Abstract
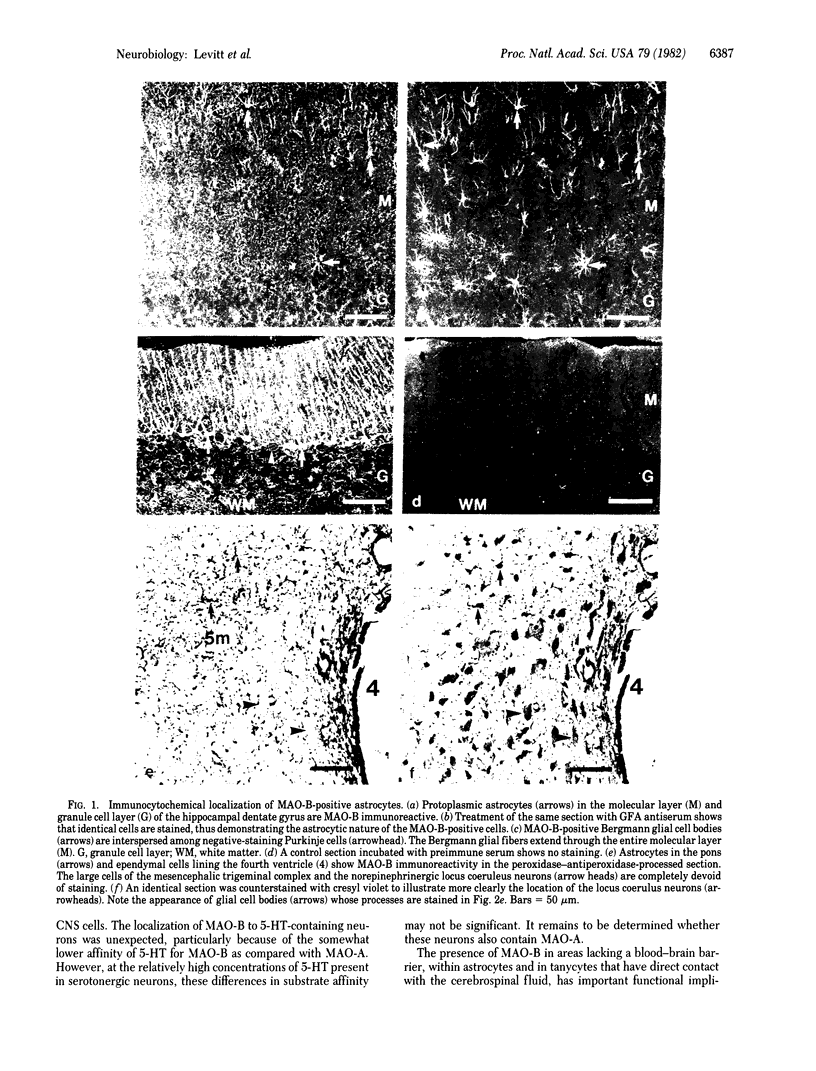
An antiserum to monoamine oxidase B (MAO-B) was used to define the distribution of this metabolic enzyme in the adult rat brain immunocytochemically. MAO-B is specifically located in two major central nervous system cell classes, astrocytes and serotonin-containing neurons. Double-immunofluorescence experiments using antisera to glial fibrillary acidic protein and MAO-B showed that both protoplasmic and fibrillary astrocytes throughout the brain contain MAO-B, whereas oligodendrocytes do not contain the enzyme. Areas lacking a blood-brain barrier, such as the specialized circumventricular organs, also contain MAO-B-positive cells. A double-immunofluorescence experiment using antisera to serotonin and MAO-B enabled the positive identification of neurons containing both molecules. The catecholamine-containing neurons of the brain did not contain detectable amounts of MAO-B. The specific distribution of MAO-B in the adult central nervous system indicates that the role of MAO-B in monoamine metabolism may be more specifically defined than previously believed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball E. H., Singer S. J. Mitochondria are associated with microtubules and not with intermediate filaments in cultured fibroblasts. Proc Natl Acad Sci U S A. 1982 Jan;79(1):123–126. doi: 10.1073/pnas.79.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berod A., Hartman B. K., Pujol J. F. Importance of fixation in immunohistochemistry: use of formaldehyde solutions at variable pH for the localization of tyrosine hydroxylase. J Histochem Cytochem. 1981 Jul;29(7):844–850. doi: 10.1177/29.7.6167611. [DOI] [PubMed] [Google Scholar]
- Brown G. K., Powell J. F., Craig I. W. Molecular weight differences between human platelet and placental monoamine oxidase. Biochem Pharmacol. 1980 Oct 1;29(19):2595–2603. doi: 10.1016/0006-2952(80)90073-8. [DOI] [PubMed] [Google Scholar]
- Campbell I. C., Robinson D. S., Lovenberg W., Murphy D. L. The effects of chronic regimens of clorgyline and pargyline on monoamine metabolism in the rat brain. J Neurochem. 1979 Jan;32(1):49–55. doi: 10.1111/j.1471-4159.1979.tb04508.x. [DOI] [PubMed] [Google Scholar]
- Cawthon R. M., Pintar J. E., Haseltine F. P., Breakefield X. O. Differences in the structure of A and B forms of human monoamine oxidase. J Neurochem. 1981 Aug;37(2):363–372. doi: 10.1111/j.1471-4159.1981.tb00464.x. [DOI] [PubMed] [Google Scholar]
- Demarest K. T., Smith D. J., Azzaro A. J. The presence of the type A form of monoamine oxidase within nigrostriatal dopamine-containing neurons. J Pharmacol Exp Ther. 1980 Nov;215(2):461–468. [PubMed] [Google Scholar]
- Eng L. F., Rubinstein L. J. Contribution of immunohistochemistry to diagnostic problems of human cerebral tumors. J Histochem Cytochem. 1978 Jul;26(7):513–522. doi: 10.1177/26.7.357640. [DOI] [PubMed] [Google Scholar]
- Frankfurt M., Lauder J. M., Azmitia E. C. The immunocytochemical localization of serotonergic neurons in the rat hypothalamus. Neurosci Lett. 1981 Jul 17;24(3):227–232. doi: 10.1016/0304-3940(81)90161-0. [DOI] [PubMed] [Google Scholar]
- Fuller R. W., Hemrick-Luecke S. K. Elevation of epinephrine concentration in rat brain by LY51641, a selective inhibitor of type A monoamine oxidase. Res Commun Chem Pathol Pharmacol. 1981 May;32(2):207–221. [PubMed] [Google Scholar]
- Ghandour M. S., Langley O. K., Labourdette G., Vincendon G., Gombos G. Specific and artefactual cellular localizations of S 100 protein: an astrocyte marker in rat cerebellum. Dev Neurosci. 1981;4(1):66–78. doi: 10.1159/000112742. [DOI] [PubMed] [Google Scholar]
- Hatten M. E., Liem R. K. Astroglial cells provide a template for the positioning of developing cerebellar neurons in vitro. J Cell Biol. 1981 Sep;90(3):622–630. doi: 10.1083/jcb.90.3.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. V., Walsh M. L., Bockus B. J., Chen L. B. Monitoring of relative mitochondrial membrane potential in living cells by fluorescence microscopy. J Cell Biol. 1981 Mar;88(3):526–535. doi: 10.1083/jcb.88.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G. P., Hartman B. K., Creveling C. R. Immunohistochemical demonstration of catechol-o-methyltransferase in mammalian brain. Brain Res. 1979 May 11;167(2):241–250. doi: 10.1016/0006-8993(79)90819-9. [DOI] [PubMed] [Google Scholar]
- Kaplan G. P., Hartman B. K., Creveling C. R. Immunohistochemical localization of catechol-O-methyltransferase in circumventricular organs of the rat: potential variations in the blood-brain barrier to native catechols. Brain Res. 1981 Dec 21;229(2):323–335. doi: 10.1016/0006-8993(81)90997-5. [DOI] [PubMed] [Google Scholar]
- Levitt P., Cooper M. L., Rakic P. Coexistence of neuronal and glial precursor cells in the cerebral ventricular zone of the fetal monkey: an ultrastructural immunoperoxidase analysis. J Neurosci. 1981 Jan;1(1):27–39. doi: 10.1523/JNEUROSCI.01-01-00027.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt P., Rakic P. Immunoperoxidase localization of glial fibrillary acidic protein in radial glial cells and astrocytes of the developing rhesus monkey brain. J Comp Neurol. 1980 Oct 1;193(3):815–840. doi: 10.1002/cne.901930316. [DOI] [PubMed] [Google Scholar]
- Meltzer H. Y., Arora R. C. Distribution of type A and type B monoamine oxidase activities in rat and chicken skeletal muscle and nerves. Biochem Pharmacol. 1979 Nov 15;28(22):3261–3264. doi: 10.1016/0006-2952(79)90119-9. [DOI] [PubMed] [Google Scholar]
- Murphy D. L. Substrate-selective monoamine oxidases--inhibitor, tissue, species and functional differences. Biochem Pharmacol. 1978;27(15):1889–1893. doi: 10.1016/0006-2952(78)90001-1. [DOI] [PubMed] [Google Scholar]
- Pintar J. E., Breakefield X. O. Monoamine oxidase (MAO) activity as a determinant in human neurophysiology. Behav Genet. 1982 Feb;12(1):53–68. doi: 10.1007/BF01065740. [DOI] [PubMed] [Google Scholar]
- Salach J. I., Weyler W. Iron content and spectral properties of highly purified bovine liver monoamine oxidase. Arch Biochem Biophys. 1981 Nov;212(1):147–153. doi: 10.1016/0003-9861(81)90353-2. [DOI] [PubMed] [Google Scholar]
- Schoepp D. D., Azzaro A. J. Alteration of dopamine synthesis in rat striatum subsequent to selective type A monoamine oxidase inhibition. J Neurochem. 1981 Aug;37(2):527–530. doi: 10.1111/j.1471-4159.1981.tb00490.x. [DOI] [PubMed] [Google Scholar]
- Steinbusch H. W., Verhofstad A. A., Joosten H. W. Localization of serotonin in the central nervous system by immunohistochemistry: description of a specific and sensitive technique and some applications. Neuroscience. 1978;3(9):811–819. doi: 10.1016/0306-4522(78)90033-7. [DOI] [PubMed] [Google Scholar]














