Abstract
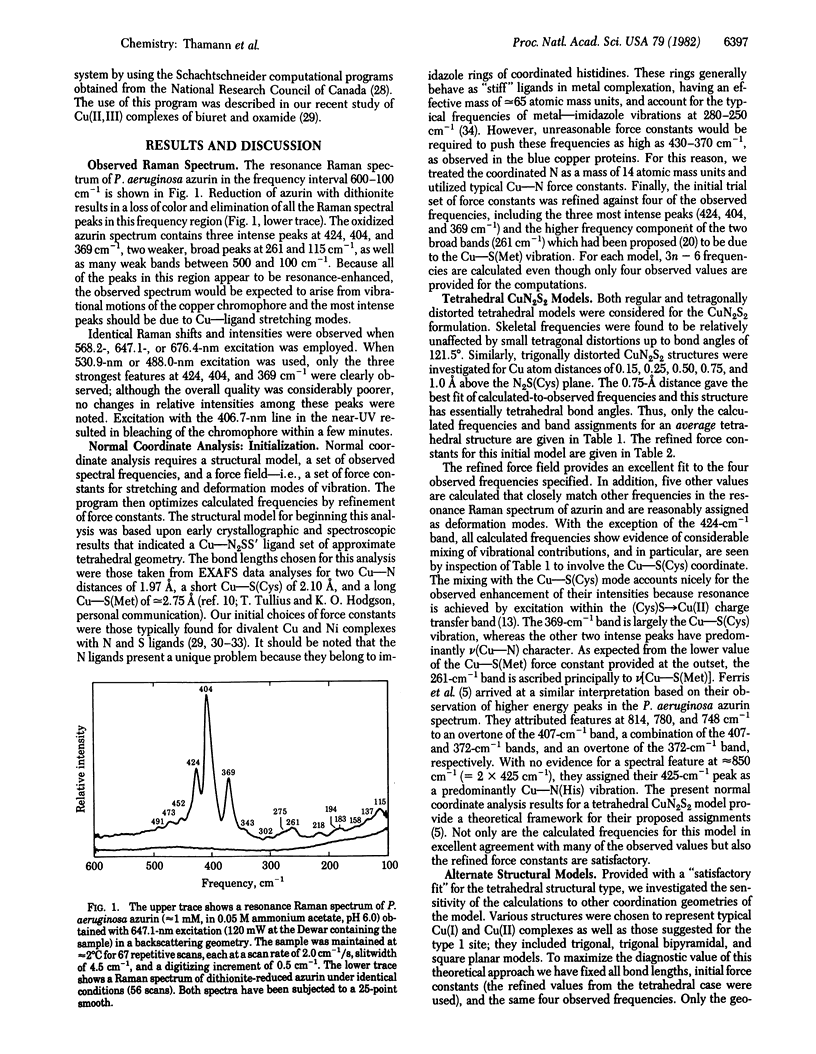
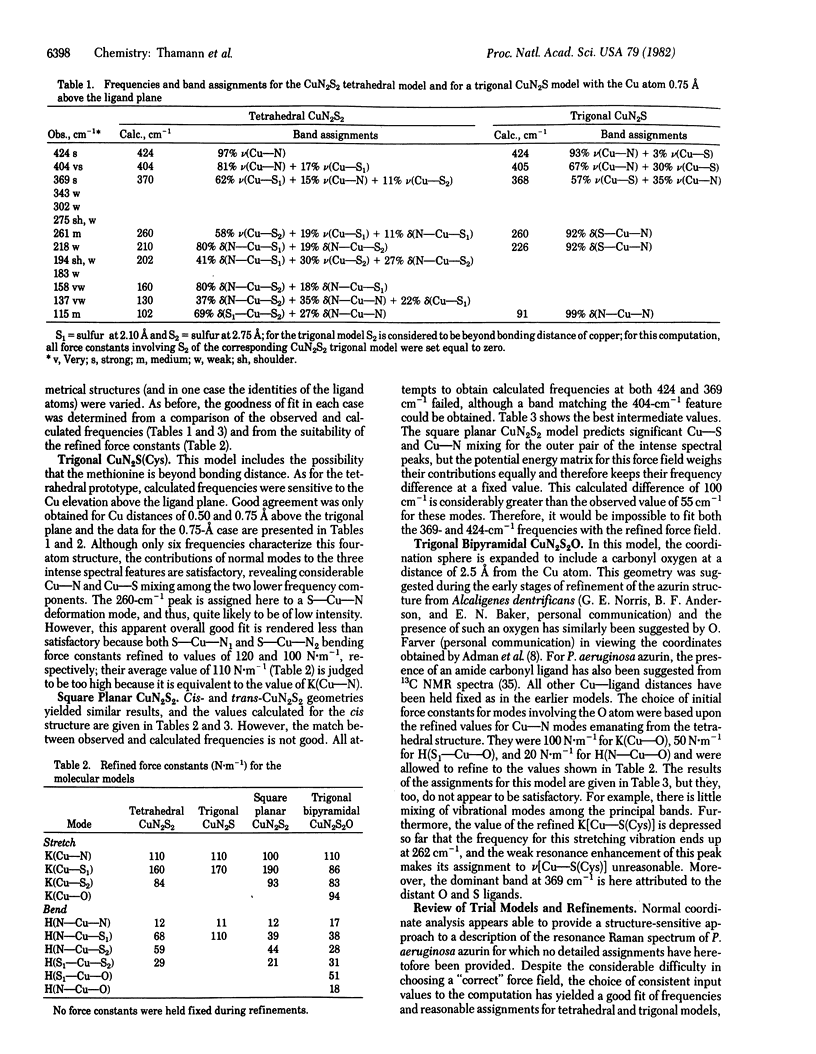
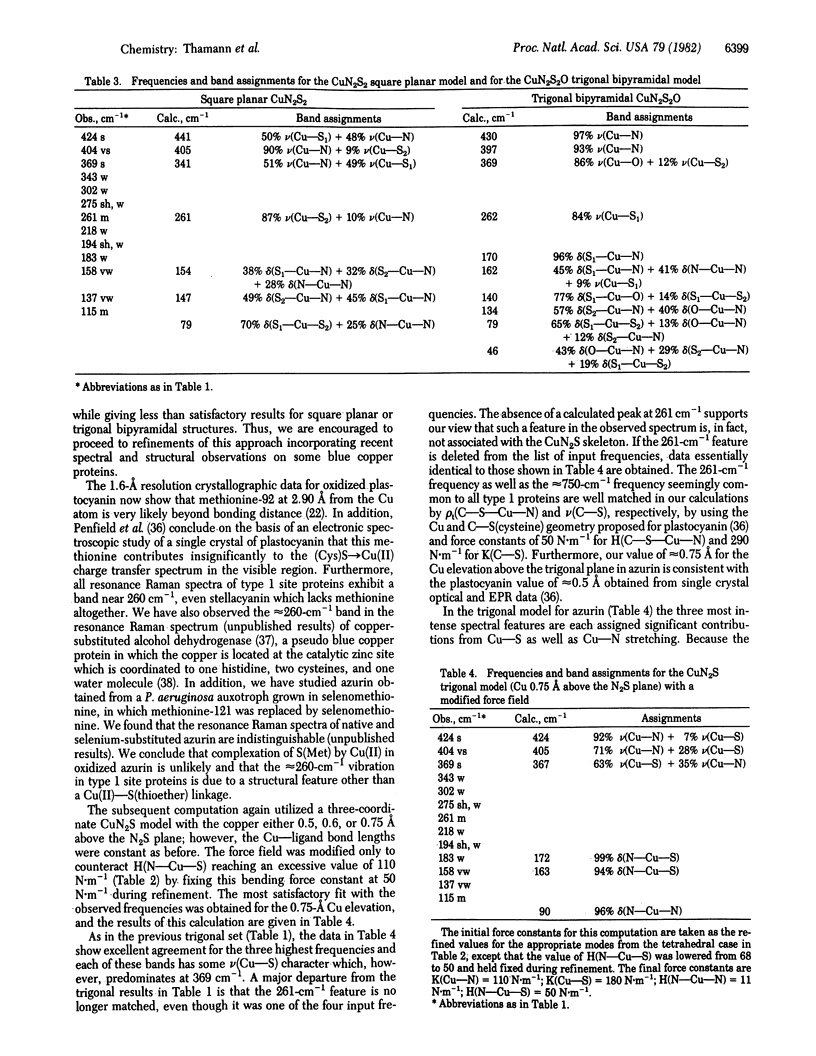
Normal coordinate analysis that utilizes a general valence force field and the Wilson FG matrix method has been applied to several structural models representing the active site of the blue copper protein, azurin. The models included tetrahedral and square planar CuN2SS', trigonal CuN2S, and trigonal bipyramidal CuN2SS'O structures in which the Ns are imidazole nitrogens of histidines, S is the thiolate sulfur of cysteine, S' is the thioether sulfur of methionine, and O is a peptide carbonyl oxygen. For constant Cu--ligand bond lengths and initial force constants, the force field was refined against the most intense of the observed frequencies (424, 404, 369, and 261 cm-1) in the resonance Raman spectrum of Pseudomonas aeruginosa azurin. The most satisfactory fit between observed and calculated frequencies occurs for tetrahedral and trigonal structures. The calculations provide detailed assignments for the resonance Raman spectrum of azurin and reveal considerable mixing of Cu--S(Cys) and Cu--N(His) vibrational modes. The trigonal model is favored because it is shown that the approximately equal to 260-cm-1 vibration is an invariant feature in the resonance Raman spectra of blue copper proteins, even those lacking a methionine in the vicinity of the copper atom. The present analysis ascribes the high frequencies of the Cu--ligand stretching modes and the resonance enhancement to the coupled nature of their vibrations and the Franck-Condon overlaps with predominant (Cys)S leads to Cu(II) charge transfer bands in the visible region.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler R. P., Wynn M. The amino acid sequences of cytochromes c-551 from three species of Pseudomonas. Biochem J. 1973 Mar;131(3):485–498. doi: 10.1042/bj1310485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergaman C., Gandvik E. K., Nyman P. O., Strid L. The amino acid sequence of Stellacyanin from the lacquer tree. Biochem Biophys Res Commun. 1977 Aug 8;77(3):1052–1059. doi: 10.1016/s0006-291x(77)80084-3. [DOI] [PubMed] [Google Scholar]
- Ferris N. S., Woodruff W. H., Tennent D. L., McMillin D. R. Native azurin and its Ni(II) derivative: a resonance Raman study. Biochem Biophys Res Commun. 1979 May 14;88(1):288–296. doi: 10.1016/0006-291x(79)91728-5. [DOI] [PubMed] [Google Scholar]
- Freedman T. B., Loehr J. S., Loehr T. M. A resonance Raman study of the copper protein, hemocyanin. New evidence for the structure of the oxygen-binding site. J Am Chem Soc. 1976 May 12;98(10):2809–2815. doi: 10.1021/ja00426a023. [DOI] [PubMed] [Google Scholar]
- Loehr T. M., Keyes W. E., Pincus P. A. A computer-controlled laser Raman spectrophotometer with interactive-graphics data analysis. Anal Biochem. 1979 Jul 15;96(2):456–463. doi: 10.1016/0003-2697(79)90606-7. [DOI] [PubMed] [Google Scholar]
- Maret W., Dietrich H., Ruf H. H., Zeppezauer M. Active site-specific reconstituted copper(II) horse liver alcohol dehydrogenase: a biological model for type 1 Cu2+ and its changes upon ligand binding and conformational transitions. J Inorg Biochem. 1980 Jun;12(3):241–252. doi: 10.1016/s0162-0134(00)80205-6. [DOI] [PubMed] [Google Scholar]
- Markley J. L., Ulrich E. L., Berg S. P., Krogmann D. W. Nuclear magnetic resonance studies of the copper binding sites of blue copper proteins: oxidized, reduced, and apoplastocyanin. Biochemistry. 1975 Oct 7;14(20):4428–4433. doi: 10.1021/bi00691a014. [DOI] [PubMed] [Google Scholar]
- McMillin D. R., Rosenberg R. C., Gray H. B. Preparation and spectroscopic studies of cobalt(II) derivatives of blue copper proteins. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4760–4762. doi: 10.1073/pnas.71.12.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskowski V., Tang S. P., Spiro T. G., Shapiro E., Moss T. H. The copper coordination group in "blue" copper proteins: evidence from resonance Raman spectra. Biochemistry. 1975 Mar 25;14(6):1244–1250. doi: 10.1021/bi00677a024. [DOI] [PubMed] [Google Scholar]
- Parr S. R., Barber D., Greenwood C. A purification procedure for the soluble cytochrome oxidase and some other respiratory proteins from Pseudomonas aeruginosa. Biochem J. 1976 Aug 1;157(2):423–430. doi: 10.1042/bj1570423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peisach J., Mims W. B. The linear electric field effect in stellacyanin, azurin and in some simple model compounds. Eur J Biochem. 1978 Mar;84(1):207–214. doi: 10.1111/j.1432-1033.1978.tb12158.x. [DOI] [PubMed] [Google Scholar]
- Siiman O., Young N. M., Carey P. R. Resonance Raman studies of "blue" copper proteins. J Am Chem Soc. 1974 Aug 21;96(17):5583–5585. doi: 10.1021/ja00824a053. [DOI] [PubMed] [Google Scholar]
- Siiman O., Young N. M., Carey P. R. Resonance raman spectra of "blue" copper proteins and the nature of their copper sites. J Am Chem Soc. 1976 Feb 4;98(3):744–748. doi: 10.1021/ja00419a017. [DOI] [PubMed] [Google Scholar]
- Tosi L., Garnier A. Circular dichroism and resonance Raman spectra of the Cu(II)-Cu(I) complex of D-penicillamine. The CuS(CYs) stretching mode in blue copper proteins. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1273–1279. doi: 10.1016/0006-291x(79)91204-x. [DOI] [PubMed] [Google Scholar]
- Tosi L., Garnier A., Herve M., Steinbuch M. Ceruloplasmin-anicn interaction. A resonance Raman spectroscopic study. Biochem Biophys Res Commun. 1975 Jul 8;65(1):100–106. doi: 10.1016/s0006-291x(75)80066-0. [DOI] [PubMed] [Google Scholar]
- Tullius T. D., Frank P., Hodgson K. O. Characterization of the blue copper site in oxidized azurin by extended x-ray absorption fine structure: Determination of a short Cu-S distance. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4069–4073. doi: 10.1073/pnas.75.9.4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugurbil K., Norton R. S., Allerhand A., Bersohn R. Studies of individual carbon sites of azurin from Pseudomonas aeruginosa by natural-abundance carbon-13 nuclear magnetic resonance spectroscopy. Biochemistry. 1977 Mar 8;16(5):886–894. doi: 10.1021/bi00624a012. [DOI] [PubMed] [Google Scholar]


