Abstract
Currently, the most effective therapy for the treatment of morbid obesity to induce significant and maintained body weight loss with a proven mortality benefit is bariatric surgery1,2. Consequently, there has been a steady rise in the number of bariatric operations done worldwide in recent years with the Roux-en-Y gastric bypass (gastric bypass) being the most commonly performed operation3. Against this background, it is important to understand the physiological mechanisms by which gastric bypass induces and maintains body weight loss. These mechanisms are yet not fully understood, but may include reduced hunger and increased satiation4,5, increased energy expenditure6,7, altered preference for food high in fat and sugar8,9, altered salt and water handling of the kidney10 as well as alterations in gut microbiota11. Such changes seen after gastric bypass may at least partly stem from how the surgery alters the hormonal milieu because gastric bypass increases the postprandial release of peptide-YY (PYY) and glucagon-like-peptide-1 (GLP-1), hormones that are released by the gut in the presence of nutrients and that reduce eating12.
During the last two decades numerous studies using rats have been carried out to further investigate physiological changes after gastric bypass. The gastric bypass rat model has proven to be a valuable experimental tool not least as it closely mimics the time profile and magnitude of human weight loss, but also allows researchers to control and manipulate critical anatomic and physiologic factors including the use of appropriate controls. Consequently, there is a wide array of rat gastric bypass models available in the literature reviewed elsewhere in more detail 13-15. The description of the exact surgical technique of these models varies widely and differs e.g. in terms of pouch size, limb lengths, and the preservation of the vagal nerve. If reported, mortality rates seem to range from 0 to 35%15. Furthermore, surgery has been carried out almost exclusively in male rats of different strains and ages. Pre- and postoperative diets also varied significantly.
Technical and experimental variations in published gastric bypass rat models complicate the comparison and identification of potential physiological mechanisms involved in gastric bypass. There is no clear evidence that any of these models is superior, but there is an emerging need for standardization of the procedure to achieve consistent and comparable data. This article therefore aims to summarize and discuss technical and experimental details of our previously validated and published gastric bypass rat model.
Keywords: Medicine, Issue 64, Physiology, Roux-en-Y Gastric bypass, rat model, gastric pouch size, gut hormones
Protocol
1. Preoperative Care
Remove food from rat overnight prior to surgery.
Induce anaesthesia in chamber with 4-5% isoflurane and O2 flow of 2 l/min.
Shave abdomen from sternum to pelvis using electric razor.
Place anaesthetised rat in supine position on isothermal heating pad.
Apply eye ointment (Vitagel) before placing the rats' snout in nosecone.
Maintain anaesthesia with isoflurane concentration of 2-3% and O2 flow of 2 l/min.
Disinfect skin with Betadine-Solution.
Confirm depth of anaesthesia with forceps pinch between toes of hind leg.
Administer 5.7 mg/kg Enrofloxacin intraperitoneally as perioperative antibiotic prophylaxis, and 1 mg/kg Flunixin for analgesia.
2. Median Laparotomy
Perform midline incision using scalpel starting just below xyphoid process (Blade No. 10).
Mobilise skin circumferentially from underlying abdominal muscles using Metzenbaum scissors.
Open abdominal cavity.
Install retractors to facilitate best possible exposure of the operation field.
3. Biliopancreatic and Alimentary Limb
Identify where the duodenum or proximal jejunum passes under the colon.
Transect small bowel about 10 cm aborally from here and ligate both ends of the gut (PDS 5-0).
Place proximal stump of the two ends in left upper quadrant of abdomen as it will be later used to form the biliopancreatic limb of Roux-en-Y reconstruction.
Place distal stump of the two ends in right upper quadrant of abdomen as it will be later used to form the alimentary limb of Roux-en-Y reconstruction.
4. Jejuno-Jejunostomy
Identify caecum with ileocoecal valve and ileum.
Follow ileum orally for approximately 25 cm. The Jejuno-Jejunostomy will be placed here as starting point of the common channel of Roux-en-Y reconstruction.
Retrieve biliopancreatic limb from left upper quadrant of abdomen and position it next to common channel where you plan to perform Jejuno-Jejunostomy.
Secure biliopancreatic limb and common channel with retention stitch (PDS 6-0).
Incise both loops over approximate 10 mm by using micro scissors.
Create Jejuno-Jejunostomy by performing side-to-side anastomosis using interrupted sutures (PDS 6-0).
First complete dorsal side and then ventral side of anastomosis.
5. Gastric Pouch
Identify gastro-oesophageal junction.
Mobilise this area by dissecting gastro-hepatic and gastro-splenic ligaments using Metzenbaum scissors.
Move left gastric artery and vagal fibres of left para-oesophageal bundle laterally to prevent major bleedings and vagal nerve damage when small gastric pouch is created.
Expose gastro-oesophageal junction by placing cotton swab retro-oesophageally.
Coagulate small vessels of frontal stomach by using commercially available cautery device - also to prevent bleedings.
Transect stomach approximately 5 mm below gastro-oesophageal junction creating gastric pouch of a size of no more than 2-3% of original stomach size using delicate, curved scissors.
Close gastric remnant (PDS 5-0).
6. Gastro-Jejunostomy
Retrieve alimentary limb from right upper quadrant of abdomen and position it next to gastric pouch.
Create Gastro-Jejunostomy by performing end-to-side anastomosis (PDS 7-0).
First complete back side and then front side of anastomosis.
7. Abdominal Closure
Reduce anaesthesia by reducing isoflurane concentration to 1.5%.
Close muscle layer of abdominal wall using continuous sutures (PDS 4-0).
Administer 100 μl of 0.3 mg/ml buprenorphine solution subcutaneously for analgesia.
Further reduce isoflurane concentration down to 1%.
Close the skin using interrupted sutures (Vicryl 4-0).
8. Postoperative Care
Stop isoflurane and continue with O2.
Administer 5 ml of warm saline for fluid replacement in three subcutaneous depots.
Position rat under red light until full recovery.
Return the rat to home cage.
9. Representative Results
Animals and housing
Male Wistar rats (Harlan Laboratories Inc., Blackthorn, UK; Elevage Janvier, Le-Genest-St. Isle, France) weighing between 350 and 500 g were individually housed under a 12 h /12 h light-dark cycle at a room temperature of 21±2 °C. Water and standard chow were available ad libitum, unless otherwise stated. All experiments were performed under a license issued by the Home Office, UK (PL70-6669) or approved by the Veterinary Office of the Canton Zurich, Switzerland. All rats were given one week of acclimatization before being randomized to gastric bypass or sham-operation. After surgery, rats received liquid diet for 3 days before access to normal chow was reinstalled.
Body weight
Data of our rat gastric bypass model are consistent with previous findings that gastric bypass surgery is effective to reduce body weight and especially to maintain body weight loss (Figure 2). Average pre-surgical body weight of rats used for gastric bypass and sham-operations was similar (sham: 433.4 ± 8.3 g vs. bypass: 420.7 ± 8.4 g, p= 0.28). Five days after surgery sham-operated controls weighed significantly more compared to gastric bypass rats (sham: 422.2 ± 8.3 g vs. bypass: 374.7 ± 7.6 g, p<0.001). On postoperative day 60, difference in body weight was almost 170 g (sham: 533.2 ± 8.1 g vs. bypass: 366.2 ± 10.8 g, p<0.001).
Food intake
Food intake followed similar patterns as body weight and was reduced in gastric bypass rats when compared to sham-operated ad libitum fed rats. Figure 3 shows the average daily food intake for both groups (postoperative day 1-60). Daily food intake was consistently lower after gastric bypass (sham: 29.9 ± 0.2 g vs. bypass: 25.7 ± 0.3 g, p<0.001).
Gut hormones
Blood from all rats was collected on the day of study termination 8,16. Animals had ad libitum food access the night before and were decapitated at the beginning of the light cycle on postoperative day 60. Blood was obtained, immediately centrifuged at 3000 rpm for 10 minutes at 4°C, and stored at -20°C until the samples were assayed in duplicate in a single run. PYY-like immunoreactivity was measured with a specific and sensitive radioimmunoassay, which measures, both the full length (PYY1-36) and the fragment (PYY3-36). GLP-1 was measured by established in-house radioimmunoassays17,18. Differences in food intake may be partly explained by increased postprandial plasma levels of peptide YY (PYY) and glucagon-like peptide 1 (GLP-1) as gastric bypass rats showed significantly higher levels for PYY (sham: 26 ± 2 pmol/L vs. bypass: 141 ± 14 pmol/L, p<0.001) and GLP-1 (sham: 40 ± 5 pmol/L vs. bypass: 215 ± 23 pmol/L, p<0.001; Figure 4).
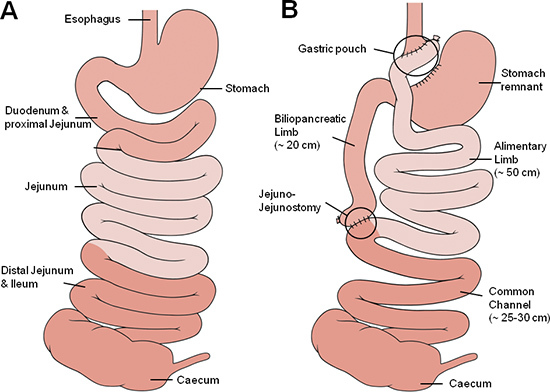 Figure 1. Gastric bypass anatomy. Schematic illustration of the small bowel anatomy before (A) and after (B) gastric bypass operation. The different shades of red approximately represent corresponding segments of the small bowel with the medium red representing the foregut (oesophagus, stomach duodenum and proximal jejunum), the light red representing the midgut (proximal and mid jejunum, proximal ileum) and the dark red representing the hindgut (ileum, caecum).
Figure 1. Gastric bypass anatomy. Schematic illustration of the small bowel anatomy before (A) and after (B) gastric bypass operation. The different shades of red approximately represent corresponding segments of the small bowel with the medium red representing the foregut (oesophagus, stomach duodenum and proximal jejunum), the light red representing the midgut (proximal and mid jejunum, proximal ileum) and the dark red representing the hindgut (ileum, caecum).
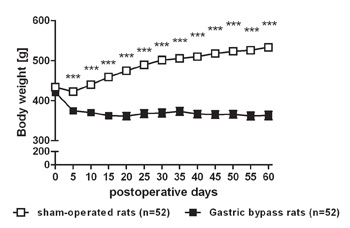 Figure 2. Body weight loss after gastric bypass surgery in rats. Body weight change for a representative group of rats after gastric bypass (- -) (n=52) and sham-operated rats (- -) (n=52) throughout an observation period of 60 days. Data were pooled from previous publications6,8-10 and are shown as mean values ± SEM (*** = p<0.001).
Figure 2. Body weight loss after gastric bypass surgery in rats. Body weight change for a representative group of rats after gastric bypass (- -) (n=52) and sham-operated rats (- -) (n=52) throughout an observation period of 60 days. Data were pooled from previous publications6,8-10 and are shown as mean values ± SEM (*** = p<0.001).
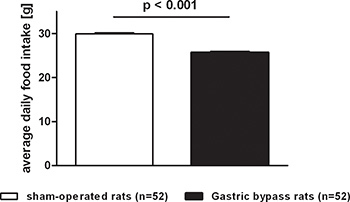 Figure 3. Average food intake after gastric bypass surgery in rats. Average daily food intake of a representative group of rats after gastric bypass (black, n=52) and sham-operated rats (white, n=52) throughout a postoperative period of 60 days. Data were pooled from previous publications6,8-10 and are shown as mean values ± SEM.
Figure 3. Average food intake after gastric bypass surgery in rats. Average daily food intake of a representative group of rats after gastric bypass (black, n=52) and sham-operated rats (white, n=52) throughout a postoperative period of 60 days. Data were pooled from previous publications6,8-10 and are shown as mean values ± SEM.
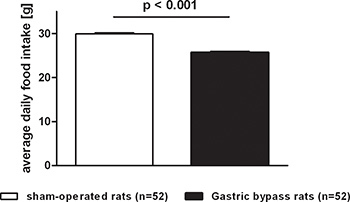 Figure 4. Postprandial PYY and GLP-1 serum levels after gastric bypass surgery in rats. Postprandial PYY and GLP-1 serum level for gastric bypass rats (black, n=18) and sham-operated rats (white, n=22). Data were pooled from previous publications8,16 and are shown as mean values ± SEM.
Figure 4. Postprandial PYY and GLP-1 serum levels after gastric bypass surgery in rats. Postprandial PYY and GLP-1 serum level for gastric bypass rats (black, n=18) and sham-operated rats (white, n=22). Data were pooled from previous publications8,16 and are shown as mean values ± SEM.
Discussion
The Roux-en-Y gastric bypass procedure in humans was first described by Mason in 1967 and modified to its current form by Torres in 198319. Currently, the procedure consists of a small gastric pouch and the bypass of the proximal small bowel. A schematic illustration of the pre- and postoperative anatomy is given in Figure 1.
Gastric bypass in humans induces and maintains body weight loss of approximately 15-30%2. The majority of body weight is lost during the first months after surgery partly due to reduced food intake, altered food preference and presumably increased energy expenditure4-6,8-10. Similarly to what has been observed in humans, our gastric bypass rat model induces a significant reduction in food intake and body weight. In contrast, gastric bypass models of others display a constant weight regain paralleling the body weight of sham-operated controls shortly after gastric bypass with no differences in food intake between sham-operated and gastric bypass operated rats20.
Postoperative variations in the course of body weight and food intake between published rat models may be partly related to differences in gastric pouch sizes. Large pouch sizes have been reported to cause insufficient weight loss or weight regain in humans21. The creation of a small gastric pouch in rats is technically demanding but feasible and a variety of different techniques has been described15. The pouch size of gastric bypass models in the rat literature ranges from <5% to more than 20% of the initial stomach size15. While we use curved microsurgical scissors, the majority of authors transect the stomach by using human stapler devices resulting in a preserved gastric pouch of at least 20% of the original stomach volume7,15,20,22-24. This is in contrast to how the gastric bypass procedure is usually performed in humans where at least 90% of the stomach is bypassed25 and many surgeons report that only 1-2% of the stomach is left contiguous with the small intestine26. Our rat model of gastric bypass therefore closely mimics the surgical procedure used in humans by creating a very small gastric pouch consisting of <5% of the original stomach volume27. Thus, in our rat model and in human patients, food moves directly into the jejunum rather than being diluted in the pouch by other food and fluids before it is then more slowly transported into the jejunum. Thus, if left too big, the gastric pouch may retain some storage capacity, consequently resulting in a different physiological state compared to human gastric bypass. Accordingly, gastric bypass rats with a pouch size of 20% or more of the original stomach size have been shown to still retain ingested contrast medium in their pouch long after ingestion has stopped7. Interestingly, differences in the gastric pouch size have also been shown to affect weight loss in humans21. Therefore, differences in gastric pouch sizes may affect the transit time of food to the small intestine, which in turn could impact on food intake and food preferences after gastric bypass.
Altered levels of gut hormones after gastric bypass in humans have been consistently demonstrated4,5,28, while some but not all of the published rat models of gastric bypass reported alterations in gut hormone levels15. If investigated, elevated levels of PYY and GLP-1 were found in both fasting and fed rats after gastric bypass15 which is in agreement with the findings in our gastric bypass model8,16,27. Elevated levels of PYY and GLP-1 have been previously demonstrated to increase satiation and reduce hunger in part mediated through actions on the hypothalamic arcuate nucleus and paraventricular nucleus, respectively29, but also in part through vagal afferents30. However, it remains unclear whether bypassing the hormonally active duodenum and proximal jejunum or whether increasing the delivery of undiluted bile and undigested foods to the distal small bowel, or both, stimulate the enteroendocrine L-cells to secrete more gut hormones such as PYY and GLP-1 after gastric bypass31,32. The effect of gastric bypass surgery on gut hormone levels has been systematically reviewed elsewhere33.
The impact of the different intestinal limb lengths in terms of body weight loss in humans is still controversially debated34-36, and there are also considerable differences in the limb lengths across the available gastric bypass rat models with an alimentary limb length varying between 10 cm and 50 cm, the biliopancreatic limb length ranging between 10 cm and 40 cm and a common channel between 18 cm and 34 cm15. A relatively short common channel of 25-30 cm characterizes our gastric bypass model which may suggest that the observed body weight loss may partly be the result of caloric malabsorption; however, we believe that caloric malabsorption is not a major mechanism of body weight loss in our rat model because bomb calorimetry demonstrated no differences in fresh faecal mass and calorie content between gastric bypass and sham-operated control rats when fed normal low fat chow6. However, others reported a small degree of fat malabsorption in gastric bypass models with a longer common channel (~50 cm) when rats were given a high fat diet7. Thus, caloric malabsorption may relate more to the dietary fat content than to the limb length.
To date, the relevance of the vagal nerve for body weight loss after gastric bypass is incompletely understood. We therefore selectively separate and ligate the left gastric vessels in our gastric bypass model for two reasons: First, to prevent major bleedings and second, to preserve the vagal fibers in the dorsal vagal trunk. We were able demonstrate that this selective technique leads to greater and more sustained body weight loss in gastric bypass rats suggesting that preserving vagal fibers of the dorsal vagal trunk during gastric bypass operations may be important27. This observation is consistent with previous reports showing that the ablation of the vagal-brainstem-hypothalamic pathway attenuates the inhibitory effects of PYY and GLP-1 on food intake30 and that specific vagal deafferentation abolishes the eating inhibitory effect of intraperitoneally injected GLP-137. However, while less interference with the stomach would presumably cause less damage to the vagus nerve, the extent to which vagal afferents are damaged by models with a greater gastric pouch than our model remains to be explicitly tested27.
The gastric bypass-related mortality of our model is approximately 15%27. Mortality rates after gastric bypass operations in rats are rarely stated by the authors, but seem to range from 0 to 35%15. In our hands mortality was predominantly due to leakage or stenosis of the gastro-jejunal anastomosis, bleeding after transection of the stomach, wound complications, anaesthesiological incidents and persistent excessive weight loss leading to compromised animal welfare15.
We are aware of the fact that our gastric bypass model carries various limitations. Firstly, although we strongly advocate the formation of a small gastric pouch, no formal proof whether the pouch still contains gastric mucosa has been made yet. In addition, the actual impact of the gastric pouch size as a single variable has not been analyzed. Secondly, the high technical demand of the small pouch technique in our model in comparison to the stapler technique used by others may limit its availability to research groups that have an adequately trained and skilled operator at their availability. Thirdly, many research groups focus on changes in glucose homeostasis after gastric bypass surgery. However, so far, we did not use our model to investigate postoperative glucose or lipid profiles; hence, the suitability of our model to answer such questions remains unknown. Finally, most of our experiments were performed in animals fed a standard low fat chow diet.
In conclusion, there is a large variety of gastric bypass rat models. Several components acting in concert lead to the observed physiological changes after gastric bypass, but the relative contribution of these components and their interaction remains unknown. The variety in published rat gastric bypass models complicates identification of specific physiological mechanisms involved in body weight loss after gastric bypass. Thus, there is an emerging need for standardization of the procedure to achieve consistent and comparable data. Thus far there is no evidence that any of the models is superior.
Disclosures
No conflicts of interest declared.
Acknowledgments
Marco Bueter and Florian Seyfried were supported by the Deutsche Forschungsgemeinschaft (DFG). Thomas A Lutz was supported by the Swiss National Research Foundation (SNF). Marco Bueter and Thomas A Lutz further receive funding from the National Institute of Health (NIH) and from the Zurich Center for Integrative Human Physiology (ZIHP). Carel W le Roux was supported by a Department of Health Clinician scientist award. Imperial College London receives support from the NIHR Biomedical Research Centre funding scheme.
References
- Adams TD, Gress RE, Smith SC. Long-term mortality after gastric bypass surgery. N. Engl. J. Med. 2007;357:753–761. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- Sjostrom L, Lindroos AK, Peltonen M. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N. Engl. J. Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- Buchwald H, Oien DM. Metabolic/bariatric surgery Worldwide. Obes. Surg. 2008;19:1605–1611. doi: 10.1007/s11695-009-0014-5. [DOI] [PubMed] [Google Scholar]
- Welbourn R, Werling M. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann. Surg. 2007;246:780–785. doi: 10.1097/SLA.0b013e3180caa3e3. [DOI] [PubMed] [Google Scholar]
- le Roux CW, Aylwin SJ, Batterham RL. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann. Surg. 2006;243:108–114. doi: 10.1097/01.sla.0000183349.16877.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueter M, Lowenstein C, Olbers T. Gastric bypass increases energy expenditure in rats. Gastroenterology. 2010;138:1845–1853. doi: 10.1053/j.gastro.2009.11.012. [DOI] [PubMed] [Google Scholar]
- Stylopoulos N, Hoppin AG, Kaplan LM. Roux-en-Y Gastric Bypass Enhances Energy Expenditure and Extends Lifespan in Diet-induced Obese Rats. Obesity (Silver Spring) 2009;17:1839–1847. doi: 10.1038/oby.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueter M, Miras AD, Chichger H. Alterations of sucrose preference after Roux-en-Y gastric bypass. Physiol. Behav. 2011;104:709–721. doi: 10.1016/j.physbeh.2011.07.025. [DOI] [PubMed] [Google Scholar]
- le Roux CW, Bueter M, Theis N. Gastric bypass reduces fat intake and preference. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;301:1057–1066. doi: 10.1152/ajpregu.00139.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueter M, Ashrafian H, Frankel AH. Sodium and water handling after gastric bypass surgery in a rat model. Surg. Obes. Relat. Dis. 2011;7:68–73. doi: 10.1016/j.soard.2010.03.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JV, Ashrafian H, Bueter M. Metabolic surgery profoundly influences gut microbial-host metabolic cross-talk. Gut. 2011;60:1214–1223. doi: 10.1136/gut.2010.234708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafian H, le Roux CW. Metabolic surgery and gut hormones - a review of bariatric entero-humoral modulation. Physiol. Behav. 2009;97:620–631. doi: 10.1016/j.physbeh.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Ashrafian H, Bueter M, Ahmed K. Metabolic surgery: an evolution through bariatric animal models. Obes. Rev. 2010;11:907–920. doi: 10.1111/j.1467-789X.2009.00701.x. [DOI] [PubMed] [Google Scholar]
- Rao RS, Rao V, Kini S. Animal models in bariatric surgery--a review of the surgical techniques and postsurgical physiology. Obes. Surg. 2010;20:1293–1305. doi: 10.1007/s11695-010-0135-x. [DOI] [PubMed] [Google Scholar]
- Seyfried F, le Roux CW, Bueter M. Lessons learned from gastric bypass operations in rats. Obes. Facts. 2011;4:3–12. doi: 10.1159/000327301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenske WK, Bueter M, Miras AD. Exogenous peptide YY3-36 and Exendin-4 further decrease food intake, whereas octreotide increases food intake in rats after Roux-en-Y gastric bypass. Int. J. Obes. (Lond) 2011. [DOI] [PubMed]
- Kreymann B, Williams G, Ghatei MA. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet. 1987;2:1300–1304. doi: 10.1016/s0140-6736(87)91194-9. [DOI] [PubMed] [Google Scholar]
- le Roux CW, Batterham RL, Aylwin SJ. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology. 2006;147:3–8. doi: 10.1210/en.2005-0972. [DOI] [PubMed] [Google Scholar]
- Torres JC, Oca CF, Garrison RN. Gastric bypass: Roux-en-Y gastrojejunostomy from the lesser curvature. South Med. J. 1983;76:1217–1221. [PubMed] [Google Scholar]
- Guijarro A, Suzuki S, Chen C. Characterization of weight loss and weight regain mechanisms after Roux-en-Y gastric bypass in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;293:R1474–R1489. doi: 10.1152/ajpregu.00171.2007. [DOI] [PubMed] [Google Scholar]
- Roberts K, Duffy A, Kaufman J. Size matters: gastric pouch size correlates with weight loss after laparoscopic Roux-en-Y gastric bypass. Surg. Endosc. 2007;21:1397–1402. doi: 10.1007/s00464-007-9232-x. [DOI] [PubMed] [Google Scholar]
- Hajnal A, Kovacs P, Ahmed T. Gastric bypass surgery alters behavioral and neural taste functions for sweet taste in obese rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;299:G967–G979. doi: 10.1152/ajpgi.00070.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin AC, Zheng H, Pistell PJ. Roux-en-Y gastric bypass surgery changes food reward in rats. Int. J. Obes. (Lond) 2011;35:642–651. doi: 10.1038/ijo.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tichansky DS, Rebecca GA, Madan AK. Decrease in sweet taste in rats after gastric bypass surgery. Surg. Endosc. 2011;25:1176–1181. doi: 10.1007/s00464-010-1335-0. [DOI] [PubMed] [Google Scholar]
- Olbers T, Lonroth H, Fagevik-Olsen M. Laparoscopic gastric bypass: development of technique, respiratory function, and long-term outcome. Obes. Surg. 2003;13:364–370. doi: 10.1381/096089203765887679. [DOI] [PubMed] [Google Scholar]
- Madan AK, Harper JL, Tichansky DS. Techniques of laparoscopic gastric bypass: on-line survey of American Society for Bariatric Surgery practicing surgeons. Surg. Obes. Relat. Dis. 2008;4:166–172. doi: 10.1016/j.soard.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Bueter M, Lowenstein C, Ashrafian H. Vagal sparing surgical technique but not stoma size affects body weight loss in rodent model of gastric bypass. Obes. Surg. 2010;20:616–622. doi: 10.1007/s11695-010-0075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korner J, Bessler M, Cirilo LJ. Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J. Clin. Endocrinol. Metab. 2005;90:359–365. doi: 10.1210/jc.2004-1076. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Abbott CR, Monteiro M, Small CJ. The inhibitory effects of peripheral administration of peptide YY(3-36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res. 2005;1044:127–131. doi: 10.1016/j.brainres.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Adrian TE, Ballantyne GH, Longo WE. Deoxycholate is an important releaser of peptide YY and enteroglucagon from the human colon. Gut. 1993;34:1219–1224. doi: 10.1136/gut.34.9.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani H, Kasama K, Oshiro T. Serum bile acid along with plasma incretins and serum high-molecular weight adiponectin levels are increased after bariatric surgery. Metabolism. 2009;58:1400–1407. doi: 10.1016/j.metabol.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Ashrafian H, le Roux CW. Metabolic surgery and gut hormones - a review of bariatric entero-humoral modulation. Physiol. Behav. 2009;97:620–631. doi: 10.1016/j.physbeh.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Choban PS, Flancbaum L. The effect of Roux limb lengths on outcome after Roux-en-Y gastric bypass: a prospective, randomized clinical trial. Obes. Surg. 2002;12:540–545. doi: 10.1381/096089202762252316. [DOI] [PubMed] [Google Scholar]
- Gleysteen JJ. Five-year outcome with gastric bypass: Roux limb length makes a difference. Surg. Obes. Relat. Dis. 2009;5:242–247. doi: 10.1016/j.soard.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Lee S, Sahagian KG, Schriver JP. Relationship between varying Roux limb lengths and weight loss in gastric bypass. Curr. Surg. 2006;63:259–263. doi: 10.1016/j.cursur.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Hayes MR, Kanoski SE, De Jonghe BC. The common hepatic branch of the vagus is not required to mediate the glycemic and food intake suppressive effects of glucagon-like-peptide-1. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;301:R1479–R1485. doi: 10.1152/ajpregu.00356.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


