Abstract
Functional brain mapping based on changes in local cerebral blood flow (lCBF) or glucose utilization (lCMRglc) induced by functional activation is generally carried out in animals under anesthesia, usually α-chloralose because of its lesser effects on cardiovascular, respiratory, and reflex functions. Results of studies on the role of nitric oxide (NO) in the mechanism of functional activation of lCBF have differed in unanesthetized and anesthetized animals. NO synthase inhibition markedly attenuates or eliminates the lCBF responses in anesthetized animals but not in unanesthetized animals. The present study examines in conscious rats and rats anesthetized with α-chloralose the effects of vibrissal stimulation on lCMRglc and lCBF in the whisker-to-barrel cortex pathway and on the effects of NO synthase inhibition with NG-nitro-l-arginine methyl ester (l-NAME) on the magnitude of the responses. Anesthesia markedly reduced the lCBF and lCMRglc responses in the ventral posteromedial thalamic nucleus and barrel cortex but not in the spinal and principal trigeminal nuclei. l-NAME did not alter the lCBF responses in any of the structures of the pathway in the unanesthetized rats and also not in the trigeminal nuclei of the anesthetized rats. In the thalamus and sensory cortex of the anesthetized rats, where the lCBF responses to stimulation had already been drastically diminished by the anesthesia, l-NAME treatment resulted in loss of statistically significant activation of lCBF by vibrissal stimulation. These results indicate that NO does not mediate functional activation of lCBF under physiological conditions.
Keywords: whisker-to-barrel cortex pathway, cerebral glucose utilization, deoxy[14C]glucose, functional brain imaging, iodo[14C]antipyrine
Neuronal functional activation is normally associated with increases in local cerebral glucose utilization (lCMRglc) and blood flow (lCBF) in anatomic units of the activated neural pathways. These associations are now widely exploited to map regions of the brain involved in specific neural and cognitive processes. The mechanisms underlying the functional activation of lCMRglc are reasonably well understood. Glucose utilization is increased by functional activation in direct proportion to the increases in spike frequency in the afferent inputs to the activated areas, and the increases are localized in neuropil and not perikarya (1, 2). The increases in lCMRglc appear to result mainly from activation of Na+,K+-ATPase activity (2, 3), needed to restore ionic gradients in the neuronal elements degraded by the spike activity. Neuropil contains not only axonal and dendritic processes but also astroglial processes, and glutamate stimulates glucose utilization in astroglia, also due in part to activation of Na+,K+-ATPase activity by coupled uptake of Na+ ions with the glutamate released during functional activation (4, 5). Glucose utilization is also increased to support the ATP-dependent conversion to glutamine of the glutamate taken up by the astroglia (6).
The mechanisms mediating the increases in lCBF during functional activation are less clear. Roy and Sherrington (7) hypothesized that the cerebral blood flow (CBF) is intrinsically regulated by products of energy metabolism to adjust it to the altered metabolic demands associated with changes in functional activity. Subsequent findings that CBF is elevated by a rise in Pco2, a fall in pH, or a lowering of Po2, expected consequences of increased energy metabolism, and reduced by their changes in the opposite direction, to be expected with decreased metabolism (8), offered support to this hypothesis. There are now, however, many other endogenous substances known to affect cerebral blood vessels—e.g., neurotransmitters, neuromodulators, nitric oxide, adenosine, adenine nucleotides, K+, prostaglandins, vasoactive intestinal peptide, etc. Some of these have been considered as possible mediators, but not one, either alone or in combination with others, has yet been proved to account fully or even to be essential for the enhancement of CBF by neuronal activation. Nitric oxide is a current popular candidate. This potent vasodilator is synthesized in brain by two constitutive isoforms of nitric oxide synthase, one in the vascular endothelium and the other in neurons, and an inducible one in glia. There have been a number of reports that activation of local CBF by sensory stimulation is abolished or, at least, markedly diminished by chemically induced inhibition of nitric oxide synthase (9–17). Studies in knockout mice lacking either the endothelial or neuronal nitric oxide synthase genes showed no loss of functional activation of CBF, but when the remaining isoforms were inhibited with drugs, results were obtained that were interpreted as evidence of an essential role for neuronal nitric oxide synthase in the CBF response to functional activation (18, 19). All of these studies implicating nitric oxide were done, however, in anesthetized animals. Studies in unanesthetized animals with quantitative determinations of CBF have generally failed to confirm that nitric oxide is essential for the functional activation of CBF. In two studies in unanesthetized rats (20, 21), nonselective inhibition of all nitric oxide synthase isoforms by NG-nitro-L-arginine methyl ester (L-NAME) had no significant effects on percent enhancements of lCBF by vibrissal stimulation in all four anatomical structures in the whisker-to-barrel cortex sensory pathway that were examined. In another study in unanesthetized rats, inhibition of neuronal nitric oxide synthase with 7-nitroindazole did reduce but did not abolish activation of lCBF by vibrissal stimulation in the thalamus and barrel cortex, stations of the pathway containing higher level synapses, but it had no effects on activation of lCBF in the principal and spinal trigeminal nuclei, the stations with the primary synapses (22).
An obvious difference between studies supporting and not supporting a role for nitric oxide in the functional activation of CBF is the use of anesthesia, which has been reported to alter the magnitude of functional activation of energy metabolism (23). Therefore, to examine systematically the influence of anesthesia on functional activation of energy metabolism and CBF and on the role of nitric oxide, we have examined with quantitative methods the effects of anesthesia on baseline and functionally activated glucose utilization and CBF in the stations of the whisker-to-barrel cortex pathway before and after inhibition of nitric oxide synthase activity.
Materials and Methods
Chemicals.
Chemicals were obtained from the following sources: 2-deoxy-d-[1-14C]glucose (2-deoxy[14C]glucose; specific activity, 53 mCi/mmol) and 4-iodo[N-methyl-14C]antipyrine, iodo[14C]antipyrine; specific activity, 54 mCi/mmol) from DuPont NEN (Boston, MA); (L-NAME) and α-chloralose were obtained from Sigma.
Animal Preparation.
All procedures performed on the animals were in strict accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and approved by the local Animal Care and Use Committee.
Normal adult male Sprague–Dawley rats (308–398 g) were purchased from Charles River Laboratories and maintained in a climate-controlled room on a normal 12-h light/dark cycle with food and water available ad libitum. They were fasted but allowed free access to water for 16 h before the experiment. The rats were anesthetized with halothane (5% for induction, 0.8–1.0% for maintenance) in 70% N2O/30% O2. Rats to be studied later under α-chloralose anesthesia were intubated with an endotracheal catheter while under halothane anesthesia and then mechanically ventilated with the same anesthetic mixture until completion of the surgical preparation. Polyethylene catheters (PE 50; Clay Adams) were inserted in both femoral arteries and veins; one arterial catheter was used for recording mean arterial blood pressure and the other for sampling of arterial blood. Catheter length was fixed at 16 cm to simplify correction for lag and dead space washout in the sampling system (24, 25). One venous catheter was used for injection of tracer and L-NAME or saline vehicle and the other for α-chloralose when administered. Lidocaine ointment (5%) was applied to the surgical wounds after closure. Rats to be studied in the conscious state were fitted with a loose-fitting plaster cast that was applied to the pelvic area and taped to a lead brick to prevent locomotion; anesthetized rats did not require such restraint. Body temperature was continuously monitored by a rectal probe and maintained at 37°C throughout the surgical and subsequent experimental procedures by a thermostatically controlled heating lamp or heating pad (model 73A; Yellow Springs Instruments).
Anesthetic Conditions During Experimental Procedures.
lCBF and lCMRglc were determined in conscious unanesthetized rats and in rats under α-chloralose anesthesia. α-Chloralose anesthesia was chosen because it is believed to be least depressant of neural functions. It is often used in studies of functional activation, and we administered it as described in one such previous study (26). Immediately after termination of the halothane anesthesia, an acute dose of α-chloralose (50 mg/kg), dissolved in physiological saline, was injected i.v. and followed by a continuous i.v. infusion at a rate of 40 mg/kg per h. The rats were ventilated with 30% oxygen/70% nitrogen throughout the procedure. Unanesthetized rats received equivalent injections and infusions of saline. lCBF and lCMRglc were determined about 3 h after termination of the halothane anesthesia in the unanesthetized group and about 45 min after switching from halothane to α-chloralose in the anesthetized group.
Inhibition of Nitric Oxide Synthase.
Effects of nitric oxide synthase inhibition on baseline and functionally activated lCBF and lCMRglc were studied in unanesthetized and anesthetized rats. About 30 min before determination of lCBF or lCMRglc, L-NAME (30 mg/kg), dissolved in normal saline, was infused i.v. over a 3-min period; control rats were infused with equivalent amounts of saline.
Vibrissal Stimulation.
Vibrissae were stimulated unilaterally by stroking the whiskers on the left side of the face continuously with a soft paint brush at a frequency of 2–3/s. It was initiated simultaneously with the onset of determination of either lCBF or lCMRglc and continued throughout the 1-min period of lCBF determination or the 45 min needed for lCMRglc determination. The whiskers on the right side had previously been clipped flush with the skin to avoid spurious stimulation on the control side.
Monitoring of Physiological Variables.
Several physiological variables with influence on CBF and metabolism were measured immediately after the surgery and periodically thereafter. Mean arterial blood pressure was monitored with a blood pressure analyzer (model 300; Digi-Med, Louisville, KY) that had been calibrated with an air-damped mercury manometer. Arterial blood carbon dioxide (PaCO2) and oxygen (Pao2) tensions and pH were measured with a blood gas analyzer (model 288 Blood Gas System; CIBA-Corning Diagnostics Corp., Medfield, MA). Arterial plasma glucose levels were determined in a Glucose Analyzer 2 (Beckman Instruments, Fullerton, CA).
Measurements of lCBF and lCMRglc.
lCMRglc was determined by the quantitative autoradiographic 2-deoxy[14C]glucose method (27). lCBF was determined by the quantitative autoradiographic iodo[14C]antipyrine method as described by Sakurada et al. (28), except that the iodo[14C]antipyrine (40 μCi in 0.8 ml of physiological saline) was infused continuously via the femoral venous catheter by a computer-driven infusion pump (model 2400-003; Harvard Apparatus) programmed to produce an approximately linear rise in arterial tracer concentration throughout the 1-min experimental period. Corrections for delay and dispersion in the arterial sampling system were included in the computation of lCBF (24, 25).
lCBF and lCMRglc were determined bilaterally in four stations of the whisker-to-barrel cortex pathway, i.e., spinal trigeminal and principal sensory trigeminal nuclei, ventral posteromedial (VPM) nucleus of the thalamus, and the barrel field of the somatosensory cortex. Values for lCBF or lCMRglc in the homologous structures of the stimulated and unstimulated sides were compared to assess the effects of stimulation. lCBF or lCMRglc was also determined bilaterally in four structures outside this sensory pathway (i.e., cerebellar white matter, caudate putamen, primary motor cortex, and nucleus accumbens) to evaluate possible effects of vibrissal stimulation, L-NAME, and anesthesia on structures not directly related to the activation. Anatomical structures were identified in the autoradiograms by comparison of the thionine-stained sections of brain corresponding to the autoradiograms with the rat brain atlas of Paxinos and Watson (29).
Statistical Analyses.
Data are presented as means ± SEM. Statistical significance of differences in lCBF or lCMRglc between stimulated and unstimulated sides was determined by paired t tests with Bonferroni correction. Significance of the effects of α-chloralose and/or L-NAME on baseline values of lCBF and lCMRglc in the unstimulated control side was evaluated by unpaired t tests with Bonferroni correction. The Kruskal–Wallis test (one-way ANOVA by ranks) was used to determine statistical significance of the effects of anesthesia and/or L-NAME on percent differences in lCBF or lCMRglc between unstimulated and stimulated sides followed by Dunn's test if statistical significance (P < 0.05) was found.
Results
Physiological Variables.
Rats anesthetized with α-chloralose were hypersensitive to loud sounds, and hand clapping evoked myoclonic responses. Their arterial Pao2 and pH levels were higher than those of the unanesthetized groups, undoubtedly because of the mechanical ventilation (Table 1). L-NAME raised systemic arterial blood pressure substantially in both conscious and anesthetized groups, evidence that at least systemic endothelial nitric oxide synthase activity was effectively inhibited (Table 1). Other physiological variables were unaffected by L-NAME.
Table 1.
Physiological variables
| Physiological variables | Conscious
|
α-Chloralose anesthesia
|
||
|---|---|---|---|---|
| Saline control | l-NAME | Saline control | l-NAME | |
| Before measurement of CBF | n = 6 | n = 6 | n = 7 | n = 6 |
| Mean arterial blood pressure, mmHg | 118 ± 2 | 152 ± 2** | 114 ± 5 | 141 ± 3† |
| Paco2, mmHg | 34 ± 1 | 32 ± 1 | 32 ± 1 | 33 ± 1 |
| Pao2, mmHg | 89 ± 3 | 93 ± 2 | 138 ± 6** | 128 ± 9 |
| Arterial pH | 7.45 ± 0.01 | 7.45 ± 0.01 | 7.49 ± 0.01** | 7.48 ± 0.01 |
| Plasma glucose, mM | 7.6 ± 0.3 | 6.9 ± 0.2 | 6.5 ± 0.3 | 5.7 ± 0.3 |
| Before measurement of cerebral glucose utilization | n = 6 | n = 6 | n = 5 | n = 5 |
| Mean arterial blood pressure, mmHg | 121 ± 4 | 158 ± 4** | 121 ± 4 | 140 ± 6† |
| Paco2, mmHg | 36 ± 1 | 36 ± 1 | 33 ± 1 | 34 ± 1 |
| Pao2, mmHg | 90 ± 3 | 95 ± 2 | 128 ± 8** | 111 ± 10 |
| Arterial pH | 7.44 ± 0.01 | 7.44 ± 0.01 | 7.48 ± 0.01** | 7.45 ± 0.01 |
| Plasma glucose, mM | 7.1 ± 0.4 | 6.2 ± 0.1 | 6.2 ± 0.1 | 5.8 ± 0.3 |
Values are means ± SEM of number of animals in parentheses.
, P < 0.05;
, P < 0.01;
, P < 0.001 compared to the conscious saline controls.
, P < 0.001 compared to the α-chloralose-anesthetized saline-treated groups.
Effects of α-Chloralose Anesthesia and l-NAME on Baseline CBF and Glucose Utilization.
Unilateral vibrissal stimulation produced no statistically significant side-to-side differences in lCMRglc or lCBF in any of the structures outside the whisker-to-barrel cortex pathway (Table 2). Effects of α-chloralose anesthesia and L-NAME on baseline lCMRglc and lCBF were ascertained from their effects in these structures outside the pathway, as well as in the structures of the whisker-to-barrel cortex pathway on the unstimulated side of the brain.
Table 2.
Effects of α-chloralose anesthesia and l-NAME on CBF and glucose utilization in some representative cerebral structures outside whisker-to-barrel cortex sensory pathway
| Structure and treatment | CBF, ml/100 g/min
|
Cerebral glucose utilization, μmol/100 g/min
|
||||||
|---|---|---|---|---|---|---|---|---|
| n | Left side | Right side | % L-R diff. | n | Left side | Right side | % L-R diff. | |
| Cerebellar white matter | ||||||||
| Conscious + saline | 6 | 50 ± 2 | 50 ± 1 | 2 ± 3 | 6 | 33 ± 2 | 34 ± 2 | 3 ± 2 |
| Conscious + l-NAME | 6 | 34 ± 2** | 35 ± 1 | 2 ± 4 | 6 | 31 ± 1 | 31 ± 1 | 0.3 ± 2 |
| Anesthetized + saline | 7 | 24 ± 2** | 24 ± 3 | −1 ± 4 | 5 | 18 ± 1** | 18 ± 1 | −2 ± 2 |
| Anesthetized + l-NAME | 6 | 18 ± 1 | 18 ± 1 | 4 ± 3 | 5 | 19 ± 1 | 18 ± 1 | −6 ± 2 |
| Caudate-putamen | ||||||||
| Conscious + saline | 6 | 164 ± 13 | 165 ± 11 | 0.5 ± 2 | 6 | 120 ± 3 | 121 ± 4 | 1 ± 1 |
| Conscious + l-NAME | 6 | 139 ± 8 | 138 ± 9 | −1 ± 2 | 6 | 129 ± 4 | 129 ± 4 | −0.2 ± 1 |
| Anesthetized + saline | 7 | 68 ± 5** | 66 ± 6 | −4 ± 3 | 5 | 44 ± 3** | 43 ± 3 | −1 ± 1 |
| Anesthetized + l-NAME | 6 | 47 ± 1† | 44 ± 2 | −6 ± 2 | 5 | 49 ± 1 | 47 ± 1 | −3 ± 2 |
| Motor cortex | ||||||||
| Conscious + saline | 6 | 165 ± 10 | 197 ± 17 | 19 ± 5 | 6 | 103 ± 2 | 108 ± 3 | 5 ± 2 |
| Conscious + l-NAME | 6 | 139 ± 11 | 152 ± 14 | 9 ± 3 | 6 | 110 ± 3 | 115 ± 3 | 4 ± 1 |
| Anesthetized + saline | 7 | 58 ± 4** | 57 ± 4 | −1 ± 1 | 5 | 37 ± 3** | 37 ± 3 | 2 ± 2 |
| Anesthetized + l-NAME | 6 | 42 ± 1† | 42 ± 1 | 1 ± 3 | 5 | 42 ± 2 | 41 ± 2 | −2 ± 2 |
| Nucleus accumbens core | ||||||||
| Conscious + saline | 6 | 172 ± 11 | 171 ± 14 | −1 ± 3 | 6 | 72 ± 2 | 73 ± 3 | 1 ± 2 |
| Conscious + l-NAME | 6 | 117 ± 13* | 112 ± 11 | −4 ± 3 | 6 | 76 ± 4 | 74 ± 3 | −2 ± 1 |
| Anesthetized + saline | 5 | 59 ± 8** | 59 ± 8 | −1 ± 2 | 5 | 32 ± 3** | 32 ± 3 | −1 ± 1 |
| Anesthetized + l-NAME | 6 | 34 ± 2 | 36 ± 1 | 5 ± 4 | 5 | 33 ± 1 | 33 ± 1 | −1 ± 2 |
Values are means ± SEM of number of animals indicated. Percent L-R diff. (left-right difference) is the mean of the individual side-to-side percent differences obtained in each rat and not the percent difference between the means. Conscious saline-treated controls vs. anesthetized saline-treated or conscious l-NAME-treated groups (only left side statistically analyzed),
, P < 0.05;
, P < 0.01;
, P < 0.001 (Bonferroni corrected). Anesthetized saline-treated vs. anesthetized l-NAME-treated groups (only left side statistically analyzed),
, P < 0.05 (Bonferroni corrected).
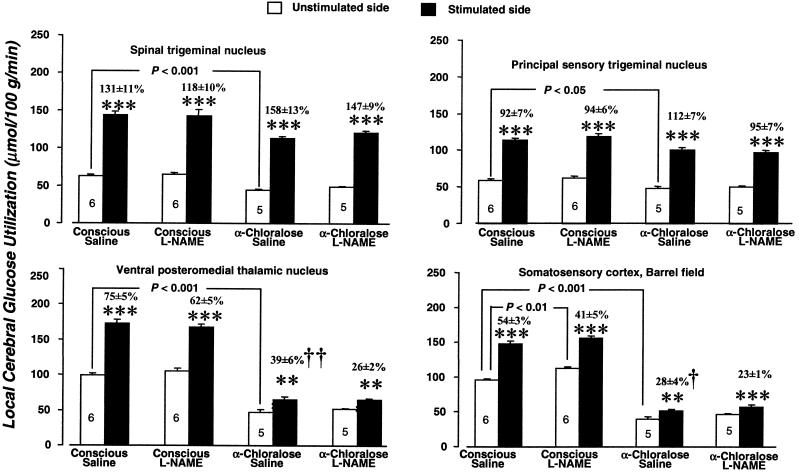
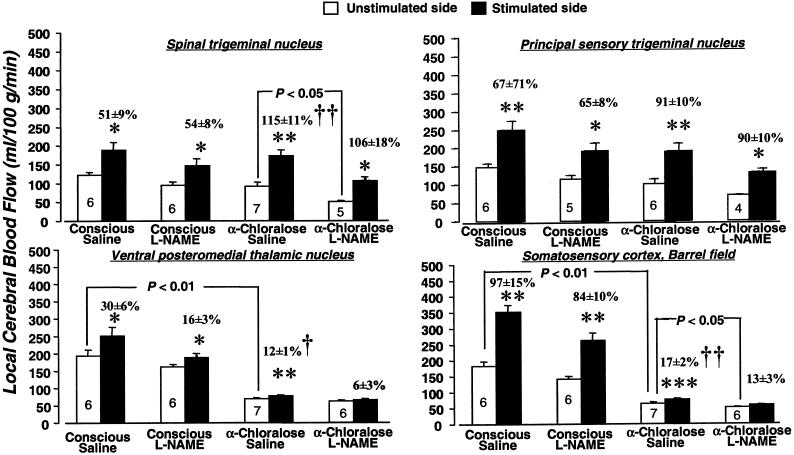
α-Chloralose anesthesia markedly lowered baseline levels of both lCMRglc and lCBF in all of the structures outside the whisker-to-barrel cortex pathway that were examined (Table 2). These effects on lCBF are similar to those previously observed with isoflurane anesthesia (30). Anesthesia also significantly reduced baseline lCMRglc in all of the stations of the pathway on the unstimulated control side (Fig. 1) but reduced lCBF statistically significantly only in the VPM thalamic nucleus and sensory cortex and not in the two trigeminal nuclei (Fig. 2).
Figure 1.
Effects of α-chloralose anesthesia and L-NAME (30 mg/kg) on baseline rates of glucose utilization and on the increases in glucose utilization due to unilateral vibrissal stimulation in four stations of the whisker-to-barrel cortex pathway. Bar heights and error bars represent mean rates of local glucose utilization + SEM obtained in the number of rats indicated. **, P < 0.01; ***, P < 0.001 for side-to-side differences between stimulated and unstimulated sides (paired t test with Bonferroni correction). Bracketed P values are for comparison of the values of glucose utilization in the structures and sides indicated by the brackets (unpaired t test with Bonferroni correction). †, P < 0.05; ††, P < 0.01, statistical significance of difference in percent increase in glucose utilization attributable to stimulation in indicated condition from percent increases due to stimulation found in conscious saline-treated controls, determined by Kruskal–Wallis test (one-way ANOVA by ranks) with Dunn's test.
Figure 2.
Effects of α-chloralose anesthesia and L-NAME (30 mg/kg) on baseline blood flow and increases in blood flow due to vibrissal stimulation in four stations of the whisker-to-barrel cortex pathway. Bar heights and error bars represent mean rates of blood flow + SEM obtained in the number of rats indicated. *, P < 0.05; **, P < 0.01; ***, P < 0.001 for side-to-side differences between stimulated and unstimulated sides (paired t test with Bonferroni correction). Bracketed P values are for comparison of the values of blood flow in the structures and sides indicated by the brackets (unpaired t test with Bonferroni correction). †, P < 0.05; ††, P < 0.01, statistical significance of difference in percent increase in blood flow attributable to stimulation in indicated condition from percent increases due to stimulation found in conscious saline-treated controls, determined by Kruskal–Wallis test (one-way ANOVA by ranks) with Dunn's test.
L-NAME administration reduced baseline lCBF in all of the structures outside the whisker-to-barrel cortex pathway in both conscious and anesthetized rats, although some of these reductions fell short of statistical significance after Bonferroni correction for multiple comparisons (Table 2). L-NAME also tended to lower lCBF in the stations of the pathway on the unstimulated side in both conscious and anesthetized rats, but the reductions were statistically significant only in the spinal trigeminal nucleus and barrel cortex of the anesthetized rats. As previously observed (31, 32) L-NAME had no effects on baseline lCMRglc (Table 2), except for a slight but statistically significant increase in the sensory cortex of the conscious rats (Fig. 1).
Functional Activation of lCBF and lCMRglc.
In conscious saline-treated rats, whisker stroking on the left side of the face markedly and statistically significantly increased both lCBF and lCMRglc in the left spinal and principal trigeminal nuclei and right VPM thalamic nucleus and sensory cortex above their levels in the contralateral homologous structures (Figs. 1 and 2). Because both anesthesia and L-NAME altered the baseline levels of lCMRglc and lCBF in these structures on the unstimulated side, the percent difference between the stimulated and unstimulated sides were used to assess their effects on the magnitude of functional activation.
α-Chloralose anesthesia did not significantly alter percent increases in lCMRglc due to vibrissal stimulation in the spinal and principal trigeminal nuclei from their values in unanesthetized rats, but it markedly reduced them from 75 ± 5% to 39 ± 6% (P < 0.01) in the VPM thalamic nucleus and from 54 ± 3% to 28 ± 4% (P < 0.05) in the barrel cortex (Fig. 1). In contrast, anesthesia tended to enhance percent differences in lCBF between stimulated and unstimulated sides in both trigeminal nuclei; the increase from 51 ± 9% to 115 ± 11% in the spinal trigeminal nucleus reaching statistical significance (P < 0.01). Anesthesia, however, markedly depressed the percent increases due to stimulation from 30 ± 6% to 12 ± 1% (P < 0.05) in the VPM thalamic nucleus and from 97 ± 15 to 17 ± 2% (P < 0.01) in the barrel cortex (Fig. 2).
L-NAME tended to reduce the percent differences in lCMRglc between stimulated and unstimulated sides in all structures of the pathway in both conscious and anesthetized rats. None of these reductions was statistically significant, however, and side-to-side differences retained their statistical significance in all of the structures (Fig. 1). Similarly, L-NAME had no statistically significant effects on percent differences in lCBF between stimulated and unstimulated sides in any of the stations of the pathway in both the conscious and anesthetized rats (Fig. 2). It did, however, tend to reduce slightly the effects of stimulation in the VPM thalamic nucleus and barrel cortex, and in anesthetized rats in which anesthesia had already markedly reduced both baseline and stimulated lCBF, this was sufficient to eliminate the statistical significance of the differences between stimulated and unstimulated sides (Fig. 2).
Discussion
Functional brain imaging, as currently used, is based largely on localizing changes in rates of CBF or glucose utilization, or indices of such changes, that are induced by alterations in specific neural functions. In humans it is usually applied in the conscious state, but its use in animals is often limited to the anesthetized state because of regulatory and/or physical constraints. General anesthetics have many effects on CBF and metabolism (33, 34), but α-chloralose is commonly used in studies involving functional activation because of its lesser effects on cardiovascular and respiratory functions and reflex activities. Ueki et al. (35) compared the effects of α-chloralose, halothane, pentobarbital, and nitrous oxide on activation of lCMRglc evoked by forepaw electrical stimulation in rats and found that the pattern of metabolic activation in the primary somatosensory cortex under α-chloralose anesthesia to be most similar to that in conscious animals. Lindauer et al. (26) compared effects of α-chloralose, halothane, and thiobarbiturate on lCBF responses to vibrissal stimulation in the sensory cortex in rats; they concluded that α-chloralose anesthesia is the one most suitable for studying lCBF coupling to neuronal functional activation because the lCBF responses were greater in α-chloralose-anesthetized (+16.9%) than in thiobarbiturate-anesthetized (+10.6%) rats and more stable than those in halothane-anesthetized rats. None of these studies, however, included direct comparisons between anesthetized and unanesthetized states.
α-Chloralose does, in fact, have many effects on neuronal functions (36). These include not only analgesia and unconsciousness, but also enhanced γ-aminobutyric acid type A receptor activity similar to that produced by barbiturates (37) and nonuniform localized reductions in lCMRglc (38). The results of the present studies confirm that α-chloralose anesthesia lowers lCMRglc throughout the brain (Table 2 and Fig. 1) and also show that it tends to lower lCBF generally but not statistically significantly in all structures examined (Table 2 and Fig. 2). Furthermore, anesthesia altered not only baseline levels of lCMRglc and lCBF but also the magnitude of their responses to neuronal functional activation that varied in the different stations of the activated neural pathway (Figs. 1 and 2). Anesthesia had relatively little effect on baseline and activated levels of lCMRglc and lCBF in the spinal and principal trigeminal nuclei, but it markedly diminished all of them in the VPM thalamic nucleus and sensory cortex to the point where effects of functional activation, particularly on lCBF, were barely, if at all, detectable (Figs. 1 and 2). It is of interest that the effects of anesthesia were mainly in the VPM thalamic nucleus and sensory cortex, which contain secondary and tertiary synapses of the pathway, but were small or absent in the brainstem nuclei, the direct projection zones from the peripheral receptors and sites of the primary synapses. Inasmuch as evoked metabolic responses to functional activation have been shown to be localized in neuropil and directly related to spike frequency in the afferent inputs to the synapses (1, 2), this finding suggests that the anesthesia inhibits mainly synaptic functions and has little effect, if any, on the output of the peripheral receptors. Such regional differences in the effects of anesthesia on the functional activation of lCBF or lCMRglc could lead to biases in identifying and localizing structures related to specific neural functional activations.
α-Chloralose anesthesia is also widely used in studies of the mechanisms of the lCBF responses to functional activation. The role of nitric oxide has been intensively examined, and most of these studies were carried out in anesthetized animals in which lCBF was not measured quantitatively but assessed indirectly by laser-Doppler flowmetry and only in the cerebral cortex. These studies generally showed that nonspecific inhibition of nitric oxide synthase activity with L-NAME or only of the neuronal isoform with 7-nitroindazole markedly depresses or eliminates lCBF responses to sensory stimulation in the sensory cortex (9–17). In contrast, those studies that were carried out in unanesthetized animals with quantitative measurements of lCBF in several stations of the activated pathway have failed to show that inhibition of nitric oxide synthase activity by L-NAME reduces the percent increases in lCBF evoked by vibrissal stimulation in any of the stations of the pathway (20–22). In the study by Gotoh et al. (22), inhibition of the neuronal nitric oxide synthase with 7-nitroindazole had no effects in the spinal and principal trigeminal nuclei but did diminish somewhat the increases in lCBF evoked by vibrissal stimulation in the VPM thalamic nucleus and barrel cortex. The restricted localization of the effects of 7-nitroindazole to the thalamus and cortex, sites of the higher level synapses in the pathway, suggests that the diminished lCBF responses might have been due more to the effects of nitric oxide deprivation on neuronal functional activation than on the mechanisms of coupling of blood flow to functional activation. Nitric oxide, produced by neurons, may be involved in interneuronal signal transduction (39–41), which might be disrupted by 7-nitroindazole, an inhibitor of neuronal nitric oxide synthase, although it has been reported that 7-nitroindazole has no effects on baseline lCMRglc in conscious rats (42) and that it does not reduce the lCMRglc responses to whisker stimulation in rats under α-chloralose anesthesia (17). The present studies show, however, that the anesthesia itself markedly reduces these responses, at least in the VPM thalamic nucleus and sensory cortex. Studies on the effects of inhibition of neuronal nitric oxide synthase on functional activation of lCMRglc in unanesthetized animals are clearly needed.
General anesthesia is used to produce analgesia and unconsciousness, which must be achieved by altering neuronal functions from their normal physiological state. It is, therefore, hardly surprising that even the most favorable of the general anesthetic agents, α-chloralose, can influence lCBF and lCMRglc responses to neuronal functional activation, at least in regions of the brain with higher level synapses. The present studies demonstrate that these influences not only can be sufficient to obscure the association of some of the cerebral structures with the function under study but also may bias the results of studies on the mechanisms of coupling of lCBF or lCMRglc to functional activity. In the anesthetized state, nitric oxide may appear to contribute to or modulate the functional activation of lCBF in some structures (43), but it clearly is not the mediator of such responses in the physiologically more normal unanesthetized state.
Acknowledgments
We express our appreciation to Dr. Kazuhide Furuya (Stroke Branch, National Institute of Neurological Disorders and Stroke, National Institutes of Health) for helpful discussions.
Abbreviations
- CBF
cerebral blood flow
- lCBF
local cerebral blood flow
- lCMRglc, local cerebral glucose utilization
l-NAME, NG-nitro-l-arginine methyl ester
- VPM
ventral posteromedial
References
- 1.Kadekaro M, Crane A M, Sokoloff L. Proc Natl Acad Sci USA. 1985;82:6010–6013. doi: 10.1073/pnas.82.17.6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sokoloff L. Neurochem Res. 1999;24:321–329. doi: 10.1023/a:1022534709672. [DOI] [PubMed] [Google Scholar]
- 3.Mata M, Fink D J, Gainer H, Smith C B, Davidsen L, Savaki H, Schwartz W J, Sokoloff L. J Neurochem. 1980;34:213–215. doi: 10.1111/j.1471-4159.1980.tb04643.x. [DOI] [PubMed] [Google Scholar]
- 4.Pellerin L, Magistretti P J. Proc Natl Acad Sci USA. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi S, Driscoll B F, Law M J, Sokoloff L. Proc Natl Acad Sci USA. 1995;92:4616–4620. doi: 10.1073/pnas.92.10.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sibson N R, Dhjankar A, Mason G F, Rothman D L, Behar K L, Shulman R G. Proc Natl Acad Sci USA. 1998;95:316–321. doi: 10.1073/pnas.95.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roy C S, Sherrington C S. J Physiol (London) 1890;11:85–108. doi: 10.1113/jphysiol.1890.sp000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kety S S, Schmidt C F. J Clin Invest. 1948;27:484–492. doi: 10.1172/JCI101995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goadsby P J, Kaube H, Hoskin K L. Brain Res. 1992;595:167–170. doi: 10.1016/0006-8993(92)91470-y. [DOI] [PubMed] [Google Scholar]
- 10.Iadecola C. Am J Physiol. 1992;263:R1156–R1161. doi: 10.1152/ajpregu.1992.263.5.R1156. [DOI] [PubMed] [Google Scholar]
- 11.Northington F J, Matherne G P, Berne R M. Proc Natl Acad Sci USA. 1992;89:6649–6652. doi: 10.1073/pnas.89.14.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raszkiewicz J L, Linville D G, Kerwin J F, Jr, Wagenaar F, Arneric S P. J Neurosci Res. 1992;33:129–135. doi: 10.1002/jnr.490330116. [DOI] [PubMed] [Google Scholar]
- 13.Dirnagl U, Lindauer L, Villringer A. Neurosci Lett. 1993;149:43–46. doi: 10.1016/0304-3940(93)90343-j. [DOI] [PubMed] [Google Scholar]
- 14.Iadecola C, Pelligrino D A, Moskowitz M A, Lassen N A. J Cereb Blood Flow Metab. 1994;14:175–192. doi: 10.1038/jcbfm.1994.25. [DOI] [PubMed] [Google Scholar]
- 15.Irikura K, Maynard K I, Moskowitz M A. J Cereb Blood Flow Metab. 1994;14:45–48. doi: 10.1038/jcbfm.1994.7. [DOI] [PubMed] [Google Scholar]
- 16.Cholet N, Bonvento G, Seylaz J. Brain Res. 1996;708:197–200. doi: 10.1016/0006-8993(95)01387-3. [DOI] [PubMed] [Google Scholar]
- 17.Cholet N, Seylaz J, Lacombe P, Bonvento G. J Cereb Blood Flow Metab. 1997;17:1191–1201. doi: 10.1097/00004647-199711000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Ma J, Ayata C, Huang P L, Fishman M C, Moskowitz M A. Am J Physiol. 1996;270:H1085–H1090. doi: 10.1152/ajpheart.1996.270.3.H1085. [DOI] [PubMed] [Google Scholar]
- 19.Ayata C, Ma J, Meng W, Huang P, Moskowitz M A. J Cereb Blood Flow Metab. 1996;16:539–541. doi: 10.1097/00004647-199607000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Wang Q, Kjaer T, Jørgensen M B, Paulson O B, Lassen N A, Diemer N H, Lou H C. Neurol Res. 1992;15:33–36. doi: 10.1080/01616412.1993.11740103. [DOI] [PubMed] [Google Scholar]
- 21.Adachi K, Takahashi S, Melzer P, Campos K L, Nelson T, Kennedy C, Sokoloff L. Am J Physiol. 1994;267:H2155–H2162. doi: 10.1152/ajpheart.1994.267.6.H2155. [DOI] [PubMed] [Google Scholar]
- 22.Gotoh J, Kuang T-Y, Nakao Y, Cohen D M, Melzer P, Itoh Y, Pak H, Pettigrew K, Sokoloff L. Am J Physiol. 2001;280:H821–H829. doi: 10.1152/ajpheart.2001.280.2.H821. [DOI] [PubMed] [Google Scholar]
- 23.Shulman R G, Rothman D L, Hyder F. Proc Natl Acad Sci USA. 1999;96:3245–3250. doi: 10.1073/pnas.96.6.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freygang W H, Sokoloff L. Adv Biol Med Phys. 1958;6:263–279. doi: 10.1016/b978-1-4832-3112-9.50011-6. [DOI] [PubMed] [Google Scholar]
- 25.Frerichs K U, Kennedy C, Sokoloff L, Hallenbeck J M. J Cereb Blood Flow Metab. 1994;14:193–205. doi: 10.1038/jcbfm.1994.26. [DOI] [PubMed] [Google Scholar]
- 26.Lindauer U, Villringer A, Dirnagl U. Am J Physiol. 1993;264:H1223–H1228. doi: 10.1152/ajpheart.1993.264.4.H1223. [DOI] [PubMed] [Google Scholar]
- 27.Sokoloff L, Reivich M, Kennedy C, Des Rosiers M H, Patlak C S, Pettigrew K D, Sakurada O, Shinohara M. J Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- 28.Sakurada O, Kennedy C, Jehle J, Brown J D, Carbin G L, Sokoloff L. Am J Physiol. 1978;234:H59–H66. doi: 10.1152/ajpheart.1978.234.1.H59. [DOI] [PubMed] [Google Scholar]
- 29.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 3rd Ed. San Diego: Academic; 1997. [DOI] [PubMed] [Google Scholar]
- 30.Wei H M, Weiss H R, Sinha A, Chi O Z. Anesth Analg. 1993;77:880–885. doi: 10.1213/00000539-199311000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Macrae I M, Dawson D A, Norrie J D, McCulloch J. J Cereb Blood Flow Metab. 1993;13:985–992. doi: 10.1038/jcbfm.1993.123. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi S, Cook M, Jehle J, Kennedy C, Sokoloff L. J Neurochem. 1995;65:414–419. doi: 10.1046/j.1471-4159.1995.65010414.x. [DOI] [PubMed] [Google Scholar]
- 33.Sokoloff L. Pharmacol Rev. 1959;11:1–85. [PubMed] [Google Scholar]
- 34.Lassen N A, Christensen M S. Br J Anaesth. 1976;48:719–734. doi: 10.1093/bja/48.8.719. [DOI] [PubMed] [Google Scholar]
- 35.Ueki M, Mies G, Hossmann K A. Acta Anaesthesiol Scand. 1992;36:318–322. doi: 10.1111/j.1399-6576.1992.tb03474.x. [DOI] [PubMed] [Google Scholar]
- 36.Balis D U, Monroe R R. Psychopharmacologia. 1964;6:1–30. doi: 10.1007/BF00710911. [DOI] [PubMed] [Google Scholar]
- 37.Garrett K M, Gan J. J Pharmacol Exp Ther. 1998;285:680–686. [PubMed] [Google Scholar]
- 38.Dudley R E, Stanley S R, Samson F. Brain Res. 1982;233:173–180. doi: 10.1016/0006-8993(82)90938-6. [DOI] [PubMed] [Google Scholar]
- 39.Garthwaite J. Trends Neurosci. 1991;14:60–67. doi: 10.1016/0166-2236(91)90022-m. [DOI] [PubMed] [Google Scholar]
- 40.Bredt D S, Snyder S H. Neuron. 1992;8:3–11. doi: 10.1016/0896-6273(92)90104-l. [DOI] [PubMed] [Google Scholar]
- 41.Dawson T M, Dawson V L, Snyder S H. Ann Neurol. 1992;32:297–311. doi: 10.1002/ana.410320302. [DOI] [PubMed] [Google Scholar]
- 42.Kelly P A T, Ritchie I M, Arbuthnott G W. J Cereb Blood Flow Metab. 1995;15:766–773. doi: 10.1038/jcbfm.1995.96. [DOI] [PubMed] [Google Scholar]
- 43.Lindauer U, Megow D, Matsuda H, Dirnagl U. Am J Physiol. 1999;277:H799–H811. doi: 10.1152/ajpheart.1999.277.2.H799. [DOI] [PubMed] [Google Scholar]