel III criteria for Asian Americans (37). Participants with any 3 of the following 5 items were identified as having MetS: waist circumference ≥90 cm in men or ≥80 cm in women, triglycerides ≥1.7 mmol/L, HDL cholesterol ≤1.03 mmol/L in men or ≤1.30 mmol/L in women, blood pressure ≥130/85 mm Hg or current use of antihypertensive medications, and fasting plasma glucose ≥5.6 mmol/L or previously diagnosed type 2 diabetes mellitus or current use of oral antidiabetic agents or insulin (37).
Statistical analysis
Natural logarithm transformation was performed for continuous variables to minimize the skewness of distribution if necessary. Spearman partial correlation coefficients were calculated to examine associations of palmitoleic acid with other erythrocyte fatty acids, adipokines, hsCRP, and metabolic traits. A general linear regression model was applied to compare palmitoleic acid concentrations in participants with different numbers of MetS components. ORs for MetS and its components according to palmitoleic acid quartiles were calculated in a multivariable logistic regression model. Potential confounding factors were considered and controlled for in the current analyses, including established demographic and lifestyle risk factors (age, sex, smoking status, drinking status, physical activity, self-reported chronic diseases, and family history of chronic diseases), dietary risk factors (total energy intake, carbohydrate intake as percentage of total energy intake, dietary fiber intake, and red meat intake), and BMI. We further controlled for region and residence, which indicated whether the participants lived in the north (Beijing) or south (Shanghai) and in an urban or rural area, respectively. In an exploratory analysis, we also included adipokines and hsCRP to explore whether these factors mediate the association between palmitoleic acid and MetS. Interactions between palmitoleic acid and these biomarkers were examined in a logistic model using the likelihood ratio test for interaction terms based on quartiles of both variables at issue. All analyses were performed with Stata version 9.2 (Stata Corp). Two-sided P values <0.05 were considered statistically significant.
RESULTS
The mean (±SD) erythrocyte palmitoleic acid value was relatively low in this Chinese population: 0.41 ± 0.20% of total fatty acids. Participants with higher erythrocyte palmitoleic acid values were older and more likely to be female, to live in southern rural areas, and to have a lower educational level (Table 1). In terms of dietary factors, elevated palmitoleic acid was associated with a higher carbohydrate intake but a lower intake of fat, protein, dietary fiber, and red meat.
TABLE 1.
Characteristics of subjects according to erythrocyte palmitoleic acid quartiles
| Quartile of palmitoleic acid |
||||
| 1 (n = 777) | 2 (n = 777) | 3 (n = 776) | 4 (n = 777) | |
| Palmitoleic acid | 0.21 ± 0.041 | 0.32 ± 0.03 | 0.43 ± 0.04 | 0.69 ± 0.19 |
| Age (y) | 57.9 ± 5.8 | 58.9 ± 6.1 | 58.7 ± 6.1 | 59.4 ± 6.0 |
| Female [n (%)] | 400 (51.5) | 447 (57.5) | 459 (59.2) | 472 (60.8) |
| Urban residents [n (%)] | 423 (54.4) | 410 (52.8) | 393 (50.6) | 348 (44.8) |
| Northern residents [n (%)] | 446 (57.4) | 394 (50.7) | 350 (45.1) | 325 (41.8) |
| Education ≥10 y [n (%)] | 223 (28.7) | 169 (21.8) | 183 (23.6) | 151 (19.4) |
| Current smoking [n (%)] | 230 (29.6) | 206 (26.5) | 196 (25.3) | 193 (24.8) |
| Current drinking [n (%)] | 133 (30.0) | 132 (29.7) | 134 (30.1) | 118 (26.6) |
| BMI (kg/m2) | 23.9 ± 3.4 | 24.3 ± 3.4 | 24.5 ± 3.6 | 25.2 ± 3.9 |
| Physical activity [n (%)] | ||||
| Low | 39 (5.0) | 61 (7.6) | 60 (7.7) | 73 (9.4) |
| Moderate | 361 (46.5) | 326 (42.0) | 329 (42.4) | 326 (42.0) |
| High | 377 (48.5) | 390 (50.2) | 387 (49.9) | 378 (48.7) |
| Self-reported chronic diseases [n (%)]2 | 238 (30.6) | 224 (28.8) | 236 (30.4) | 236 (30.4) |
| Family history of chronic diseases [n (%)]3 | 265 (34.1) | 256 (33.0) | 258 (33.3) | 264 (34.0) |
| Total energy (kcal/d) | 2258 ± 639 | 2219 ± 618 | 2235 ± 614 | 2209 ± 609 |
| Carbohydrate (% of energy) | 57.2 ± 8.9 | 59.1 ± 8.9 | 59.6 ± 9.8 | 61.4 ± 9.8 |
| Fat (% of energy) | 30.5 ± 7.9 | 29.0 ± 8.1 | 28.1 ± 8.2 | 26.7 ± 8.2 |
| Protein (% of energy) | 13.2 ± 2.5 | 12.8 ± 2.4 | 12.7 ± 2.7 | 12.3 ± 2.7 |
| Dietary fiber (g/d) | 14.1 ± 5.4 | 13.3 ± 5.3 | 12.9 ± 5.2 | 12.3 ± 5.3 |
| Red meat (g/d) | 48.6 ± 46.5 | 48.6 ± 46.1 | 45.2 ± 45.3 | 41.1 ± 41.4 |
| Erythrocyte PUFAs | 41.3 ± 3.5 | 40.6 ± 4.0 | 39.7 ± 4.0 | 38.9 ± 3.8 |
| Erythrocyte SFAs | 42.0 ± 3.9 | 42.1 ± 4.1 | 42.3 ± 3.9 | 42.0 ± 3.5 |
Mean ± SD (all such values).
Includes self-reported coronary heart disease, stroke, hypertension, and diabetes.
Includes coronary heart disease, stroke, hypertension, and diabetes in a parent or a first-degree sibling.
Erythrocyte palmitoleic acid was positively correlated with myristic acid (14:0; r = 0.55, P < 0.001), palmitic acid (16:0; r = 0.31, P < 0.001), and MUFAs, including hypogeic acid (16:1n−9; r = 0.49, P < 0.001), oleic acid (18:1n−9; r = 0.43, P < 0.001), and vaccenic acid (18:1n−7; r = 0.24, P < 0.001), but inversely correlated with arachidonic acid (20:4n−6) and DHA (22:6n−3) (Table 2). Palmitoleic acid showed a strong and positive correlation with triglycerides (r = 0.37, P < 0.001) but a negative correlation with HDL cholesterol (r = −0.14, P < 0.001). In addition, palmitoleic acid was significantly correlated with lower plasma adiponectin (r = −0.15, P < 0.001) and elevated RBP-4 (r = 0.14, P < 0.001), hsCRP (r = 0.10, P < 0.001), and PAI-1 (r = 0.05, P = 0.004), although these correlations were moderate in magnitude.
TABLE 2.
Multivariable-adjusted Spearman correlation coefficients of palmitoleic acid with other erythrocyte fatty acids and metabolic risk factors1
| Spearman correlation coefficient | P | |
| Erythrocyte fatty acids | ||
| 14:0 | 0.55 | <0.001 |
| 16:0 | 0.31 | <0.001 |
| 16:1n−9 | 0.49 | <0.001 |
| 18:1n−9 | 0.43 | <0.001 |
| 18:1n–7 | 0.24 | <0.001 |
| 18:2n−6 | −0.00 | 0.88 |
| 20:4n−6 | −0.28 | <0.001 |
| 20:5n−3 | 0.14 | <0.001 |
| 22:6n−3 | −0.13 | <0.001 |
| Metabolic traits | ||
| Systolic blood pressure | 0.07 | <0.001 |
| Diastolic blood pressure | 0.06 | <0.001 |
| Fasting glucose | −0.00 | 0.35 |
| Fasting insulin | 0.08 | <0.001 |
| HOMA-IR | 0.05 | 0.05 |
| Total cholesterol | 0.08 | <0.001 |
| HDL cholesterol | −0.14 | <0.001 |
| LDL cholesterol | 0.02 | 0.36 |
| Triglycerides | 0.37 | <0.001 |
| Adiponectin | −0.15 | <0.001 |
| RBP-4 | 0.14 | <0.001 |
| PAI-1 | 0.05 | 0.004 |
| hsCRP | 0.10 | <0.001 |
Spearman correlation coefficients were adjusted by age, sex, region, residence, and BMI. Missing data: fasting insulin and HOMA-IR (n = 4), blood pressure (n = 1), adiponectin (n = 91), and PAI-1 (n = 91). hsCRP, high-sensitivity C-reactive protein; PAI-1, plasminogen activator inhibitor type 1; RBP-4, retinol binding protein 4.
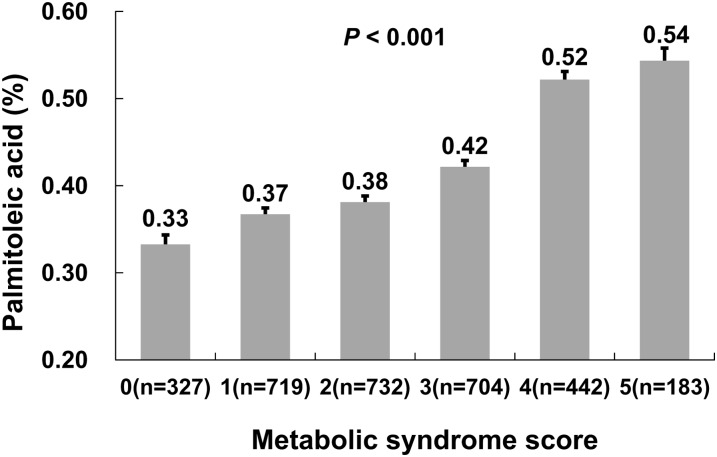
Erythrocyte palmitoleic acid increased gradually with the number of MetS components (Figure 1). The multivariable-adjusted percentage of palmitoleic acid increased from 0.33% in participants with no MetS components to 0.54% in those with all 5 components. The OR (95% CI) of MetS was 3.50 (2.66, 4.59) in the highest quartile of palmitoleic acid compared with the lowest quartile, after control for lifestyle factors, BMI, family history of chronic diseases, self-reported chronic diseases, and dietary factors (Table 3). Palmitoleic acid was also positively associated with MetS components, including hypertriglyceridemia, reduced HDL cholesterol, and elevated blood pressure. These associations were not materially attenuated by further adjustment of adipokines and hsCRP. To examine whether adiponectin, RBP-4, PAI-1, and hsCRP may modify the association between palmitoleic acid and MetS, joint classification analyses were further conducted (see Supplementary Figure 1 under “Supplemental data” in the online issue). A marginally significant interaction between palmitoleic acid and PAI-1 was identified (P-interaction = 0.04), but other interactions were not statistically significant.
FIGURE 1.
Mean (±SE) erythrocyte palmitoleic acid concentrations according to the number of metabolic syndrome components. P values were calculated from the multivariable-adjusted general linear regression model. The covariates adjusted included age, sex, region, residence, drinking, smoking, educational level, physical activity, BMI, self-reported chronic diseases, family history of diabetes and cardiovascular diseases, total energy, carbohydrate intake as a percentage of total energy intake, dietary fiber intake, and red meat intake.
TABLE 3.
ORs (and 95% CIs) of the metabolic syndrome and its components according to palmitoleic acid quartiles1
| Palmitoleic acid quartiles |
|||||
| 1 | 2 | 3 | 4 | P2 | |
| Metabolic syndrome (no. of cases) | 233 | 306 | 344 | 446 | |
| Model 13 | 1.00 | 1.57 (1.26, 1.95) | 2.04 (1.64, 2.54) | 3.76 (3.01, 4.69) | <0.001 |
| Model 24 | 1.00 | 1.58 (1.22, 2.05) | 2.02 (1.55, 2.63) | 3.50 (2.66, 4.59) | <0.001 |
| Model 35 | 1.00 | 1.50 (1.14, 1.98) | 1.77 (1.34, 2.35) | 2.54 (1.90, 3.41) | <0.001 |
| Central obesity | 326 | 362 | 381 | 431 | |
| Model 1 | 1.00 | 1.21 (0.98, 1.50) | 1.39 (1.13, 1.72) | 1.90 (1.53, 2.35) | <0.001 |
| Model 2 | 1.00 | 0.93 (0.68, 1.27) | 0.92 (0.66, 1.27) | 0.95 (0.68, 1.33) | 0.76 |
| Model 3 | 1.00 | 0.93 (0.67, 1.30) | 0.88 (0.63, 1.24) | 0.79 (0.55, 1.12) | 0.18 |
| Hypertriglyceridemia | 83 | 131 | 196 | 346 | |
| Model 1 | 1.00 | 1.81 (1.34, 2.44) | 3.17 (2.38, 4.21) | 8.48 (6.42, 11.20) | <0.001 |
| Model 2 | 1.00 | 1.80 (1.32, 2.44) | 3.08 (2.30, 4.14) | 7.88 (5.90, 10.52) | <0.001 |
| Model 3 | 1.00 | 1.74 (1.24, 2.43) | 2.97 (2.16, 4.10) | 5.95 (4.33, 8.19) | <0.001 |
| Reduced HDL cholesterol | 237 | 312 | 330 | 437 | |
| Model 1 | 1.00 | 1.53 (1.23, 1.90) | 1.66 (1.34, 2.06) | 3.05 (2.45, 3.80) | <0.001 |
| Model 2 | 1.00 | 1.48 (1.18, 1.87) | 1.54 (1.22, 1.93) | 2.57 (2.03, 3.24) | <0.001 |
| Model 3 | 1.00 | 1.40 (1.11, 1.78) | 1.41 (1.11, 1.79) | 2.13 (1.66, 2.72) | <0.001 |
| Elevated blood pressure | 463 | 557 | 541 | 589 | |
| Model 1 | 1.00 | 1.70 (1.37, 2.13) | 1.61 (1.29, 2.01) | 2.15 (1.71, 2.70) | <0.001 |
| Model 2 | 1.00 | 1.73 (1.35, 2.21) | 1.56 (1.22, 2.00) | 1.91 (1.47, 2.47) | <0.001 |
| Model 3 | 1.00 | 1.75 (1.36, 2.25) | 1.56 (1.21, 2.01) | 1.86 (1.41, 2.44) | <0.001 |
| Hyperglycemia | 333 | 305 | 304 | 314 | |
| Model 1 | 1.00 | 0.92 (0.75, 1.14) | 1.00 (0.80, 1.23) | 1.10 (0.89, 1.36) | 0.30 |
| Model 2 | 1.00 | 0.91 (0.73, 1.14) | 0.96 (0.77, 1.20) | 0.99 (0.79, 1.25) | 0.92 |
| Model 3 | 1.00 | 0.88 (0.69, 1.11) | 0.82 (0.65, 1.05) | 0.81 (0.63, 1.05) | 0.09 |
Metabolic syndrome was defined according to the updated National Cholesterol Education Program Adult Treatment Panel III criteria for Asian Americans.
Calculated by using multivariable logistic regression.
Adjusted for age, sex, region, and residence.
Adjusted as for model 1 plus physical activity, education, BMI (except when modeling associations for central obesity), smoking, drinking, family history of cardiovascular diseases and diabetes, self-reported chronic diseases, total energy, carbohydrate intake as a percentage of total energy intake, dietary fiber, and red meat.
Adjusted as for model 2 plus adiponectin, retinol binding protein 4, plasminogen activator inhibitor type 1, and high-sensitivity C-reactive protein.
DISCUSSION
We found that high erythrocyte palmitoleic acid concentrations were associated with an adverse profile of adipokines and inflammatory markers and an increased risk of MetS. These associations were independent of established lifestyle, dietary, and biological risk factors for MetS.
In mice genetically deficient in fatty acid binding protein, palmitoleic acid released from adipose tissue was identified as a “lipokine” that promotes insulin action in muscle and suppresses hepatosteatosis and adipocyte cytokine expression (4). Similarly, palmitoleic acid was also found to increase glucose utilization and peroxisome proliferator–activated receptor-γ response element activity and suppress the toxic effects of SFAs on β cells (6–8). These results from animal studies suggest the potential benefits of this particular fatty acid on insulin resistance and subsequently on MetS and diabetes. Despite the findings from animal studies, most human observational studies showed that high palmitoleic acid concentrations in various tissues were associated with unfavorable metabolic outcomes. For example, palmitoleic acid in adipose tissue and serum cholesterol were associated with insulin resistance in 2 early investigations from the Uppsala Longitudinal Study of Adult Men (12, 13). In the same study population, higher serum cholesterol ester palmitoleic acid was associated with an increased risk of developing MetS after 20 y of follow-up in 706 men (14). Furthermore, palmitoleic acid was correlated with multiple cardiometabolic risk factors, including higher BMI, larger waistline, higher blood pressure, plasma total cholesterol, triglycerides, apolipoprotein A-I, apolipoprotein B, fasting glucose, and endothelial dysfunction (11, 17, 18). Plasma or serum palmitoleic acid was also associated with a higher risk of diabetes, cardiovascular diseases, and all-cause mortality in previous studies (19–22). In the current study, we found positive associations of erythrocyte palmitoleic acid with traditional metabolic traits and risk of MetS and with adverse adipokine profiles and higher hsCRP concentrations. To our knowledge, this was the first investigation of an association of palmitoleic acid with adipokines and inflammatory markers in humans. Our findings are consistent with those of Heredia et al (38), ie, that adipose tissue palmitoleic acid was inversely associated with adiponectin expression in a rodent model. Therefore, current evidence from epidemiologic studies, including ours, does not support favorable effects of high palmitoleic acid status on metabolic disorders in humans.
Although the discrepancies between animal experiments and human studies remain largely unexplained, it has been suggested that findings regarding palmitoleic acid from animal models should not be extrapolated to humans because of the significant differences in fatty acid metabolism between the 2 species (23). In addition, human tissue concentrations of palmitoleic acid may mainly reflect de novo hepatic fatty acid synthesis mediated by SCD, which is a major source of palmitoleic acid, because the dietary contribution is minor (11, 23). Existing evidence suggests that the primary products of SCD, such as oleic acid and palmitoleic acid, are necessary components for the synthesis of triglycerides and cholesterol esters (1, 39). This may explain the associations of palmitoleic acid with elevated triglycerides and cholesterols in human studies (11, 17). Excessive accumulation of triglycerides in adipose tissue will eventually lead to obesity as well (1, 23, 39). Of note, the mechanism underlying the positive association of palmitoleic acid with triglycerides is particularly pertinent to our study population. We also found a positive association of palmitoleic acid with both carbohydrate intake and other endogenously synthesized fatty acids. The Chinese diet is traditionally characterized by a high carbohydrate intake (24), which accounted for nearly 60% of total energy in our population. High carbohydrate intake can promote hepatic de novo lipogenesis and lead to triglyceride accumulation (1, 39). This evidence may be important to disease prevention among Chinese populations. In addition, our findings raise nutritional concerns regarding the substitution of carbohydrate for fat, as suggested by current dietary guidelines (40).
Alternate explanations for the contradicting findings from existing studies warrant discussion as well. For example, the production and release of palmitoleic acid by human gluteofemoral adipose tissue is remarkably high (41). This implies that lower-body adipose tissue, but not other fatty acid pools, might be the major source of palmitoleic acid that serves as the “lipokine” in humans. Another explanation may be related to the different forms of palmitoleic acid examined in current studies. Palmitoleic acid in unesterified form was often used or measured in animal and human in vitro studies that showed favorable effects (6–8), whereas palmitoleic acid measured in most other studies was in esterified form, which may not act as a “lipokine” (11–14, 17–23, 38). Clearly, more well-designed mechanistic studies are needed to shed light on the mechanisms underlying the potentially various associations of palmitoleic acid—in different tissues or in different forms—with metabolic abnormalities.
Our study was conducted in a large representative Chinese population, with sufficient statistical power to detect weak to moderate associations. We carefully collected and adjusted demographic, lifestyle, dietary, and biological factors to control for confounding. Erythrocyte fatty acids were measured to reflect relatively long-term fatty acid status in humans. On the other hand, some limitations are worth discussing. First, a causal inference cannot be established because of the cross-sectional study design. It is possible that insulin resistance among participants with MetS activates liver SCD activity and leads to palmitoleic acid accumulation (1). However, no significant association was found between palmitoleic acid and HOMA-IR, and further adjustment of HOMA-IR did not materially change the association of palmitoleic acid with MetS and its components (data not shown). Second, palmitoleic acid was measured with some error (CV = 9.8%). However, because the measurement errors were likely to be random, the true association between palmitoleic acid and MetS should be attenuated toward the null. Finally, we did not measure unesterified palmitoleic acid and were unable to test our hypothesis that different forms of palmitoleic acid had varying effects on metabolic risk factors and MetS.
In conclusion, our cross-sectional study indicates that elevated erythrocyte palmitoleic acid is associated with an unfavorable profile of circulating adipokines and inflammatory markers and elevated risk of metabolic syndrome in middle-aged and elderly Chinese men and women.
Acknowledgments
We thank Yunhua Zhou, Shaojie Ma, He Zheng, Qianlu Jin, Yiqin Wang, Ling Lu, and Danxia Yu for their contributions at various stages of this study. We express further appreciation for the devotion of Shurong Zhou, Xinghuo Pang, and all staff members of the local CDC offices and hospitals during field work. Finally, our greatest thanks must go to our study participants themselves.
The authors’ responsibilities were as follows—GZ, QS, XY, HL, ZY, FBH, and XL: designed the research; GZ, XY, HL, ZY, QS, and XL: conducted the research; GZ: analyzed the data; GZ, QS, and XL: wrote the manuscript with help from LS and FBH; and QS and XL: had primary responsibility for the final content. All authors read and approved the final manuscript. None of the authors declared any conflicts of interest.
Footnotes
Abbreviations used: FAME, fatty acid methyl ester; hsCRP, high-sensitivity C-reactive protein; MetS, metabolic syndrome; PAI-1, plasminogen activator inhibitor type 1; RBP-4, retinol binding protein 4; SCD, stearoyl-CoA desaturase.
REFERENCES
- 1.Ntambi JM, Miyazaki M. Regulation of stearoyl-CoA desaturases and role in metabolism. Prog Lipid Res 2004;43:91–104 [DOI] [PubMed] [Google Scholar]
- 2.Goeransson G. The metabolism of fatty acids in the rat. vi. arachidonic acid. Acta Physiol Scand 1965;64:1–5 (abstr) [DOI] [PubMed] [Google Scholar]
- 3.Hagenfeldt L, Wahren J, Pernow B, Raf L. Uptake of individual free fatty acids by skeletal muscle and liver in man. J Clin Invest 1972;51:2324–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 2008;134:933–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matthan NR, Dillard A, Lecker JL, Ip B, Lichtenstein AH. Effects of dietary palmitoleic acid on plasma lipoprotein profile and aortic cholesterol accumulation are similar to those of other unsaturated fatty acids in the F1B golden Syrian hamster. J Nutr 2009;139:215–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dimopoulos N, Watson M, Sakamoto K, Hundal HS. Differential effects of palmitate and palmitoleate on insulin action and glucose utilization in rat L6 skeletal muscle cells. Biochem J 2006;399:473–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maedler K, Oberholzer J, Bucher P, Spinas GA, Donath MY. Monounsaturated fatty acids prevent the deleterious effects of palmitate and high glucose on human pancreatic beta-cell turnover and function. Diabetes 2003;52:726–33 [DOI] [PubMed] [Google Scholar]
- 8.Sauma L, Stenkula KG, Kjolhede P, Stralfors P, Soderstrom M, Nystrom FH. PPAR-gamma response element activity in intact primary human adipocytes: effects of fatty acids. Nutrition 2006;22:60–8 [DOI] [PubMed] [Google Scholar]
- 9.Stefan N, Kantartzis K, Celebi N, Staiger H, Machann J, Schick F, Cegan A, Elcnerova M, Schleicher E, Fritsche A, et al. Circulating palmitoleate strongly and independently predicts insulin sensitivity in humans. Diabetes Care 2010;33:405–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hiraoka-Yamamoto J, Ikeda K, Negishi H, Mori M, Hirose A, Sawada S, Onobayashi Y, Kitamori K, Kitano S, Tashiro M, et al. Serum lipid effects of a monounsaturated (palmitoleic) fatty acid-rich diet based on macadamia nuts in healthy, young Japanese women. Clin Exp Pharmacol Physiol 2004;31(Suppl 2):S37–8 [DOI] [PubMed] [Google Scholar]
- 11.Mozaffarian D, Cao H, King IB, Lemaitre RN, Song X, Siscovick DS, Hotamisligil GS. Circulating palmitoleic acid and risk of metabolic abnormalities and new-onset diabetes. Am J Clin Nutr 2010;92:1350–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iggman D, Arnlov J, Vessby B, Cederholm T, Sjogren P, Riserus U. Adipose tissue fatty acids and insulin sensitivity in elderly men. Diabetologia 2010;53:850–7 [DOI] [PubMed] [Google Scholar]
- 13.Vessby B, Tengblad S, Lithell H. Insulin sensitivity is related to the fatty acid composition of serum lipids and skeletal muscle phospholipids in 70-year-old men. Diabetologia 1994;37:1044–50 [DOI] [PubMed] [Google Scholar]
- 14.Warensjö E, Riserus U, Vessby B. Fatty acid composition of serum lipids predicts the development of the metabolic syndrome in men. Diabetologia 2005;48:1999–2005 [DOI] [PubMed] [Google Scholar]
- 15.Kawashima A, Sugawara S, Okita M, Akahane T, Fukui K, Hashiuchi M, Kataoka C, Tsukamoto I. Plasma fatty acid composition, estimated desaturase activities, and intakes of energy and nutrient in Japanese men with abdominal obesity or metabolic syndrome. J Nutr Sci Vitaminol (Tokyo) 2009;55:400–6 [DOI] [PubMed] [Google Scholar]
- 16.Kim OY, Lim HH, Lee MJ, Kim JY, Lee JH. Association of fatty acid composition in serum phospholipids with metabolic syndrome and arterial stiffness. Nutr Metab Cardiovasc Dis (Epub ahead of print 14 September 2011) [DOI] [PubMed] [Google Scholar]
- 17.Cambien F, Warnet JM, Vernier V, Ducimetiere P, Jacqueson A, Flament C, Orssaud G, Richard JL, Claude JR. An epidemiologic appraisal of the associations between the fatty acids esterifying serum cholesterol and some cardiovascular risk factors in middle-aged men. Am J Epidemiol 1988;127:75–86 [DOI] [PubMed] [Google Scholar]
- 18.Sarabi M, Vessby B, Millgard J, Lind L. Endothelium-dependent vasodilation is related to the fatty acid composition of serum lipids in healthy subjects. Atherosclerosis 2001;156:349–55 [DOI] [PubMed] [Google Scholar]
- 19.Siguel EN, Lerman RH. Altered fatty acid metabolism in patients with angiographically documented coronary artery disease. Metabolism 1994;43:982–93 [DOI] [PubMed] [Google Scholar]
- 20.Hodge AM, English DR, O'Dea K, Sinclair AJ, Makrides M, Gibson RA, Giles GG. Plasma phospholipid and dietary fatty acids as predictors of type 2 diabetes: interpreting the role of linoleic acid. Am J Clin Nutr 2007;86:189–97 [DOI] [PubMed] [Google Scholar]
- 21.Okada T, Furuhashi N, Kuromori Y, Miyashita M, Iwata F, Harada K. Plasma palmitoleic acid content and obesity in children. Am J Clin Nutr 2005;82:747–50 [DOI] [PubMed] [Google Scholar]
- 22.Warensjö E, Sundstrom J, Vessby B, Cederholm T, Riserus U. Markers of dietary fat quality and fatty acid desaturation as predictors of total and cardiovascular mortality: a population-based prospective study. Am J Clin Nutr 2008;88:203–9 [DOI] [PubMed] [Google Scholar]
- 23.Gong J, Campos H, McGarvey S, Wu Z, Goldberg R, Baylin A. Adipose tissue palmitoleic acid and obesity in humans: does it behave as a lipokine? Am J Clin Nutr 2011;93:186–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhai F, Wang H, Du S, He Y, Wang Z, Ge K, Popkin BM. Lifespan nutrition and changing socio-economic conditions in China. Asia Pac J Clin Nutr 2007;16(Suppl 1):374–82 [PubMed] [Google Scholar]
- 25.Guo X, Li H, Xu H, Halim V, Zhang W, Wang H, Ong KT, Woo SL, Walzem RL, Mashek DG, et al. Palmitoleate induces hepatic steatosis but suppresses liver inflammatory response in mice. PLoS ONE 2012;7:e39286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol 2011;11:85–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol 2010;314:1–16 [DOI] [PubMed] [Google Scholar]
- 28.Devaraj S, Singh U, Jialal I. Human C-reactive protein and the metabolic syndrome. Curr Opin Lipidol 2009;20:182–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res 2008;47:348–80 [DOI] [PubMed] [Google Scholar]
- 30.Ye X, Yu Z, Li H, Franco OH, Liu Y, Lin X. Distributions of C-reactive protein and its association with metabolic syndrome in middle-aged and older Chinese people. J Am Coll Cardiol 2007;49:1798–805 [DOI] [PubMed] [Google Scholar]
- 31.Yu Z, Ye X, Wang J, Qi Q, Franco OH, Rennie KL, Pan A, Li H, Liu Y, Hu FB, et al. Associations of physical activity with inflammatory factors, adipocytokines, and metabolic syndrome in middle-aged and older Chinese people. Circulation 2009;119:2969–77 [DOI] [PubMed] [Google Scholar]
- 32.The International Physical Activity Questionnaire group. Guidelines for data processing and analysis of the International Physical Activity Questionnaire (IPAQ). Available from: http://www.ipaq.ki.se/scoring.pdf (cited May 2012)
- 33.Yu Z, Lin X, Haas JD, Franco OH, Rennie KL, Li H, Xu H, Pang X, Liu H, Zhang Z, et al. Obesity related metabolic abnormalities: distribution and geographic differences among middle-aged and older Chinese populations. Prev Med 2009;48:272–8 [DOI] [PubMed] [Google Scholar]
- 34.Sun L, Franco OH, Hu FB, Cai L, Yu Z, Li H, Ye X, Qi Q, Wang J, Pan A, et al. Ferritin concentrations, metabolic syndrome, and type 2 diabetes in middle-aged and elderly Chinese. J Clin Endocrinol Metab 2008;93:4690–6 [DOI] [PubMed] [Google Scholar]
- 35.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol 1986;124:17–27 [DOI] [PubMed] [Google Scholar]
- 36.Pan A, Ye X, Franco OH, Li H, Yu Z, Wang J, Qi Q, Gu W, Pang X, Liu H, et al. The association of depressive symptoms with inflammatory factors and adipokines in middle-aged and older Chinese. PLoS ONE 2008;3:e1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106:3143–421 [PubMed] [Google Scholar]
- 38.Pérez de Heredia F, Sanchez J, Priego T, Larque E, del Puy Portillo M, Palou A, Zamora S, Garaulet M. Adiponectin is associated with serum and adipose tissue fatty acid composition in rats. J Endocrinol Invest 2009;32:659–65 [DOI] [PubMed] [Google Scholar]
- 39.Flowers MT, Ntambi JM. Stearoyl-CoA desaturase and its relation to high-carbohydrate diets and obesity. Biochim Biophys Acta 2009;1791:85–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Saturated fat, carbohydrate, and cardiovascular disease. Am J Clin Nutr 2010;91:502–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pinnick KE, Neville MJ, Fielding BA, Frayn KN, Karpe F, Hodson L. Gluteofemoral adipose tissue plays a major role in production of the lipokine palmitoleate in humans. Diabetes 2012;61:1399–403 [DOI] [PMC free article] [PubMed] [Google Scholar]