Summary
Pentraxins are innate pattern recognition molecules whose major function is to bind microbial pathogens or cellular debris during infection and inflammation and, by doing so, contribute to the clearance of necrotic cells as well as pathogens through complement activations. Fc receptors are the cellular mediators of antibody functions. While conceptually separated, both pentraxins and antibodies are important factors in controlling acute and chronic inflammations and infections. In recent years, increasing experimental evidence suggests a direct link between the innate pentraxins and humoral Fc receptors. Specifically, both human and mouse pentraxins recognize major forms of Fc receptors in solution and on cell surfaces with affinities similar to antibodies binding to their low affinity Fc receptors. Like immune complex, pentraxin aggregation and opsonization of pathogen result in Fc receptor and macrophage activation. The recently published crystal structure of a human serum amyloid P (SAP) in complex with FcγRIIA further illustrated similarities to antibody recognition. These recent findings implicate a much broader role than complement activation for pentraxins in immunity. This review summarizes the structural and functional work that bridge the innate pentraxins and the adaptive Fc receptor functions. In many ways, pentraxins can be regarded as innate antibodies.
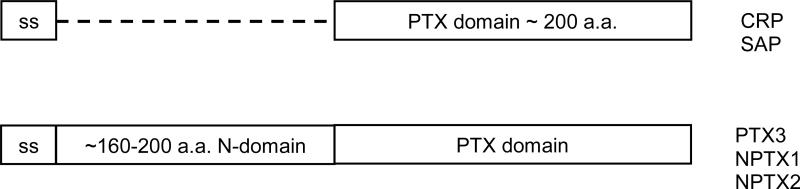
Pentraxins are a family of secreted pattern recognition proteins containing a homologous pentraxin (PTX) domain of approximately 200 amino acids (Fig. 1). Members of the family, such as C-reactive protein (CRP) (also called PTX1) and serum amyloid P component (SAP) (also called PTX2) containing only a PTX domain, are termed short chain pentraxins, while others, such as pentraxin member 3 (PTX3), and neuronal pentraxins 1 and 2, have an additional N-terminal domain preceding the PTX domain and are referred as long chain pentraxins. The structure and function of short chain pentraxins have been studied more extensively compared to the members of long chain pentraxins (1-5). Both CRP and SAP are part of acute phase proteins expressed by hepatocytes in response to inflammatory cytokine stimulation during infections (6-8). CRP recognizes phosphorylcholine moieties associated with microbial polysaccharides or necrotic cells in a calcium-dependent manner (9, 10). In addition, CRP also recognizes some nuclear antigens, such as small nuclear ribonucleoprotein and chromatin subunits (11-15). SAP is known to recognize phosphorylethanolamine (PE) and other microbial associated molecules as well as nuclear antigens in a calcium-dependent manner (11, 16-18). Both CRP and SAP can opsonize microbial pathogens or apoptotic cells and effectively clear them through complement activation (19-22).
Fig. 1.
Domain structure of pentraxins.
The structure of a pentraxin domain displays a conserved β-sandwich fold with two opposing β-sheets each consisting of 7 β-strands (2, 23, 24) (Fig. 2A). A three turn α-helix from residue 168-176 of CRP, termed ridge helix, is situated on top of the β-sandwich. Both CRP and SAP subunits display a similar donut-shaped pentameric structure, with all five ridge helices on one face and microbial ligand binding sites on the opposite face. Calcium ions form part of the phosphorylcholine-binding site (23). The face with ridge helices is shown to contain C1q-binding sites by mutational analysis (25).
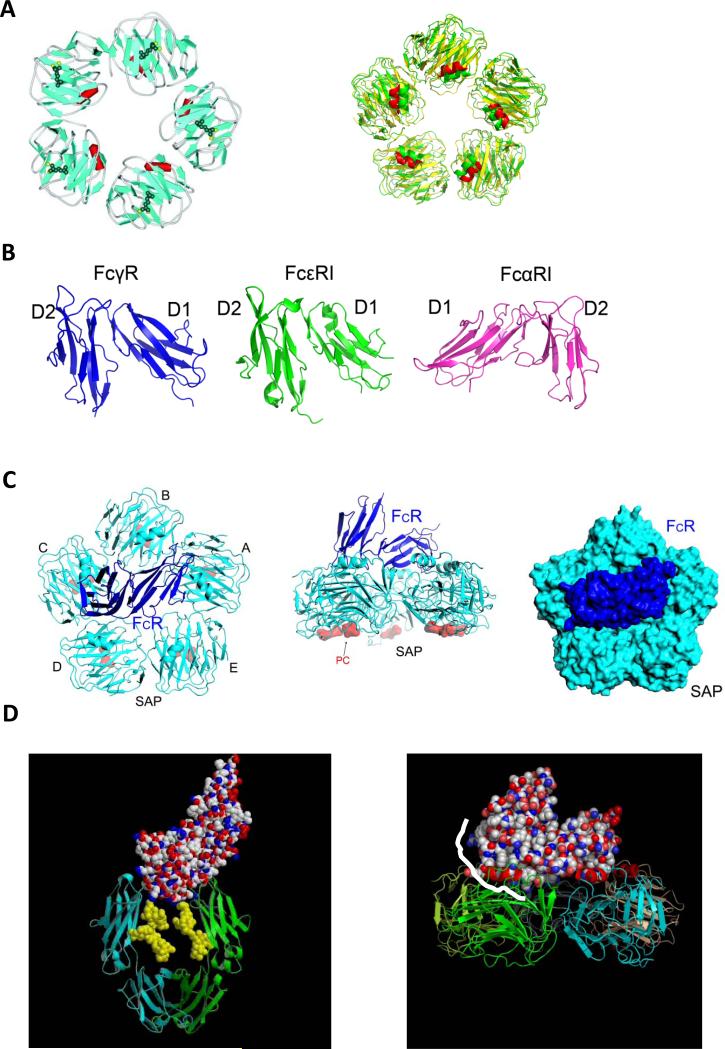
Fig. 2. Structural recognition between pentraxins and Fc receptors.
(A) crystal structure of phosphocholine bound CRP (PDB entry 1B09, left) and the structural superposition between CRP and SAP (right). (B) Structures of FcγRIIA, FcεRI (PDB entry 1F2Q) and FcαRI (PDB entry 1QVZ). (C) Structural complex between human SAP (cyan) and FcγRIIA (blue) in two orthogonal views (left and middle panels) and in space filling model (right panel). (D) Binding mode of IgG-Fc on Fc receptor (left panel) partially overlap with that of SAP (right panel). The IgG-Fc interface region is highlighted in white-line on the SAP complex structure.
Immunoglobulin Fc receptors (FcRs) are broadly expressed on hematopoietic cells and are important for antibody-mediated humoral and cellular immunity. Binding of immune complexes to Fc receptors activates effector cells leading to phagocytosis, endocytosis of IgG-opsonized particles, the release of inflammatory mediators, and antibody-dependent cellular cytotoxicity (ADCC) (26-28). The most well studied Fc receptors are FcγR and neonatal FcR (FcRn) for immunoglobulin G (IgG), FcεR for IgE, and FcαR for IgA. Fc receptors belong to the immunoglobulin superfamily, except for the FcRn and FcεRII, which are structurally related to class I major histocompatibility antigens and C-type lectins, respectively. FcγRI, FcεRI, and FcαRI display higher binding affinities to their cognate immunoglobulins than the low affinity FcγRII and FcγRIII. In addition to the affinity variations among the receptors, Fcγ receptors also display distinct IgG subtype specificities. For example, FcγRIII binds IgG1 and IgG3 better than IgG2 and IgG4 (29). All Fcγ receptors are type I transmembrane or GPI-anchored glycoproteins consisting of two or three C2-type immunoglobulin-like domains. All Fcγ receptors show a high degree of sequence identity in their extracellular portion (50-96%) but differ significantly in their cytoplasmic domains (28). Activating Fcγ receptors either contain or associate with a common signaling γ-chain that contains the immunoreceptor tyrosine-based activation motifs (ITAMs), while the inhibitory FcγRIIB contains the immunoreceptor tyrosine-based inhibitory motif (ITIM) in its cytoplasmic tail. Several structures of the extracellular portion of Fc receptors have been published (30-34). They can be divided into two structural groups depending on their relative orientation between D1 and D2 domains (Fig. 2B). Group I receptors include all Fcγ receptors and FcεRI, and group II includes FcαRI. The D1-D2 domain juxtaposition of group I receptors is opposite to that of group II, which displays natural killer cell immunoglobulin-like receptor (KIR) domain juxtaposition. The antibody-binding affinities of Fc receptors vary substantially between 10-5 and 10-9 M (26, 27, 29). Among them, FcεRI and FcγRI display high affinity IgE and IgG binding, respectively, and are presumed preoccupied with their antibodies in circulation. Others, including FcγRII, FcγRIII, and FcαRI, display intermediate and low binding affinities toward their antibodies and thus can only recognize antigen-bound immune complexes. Despite differences in antibody affinities, both the high affinity FcεRI and the lower affinity FcγRs have been shown to recognize their antibody Fc domains in similar structure modes in which one Fc receptor interacts with both heavy chains of Fc in the lower hinge region, between CH1 and CH2 domains in IgG or between Cε2 and Cε3 in IgE, near the conserved Fc glycosylation site (35-39). Aside from their well-established functions in antibody-mediated immune activations, Fc receptors were not known to recognize soluble innate pentraxins until recently.
Structural recognition of pentraxins by Fcγ receptors
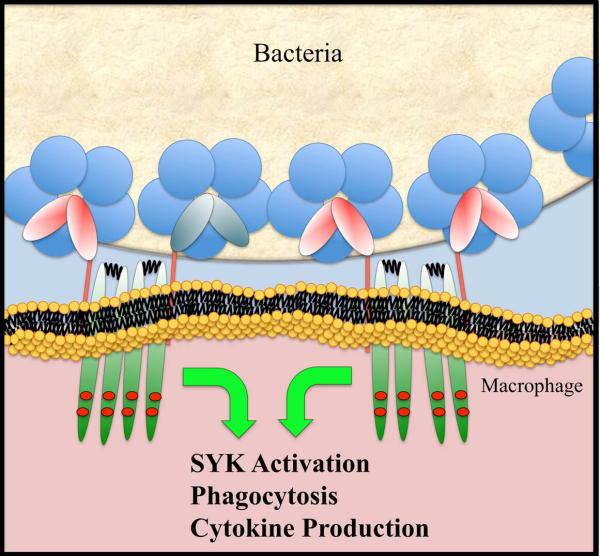
The initial recognitions between pentraxins and Fcγ receptors were demonstrated for CRP binding to COS cells transfected with either FcγRI or FcγRII (40, 41). Structurally, both CRP and SAP adopt a similar pentameric donut-shaped structure, and all Fcγ receptors display a similar structural fold consisting of two tandem Ig-like domains. The conserved structural folds within the pentraxin family and within Fcγ receptors raises the possibility of a broader recognition between pentraxins and Fcγ receptors. Using a solution BIAcore-based binding assay, the binding affinities between members of pentraxins and those Fcγ receptors were examined systematically, and the results showed the existence of both a general recognition between pentraxins and Fcγ receptors as well as specific preferences among individual members (42) (Table 1). Like γ-immunoglobulins, which recognize all isoforms of FcγR, both CRP and SAP bound to all Fcγ receptors. PTX3, in contrast, only exhibited binding to FcγRIII, suggesting a potential role in neutrophil and NK cell functions. The ability of pentraxins to bind both activating and inhibitory FcγRs resembles that of antibodies. It suggests that the outcome of pentraxin-mediated FcγR ligation depends on the expressions of the activating and inhibitory receptors. Unlike IgGs, pentraxins showed less disparity in their Fcγ receptor binding affinities. CRP bound to all isoform FcγRs equally well with 2-4 µM affinity, and SAP bound to FcγRI with an affinity of 0.5 μM, 3-5 times higher than its binding to other FcγRs. The observed affinities between pentraxins and FcγRs are similar to those between IgG and their lower affinity Fcγ receptors. This suggests that circulating pentraxins in normal conditions are too low in concentration to activate Fc receptors and the receptor activation most likely requires higher concentrations of pentraxins as measured in acute inflammation as well as target opsonization (Fig. 3).
Table 1.
Solution binding affinity between Pentraxins and Fc receptors
| Dissociation constants KD (μM) |
|||||
|---|---|---|---|---|---|
| FcγRI | FcγRIIA | FcγRIIB | FcγRIII | FcαRI | |
| CRP | 3.2 | 1.9 | 4.1 | 4.1 | 2.8 |
| SAP | 0.5 | 1.4 | 1.2 | 2.9 | 3.2 |
| PTX3 | n.d. | 19 | n.d. | 1.6 | n.d. |
| IgG1 | 0.03 | 0.32 | 0.64 | 0.38 | - |
| IgA | - | - | - | - | 0.12 |
n.d. Not detectable
Fig. 3.
Schematic diagram showing CRP activation of Fc receptors.
The structural recognition between pentraxins and FcγRs was revealed in the crystal structure of the complex between human SAP and the extracellular domain of FcγRIIA (42) (Fig. 2C). FcγRIIA docks diagonally across the pentameric effector face of SAP contacting two of the five pentraxin subunits. The complex was formed without significant conformational changes in SAP or the receptor. The overall Fcγ receptor contact area is approximately equally divided between the two diagonal SAP subunits. Further, both SAP subunits use similar residues, including Tyr 173 and Gln 174 from the ridge helix on the effector face to contact FcγRIIA. In addition, the C-terminal residues 200-204 of SAP are involved in contacting the D2 domain of the Fc receptor. Further mutations at the putative interface residues on CRP, Tyr 175 and Leu 176, significantly impaired its Fc receptor binding, suggesting that CRP binds in a similar mode to FcγR as SAP. Despite the structural differences between antibodies and pentraxins, their recognition by Fcγ receptors share common features. Both antibodies and pentraxins are homo-oligomers, dimers, and pentamers. Yet, each antibody or SAP interacts with only one Fcγ receptor molecule. The receptor binds to symmetrically located sites from two subunits of antibody-Fc and SAP, such that each receptor binding precludes additional receptors from binding to the same ligand. The receptor binding stoichiometry obligates immune-complex formation or pentraxin-opsonization for Fc receptor activation. The SAP and FcγRIIA complex structure also revealed that the pentraxin binding site partially overlaps with that of IgG on the Fcγ receptor (Fig. 2D). Namely, antibodies and pentraxins are bound in closely related orientations with respect to Fcγ receptors, suggesting a potential synergistic Fc receptor activation between antibodies and pentraxins in situations where a pathogenic surface is coated with both antibodies and pentraxins. On the other hand, the overlapping binding site between IgG-Fc and SAP also predicts a direct competition between their binding to Fc receptors. Indeed, free IgGs and soluble pentraxins competed for FcγR binding both in solution and in phagocytosis assays. Further, CRP treatment in autoimmune ITP (immune thrombocytopenia) mice was shown to protect the animal against antibody-induced thrombocytopenia similar to that of intravenous immunoglobulin (IVIg) treatment (43).
Activation of Fcγ receptors by pentraxins
The involvement of pentraxins as acute phase proteins in complement activation was well established 20 years ago. In contrast, the role of pentraxins in Fc receptor-mediated immune function has been controversial, partially due to the difficulty in earlier functional assays to separate the FcR from the Toll-like receptor (TLR) activation pathway, as pentraxins bind microbial pathogens that can directly activate TLR pathways. The SAP complexed Fc receptor structure and its structural similarity to antibody-FcR complex provided concrete evidence for pentraxin activation of Fcγ receptors. The structural recognition resulted in the internalization of SAP-opsonized zymosan into endosomes in human monocyte-derived macrophages (MDM). Moreover, monocytes treated with aggregated SAP secreted IL-10, IL-8, and IL-6, and the cytokine release was inhibited by FcγR blocking antibodies as well as by a Syk inhibitor. Further evidence supporting pentraxin activation of Fcγ receptors rather than innate TLR or NOD receptors came from SAP stimulation of bone marrow-derived macrophages (BMDMs) from myeloid differentiation factor 88 (MyD88)–/– or receptor-interacting protein 2 (RIP2)–/– mice, showing similar levels of IL-6 and CCL2 productions from the knockout compared to wildtype BMDMs. Interestingly, tumor necrosis factor-α (TNF-α) secretion was reduced by half from MyD88–/– compared to the wildtype BMDMs, indicating a potential synergistic activation between FcRs and TLRs.
Pentraxins recognize and activate FcαRI
The broad recognition of pentraxins by all isoforms of Fcγ receptors prompted us to further investigate if pentraxins could be recognized by other antibody receptors. Both human and mouse type I IgE receptor, FcεRI, is a close structure homolog of Fcγ receptors, with two similar Ig-like domains. Both Fcγ and Fcε receptors recognize their antibodies with similar structural modes, and they share a common signaling γ-chain. In human, the type I IgA receptor FcαRI (CD89) also signals through the common FcR γ-chain, but it resides in a region of chromosome close to the leukocyte receptor complex (LRC), which encodes KIRs and an activating NK cell receptor, NKp46. FcαRI also shares ~30% sequence identity to KIR and NKp46. Despite a close resemblance of FcεRI to Fcγ receptors, none of the pentraxins showed detectable binding to soluble FcεRI in solution. Yet, both CRP and SAP bound the soluble FcαRI with μM affinities but not its closely related KIR and NKp46 (44) (Table 1). Further, FcαRI appears to recognize the same effector face of CRP as Fcγ receptors. Unlike Fcγ receptors whose pentraxin-binding site partially overlap with that of IgG, FcαRI uses its N-terminal domain to bind IgA in a distinct mode from Fcγ receptors (34), and the receptor binding to IgA does not compete with its binding to CRP. In addition, CRP binding was also observed on FcαRI transfected RBL cells and CRP crosslinking of FcαRI resulted in the activation of ERK, cytokine production, and degranulation in the transfected RBL cells. In human, FcαRI is expressed primarily on circulating neutrophils and monocytes, and CRP crosslinking on neutrophils induced cell surface relocation of intracellular FcαRI, phagocytosis, and TNF-α production. Similar to Fcγ receptors, the observed μM affinity between pentraxins and FcαRI means target opsonization and acute inflammation are required for pentraxins to activate FcαRI.
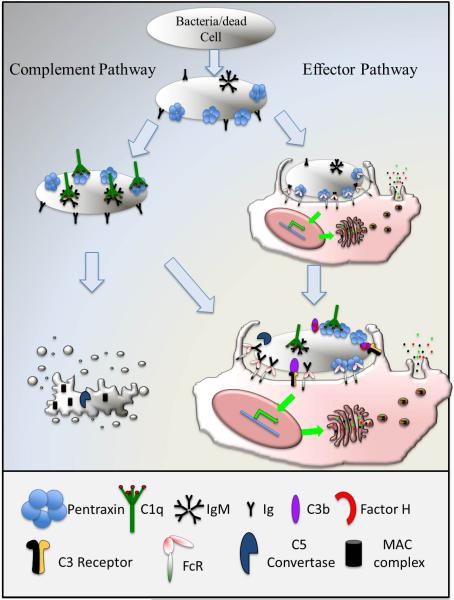
Both antibodies and pentraxins bind C1q and activate the classic complement pathway. Both activate Fc receptors (Fig. 4). Through gene recombination and somatic mutations, antibodies possess exquisite antigen specificities. In contrast, pentraxins broadly recognize pathogenic patterns and lack antigenic specificity. Antibody production depends on the differentiation and expansion of memory B cells that can take days. Pentraxins are acute phase proteins that are rapidly activated in response to infections, thus representing the first line of defense. Antibodies are highly diverse among species, while pentraxins are rather conserved. Thus, pentraxins represent an ancient innate counterpart of antibodies.
Fig. 4.
Comparison of pentraxins and antibodies in complement and Fc receptor activation.
Associations between CRP, Fc receptors, and inflammatory diseases
Establishing FcRs as pentraxin receptors provides a basis for understanding the roles of pentraxins in disease. FcR biology has been extensively studied, and functions of opsonization, phagocytosis, and immune complex-mediated inflammation have been established. As innate immune molecules and acute phase reactants, pentraxins provide a rapid response during a critical period of host reaction to infection or injury prior to the development of antibody. The ability of pentraxins to bind to damaged cells at sites of tissue injury and activate complement and FcRs suggest that they may play a regulatory role in inflammation. CRP is a clinical marker for infection and has been increasingly used as a risk indicator in chronic inflammatory diseases. The ligand-binding specificity of the classical pentraxins, CRP and SAP, for apoptotic and necrotic cells and nucleoprotein autoantigens initially suggested that they would play a role as pattern recognition molecules to regulate systemic autoimmunity in diseases such as SLE, a prototypic immune complex disease associated with high levels of autoantibodies. These autoantibodies recognize autoantigens released from or presented on apoptotic or necrotic cells. The clearance of these effete cells is central to avoiding inflammation and immunization with these self-antigens. The pentraxins, CRP and SAP, bind to apoptotic cells, chromatin and snRNPs, which are the major targets of autoantibodies in SLE. In human SLE, it has been recognized for many years that CRP levels are inappropriately low for the extent of inflammation. In clinical studies CRP was detected in the glomerular lesions of lupus nephritis and in the tubular interstitium (45). There are no genetic deficiencies or coding polymorphisms of CRP, but several studies suggest a link between genetically determined low baseline CRP levels and risk of SLE and lupus nephritis (46-49). Experimental studies in lupus-prone mouse strains provide evidence that CRP is protective in this disease. CRP was first shown to prevent accelerated disease in NZB/W mice exposed to chromatin, a disease accelerant (50). Additional studies using injected CRP in mouse lupus models demonstrated protective and therapeutic effects of CRP in SLE (51, 52) Studies using CRP transgenic NZB/W mice confirmed the protective effect of CRP in this model (53). One mechanism for these effects of CRP in SLE is that CRP contributes to the clearance of apoptotic autoantigens through activation of complement and FcγRs (10, 54). The injection of CRP in mouse lupus models produced a rapid sustained decrease in proteinuria in mice treated after the development of renal disease (51, 52). In addition to the therapeutic implications, these results suggest that CRP had mechanisms of suppression in addition to clearance of autoantigens.
In addition to SLE, the CRP transgene (tg) was associated with a delayed onset and decreased severity in an induced experimental autoimmune encephalomyelitis (EAE) mouse model (55). These effects were found to be independent of complement activation but dependent on FcγRIIb (56). More recently, the role of CRP in collagen-induced arthritis (CIA) was studied. CIA is a widely used model of human rheumatoid arthritis, which is induced by injection of type II collagen in complete Freund's adjuvant into mice. Like the EAE model, CRPtg resulted in a delayed and less severe arthritis than wildtype mice. Further, mice deficient in CRP developed a more rapid disease course than wildtype mice (57).
Studies in non-autoimmune models, including endotoxin shock, nephrotoxic nephritis, and immune thrombocytopenia (ITP), have demonstrated that CRP binding to FcγR on macrophages initiates anti-inflammatory pathways (43, 58, 59). These induced models of inflammation allowed dissection of the mechanism of CRP suppression. In all three models, signaling through an activating FcγR, specifically FcγRI, was required. Macrophage depletion or genetic deficiency of IL-10 abrogated the effects of CRP in nephrotoxic nephritis (59), and induction of IL-10 in vivo by injection of CRP and endotoxin required FcγRI. In ITP, CRP-mediated suppression of platelet clearance was transferred by spleen cells or macrophages, and this effect required FcγRI and Syk activation in the donor cells (43). The regulatory FcγRIIb was required for suppression of thrombocytopenia in the recipient mouse, as it is in IVIg suppression of ITP (60). However, the initiating cell and receptor are different for IVIg (61). Interestingly, IgG immune complexes activated macrophages in the presence of TLR agonist also induced IL-10 production (62). The finding that CRP is protective in multiple inflammatory models and the highly altered phenotype of mice deficient in or overexpressing CRP suggest that it has a more fundamental role in the regulation of inflammation. Although CRP interacts with the autoantigens in SLE, it can suppress a variety of conditions in which autoimmunization is not thought to play a role. In each of these cases, CRP interaction with FcR is essential, providing evidence that pentraxins and FcRs provide an innate mechanism for regulating inflammatory responses.
Although SAP has been less extensively studied in autoimmune models, its receptor binding properties, complement activation, and binding to nuclear autoantigens suggest that it may have similar activities to those of CRP. A mouse deficient in SAP was initially found to ‘spontaneously develop antinuclear autoimmunity and severe glomerulonephritis’ (63). However, it was later shown that the phenotype was strain dependent, and SAP deficiency did not induce a full lupus phenotype in other mouse strains. Most recently, mouse and human SAP were found to bind to DNA-derived from activated lymphocytes (ALD-DNA) and promote anti-inflammatory macrophages. These macrophages were able to suppress ALD-DNA-induced nephritis through IL-10 (64).
While there is convincing experimental evidence supporting pentraxin function through FcRs, links between pentraxins and FcR functions in diseases remain to be established. Intuitively, such connections may be apparent in diseases lacking obvious antibody components, such as cardiovascular diseases. For example, CRP appeared to be a risk factor associated with a known genetic R/H polymorphism in FcγRIIA (65). Individuals homozygous of arginine 131 genotype of the receptor showed an increased risk in acute coronary syndromes (ACS) with an odds ratio of 2.86 compared to non-R/R131 alleles (65). The structure of SAP-FcγRIIA complex suggests a close proximity of residue 131 to the putative CRP binding site and solution binding showed that the Arg variant of FcγRIIA bound significantly better than a histidine variant (42). The R/H polymorphism is also known to affect antibody binding, but interestingly, with opposite effects. Namely, R131 variant bound worse to antibodies but better to CRP than the H131 variant of FcγRIIA. The association of the R/R131 genotype with an increased risk in ACS is consistent with the involvement of CRP in the disease.
Summary
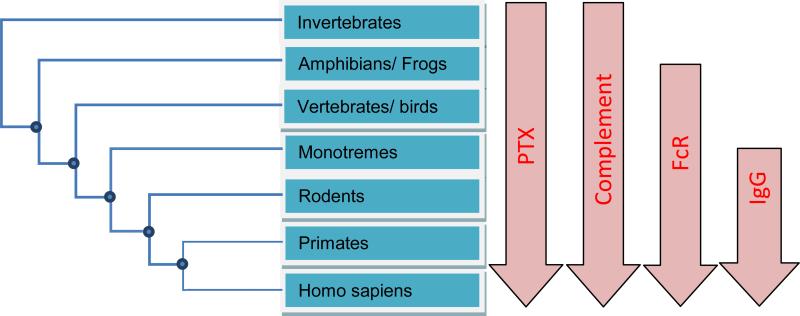
Pentraxins are indeed ancient immune mediators. They exist in species ranging from mammals, vertebrates, lizards, to horseshoe crabs (Fig. 5). Likewise, complement components, such as C1q, are ancient immune systems. Antibodies, particularly IgGs, are relatively recent evolution events and are highly diversified among species. Their conservation extends primarily in mammalian species. Although vertebrates like chicken produce antibody-like IgY molecules, they are quite different from mammalian antibodies and do not use homologous FcRs. FcRs can be traced back to lower species of mammals, such as monotremes, and FcR-like genes exist in frogs. It is seems that pentraxins existed before adaptive immune systems and function to activate complement pathways in the lower organisms that do not have cellular-based adaptive immune systems. When cellular-based immunity appeared, the effector FcRs utilized innate pentraxins for their activations. As multicellular-based immune functions diversify, antibodies appeared and their powerfully antigenic specificity and B-cell expansion presented clear advantage over the limited pentraxins and dictated FcR evolution. In a way, antibodies can be viewed as antigen-specific pentraxins that expand in response to specific pathogens. However, the activation of innate pentraxins during infections is much more rapid than the activation of antibodies, making innate pentraxins a first line of defense mediated by cellular immunity against infections.
Fig. 5.
Evolution of pentraxins and antibody-mediated immunity.
Acknowledgement
The authors do not have conflicts of interests to declare. The work was supported in part by the Intramural Research Funding of National Institute of Allergy and Infectious Diseases, National Institutes of Health, and by an National Institutes of Health Grant R21AI085414.
References
- 1.Du Clos TW, Mold C. Pentraxins (CRP, SAP) in the process of complement activation and clearance of apoptotic bodies through Fcgamma receptors. Curr Opin Organ Transplant. 2010 doi: 10.1097/MOT.0b013e32834253c7. 10.1097/MOT.0b013e32834253c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shrive AK, et al. Three dimensional structure of human C-reactive protein. Nat Struct Biol. 1996;3:346–354. doi: 10.1038/nsb0496-346. [DOI] [PubMed] [Google Scholar]
- 3.Mikolajek H, Kolstoe SE, Pye VE, Mangione P, Pepys MB, Wood SP. Structural basis of ligand specificity in the human pentraxins, C-reactive protein and serum amyloid P component. J Mol Recognit. 2011;24:371–377. doi: 10.1002/jmr.1090. [DOI] [PubMed] [Google Scholar]
- 4.Marnell L, Mold C, Du Clos TW. C-reactive protein: ligands, receptors and role in inflammation. Clin Immunol. 2005;117:104–111. doi: 10.1016/j.clim.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Jaillon S, et al. The humoral pattern recognition receptor PTX3 is stored in neutrophil granules and localizes in extracellular traps. J Exp Med. 2007;204:793–804. doi: 10.1084/jem.20061301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganapathi MK, Rzewnicki D, Samols D, Jiang SL, Kushner I. Effect of combinations of cytokines and hormones on synthesis of serum amyloid A and C-reactive protein in Hep 3B cells. J Immunol. 1991;147:1261–1265. [PubMed] [Google Scholar]
- 7.Du Clos TW, Mold C. C-reactive protein: an activator of innate immunity and a modulator of adaptive immunity. Immunol Res. 2004;30:261–277. doi: 10.1385/IR:30:3:261. [DOI] [PubMed] [Google Scholar]
- 8.Ganapathi MK, et al. Role of interleukin-6 in regulating synthesis of C-reactive protein and serum amyloid A in human hepatoma cell lines. Biochem Biophys Res Commun. 1988;157:271–277. doi: 10.1016/s0006-291x(88)80043-3. [DOI] [PubMed] [Google Scholar]
- 9.Volanakis JE, Kaplan MH. Specificity of C-reactive protein for choline phosphate residues of pneumococcal C-polysaccharide. Proc Soc Exp Biol Med. 1971;136:612–614. doi: 10.3181/00379727-136-35323. [DOI] [PubMed] [Google Scholar]
- 10.Mold C, Baca R, Du Clos TW. Serum amyloid P component and C-reactive protein opsonize apoptotic cells for phagocytosis through Fcgamma receptors. J Autoimmun. 2002;19:147–154. doi: 10.1006/jaut.2002.0615. [DOI] [PubMed] [Google Scholar]
- 11.Du Clos TW. The interaction of C-reactive protein and serum amyloid P component with nuclear antigens. Mol Biol Rep. 1996;23:253–260. doi: 10.1007/BF00351177. [DOI] [PubMed] [Google Scholar]
- 12.Du Clos TW. C-reactive protein reacts with the U1 small nuclear ribonucleoprotein. J Immunol. 1989;143:2553–2559. [PubMed] [Google Scholar]
- 13.Du Clos TW, Zlock LT, Rubin RL. Analysis of the binding of C-reactive protein to histones and chromatin. J Immunol. 1988;141:4266–4270. [PubMed] [Google Scholar]
- 14.Pepys MB, Booth SE, Tennent GA, Butler PJ, Williams DG. Binding of pentraxins to different nuclear structures: C-reactive protein binds to small nuclear ribonucleoprotein particles, serum amyloid P component binds to chromatin and nucleoli. Clin Exp Immunol. 1994;97:152–157. doi: 10.1111/j.1365-2249.1994.tb06594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du Clos TW, Marnell L, Zlock LR, Burlingame RW. Analysis of the binding of C-reactive protein to chromatin subunits. J Immunol. 1991;146:1220–1225. [PubMed] [Google Scholar]
- 16.Schwalbe RA, Dahlback B, Coe JE, Nelsestuen GL. Pentraxin family of proteins interact specifically with phosphorylcholine and/or phosphorylethanolamine. Biochemistry. 1992;31:4907–4915. doi: 10.1021/bi00135a023. [DOI] [PubMed] [Google Scholar]
- 17.Pepys MB, Butler PJ. Serum amyloid P component is the major calcium-dependent specific DNA binding protein of the serum. Biochem Biophys Res Commun. 1987;148:308–313. doi: 10.1016/0006-291x(87)91111-9. [DOI] [PubMed] [Google Scholar]
- 18.Dong A, Caughey WS, Du Clos TW. Effects of calcium, magnesium, and phosphorylcholine on secondary structures of human C-reactive protein and serum amyloid P component observed by infrared spectroscopy. J Biol Chem. 1994;269:6424–6430. [PubMed] [Google Scholar]
- 19.Kaplan MH, Volanakis JE. Interaction of C-reactive protein complexes with the complement system. I. Consumption of human complement associated with the reaction of C-reactive protein with pneumococcal C-polysaccharide and with the choline phosphatides, lecithin and sphingomyelin. J Immunol. 1974;112:2135–2147. [PubMed] [Google Scholar]
- 20.Hutchinson WL, Noble GE, Hawkins PN, Pepys MB. The pentraxins, C-reactive protein and serum amyloid P component, are cleared and catabolized by hepatocytes in vivo. J Clin Invest. 1994;94:1390–1396. doi: 10.1172/JCI117474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du Clos TW. Function of C-reactive protein. Ann Med. 2000;32:274–278. doi: 10.3109/07853890009011772. [DOI] [PubMed] [Google Scholar]
- 22.Mold C, Gewurz H, Du Clos TW. Regulation of complement activation by C-reactive protein. Immunopharmacology. 1999;42:23–30. doi: 10.1016/s0162-3109(99)00007-7. [DOI] [PubMed] [Google Scholar]
- 23.Thompson D, Pepys MB, Wood SP. The physiological structure of human C-reactive protein and its complex with phosphocholine. Structure. 1999;7:169–177. doi: 10.1016/S0969-2126(99)80023-9. [DOI] [PubMed] [Google Scholar]
- 24.Emsley J, White HE, O'Hara BP, Oliva G, Srinivasan N, Tickle IJ, et al. Structure of pentameric human serum amyloid P component. Nature. 1994;367:338–345. doi: 10.1038/367338a0. [DOI] [PubMed] [Google Scholar]
- 25.Bang R, Marnell L, Mold C, Stein MP, Du Clos KT, Chivington-Buck C, et al. Analysis of binding sites in human C-reactive protein for Fc{gamma}RI, Fc{gamma}RIIA, and C1q by site-directed mutagenesis. J Biol Chem. 2005;280:25095–25102. doi: 10.1074/jbc.M504782200. [DOI] [PubMed] [Google Scholar]
- 26.Daeron M. Fc receptor biology. Annu Rev Immunol. 1997;15:203–234. doi: 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- 27.Hulett MD, Hogarth PM. Molecular basis of Fc receptor function. Adv Immunol. 1994;57:1–127. doi: 10.1016/s0065-2776(08)60671-9. [DOI] [PubMed] [Google Scholar]
- 28.Ravetch JV, Kinet JP. Fc receptors. Annu Rev Immuno. 1991;9:457–492. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- 29.Tamm A, Schmidt RE. IgG binding sites on human Fc gamma receptors. Int Rev Immunol. 1997;16:57–85. doi: 10.3109/08830189709045703. [DOI] [PubMed] [Google Scholar]
- 30.Maxwell KF, Powell MS, Hulett MD, Barton PA, McKenzie IF, Garrett TP, et al. Crystal structure of the human leukocyte Fc receptor, Fc gammaRIIa. Nat Struct Biol. 1999;6:437–442. doi: 10.1038/8241. [DOI] [PubMed] [Google Scholar]
- 31.Sondermann P, Huber R, Jacob U. Crystal structure of the soluble form of the human fcgamma-receptor IIb: a new member of the immunoglobulin superfamily at 1.7 A resolution. EMBO J. 1999;18:1095–1103. doi: 10.1093/emboj/18.5.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Boesen CC, Radaev S, Brooks AG, Fridman WH, Sautes-Fridman C, et al. Crystal structure of the extracellular domain of a human Fc gamma RIII. Immunity. 2000;13:387–395. doi: 10.1016/s1074-7613(00)00038-8. [DOI] [PubMed] [Google Scholar]
- 33.Garman SC, Kinet JP, Jardetzky TS. Crystal structure of the human high-affinity IgE receptor. Cell. 1998;95:951–961. doi: 10.1016/s0092-8674(00)81719-5. [DOI] [PubMed] [Google Scholar]
- 34.Herr AB, Ballister ER, Bjorkman PJ. Insights into IgA-mediated immune responses from the crystal structures of human FcalphaRI and its complex with IgA1-Fc. Nature. 2003;423:614–620. doi: 10.1038/nature01685. [DOI] [PubMed] [Google Scholar]
- 35.Radaev S, Motyka S, Fridman WH, Sautes-Fridman C, Sun PD. The structure of a human type III Fcgamma receptor in complex with Fc. J Biol Chem. 2001 May 11;276:16469–16477. doi: 10.1074/jbc.M100350200. [DOI] [PubMed] [Google Scholar]
- 36.Sondermann P, Huber R, Oosthuizen V, Jacob U. The 3.2-A crystal structure of the human IgG1 Fc fragment-Fc gammaRIII complex. Nature. 2000;406:267–273. doi: 10.1038/35018508. [DOI] [PubMed] [Google Scholar]
- 37.Garman SC, Wurzburg BA, Tarchevskaya SS, Kinet JP, Jardetzky TS. Structure of the Fc fragment of human IgE bound to its high-affinity receptor Fc epsilonRI alpha. Nature. 2000;406:259–266. doi: 10.1038/35018500. [DOI] [PubMed] [Google Scholar]
- 38.Holdom MD, Davies AM, Nettleship JE, Bagby SC, Dhaliwal B, Girardi E, et al. Conformational changes in IgE contribute to its uniquely slow dissociation rate from receptor FcvarepsilonRI. Nat Struct Mol Biol. 2011;18:571–576. doi: 10.1038/nsmb.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramsland PA, Farrugia W, Bradford TM, Sardjono CT, Esparon S, Trist HM, et al. Structural basis for Fc gammaRIIa recognition of human IgG and formation of inflammatory signaling complexes. J Immunol. 2011;187:3208–3217. doi: 10.4049/jimmunol.1101467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marnell LL, Mold C, Volzer MA, Burlingame RW, Du Clos TW. C-reactive protein binds to Fc gamma RI in transfected COS cells. J Immunol. 1995;155:2185–2193. [PubMed] [Google Scholar]
- 41.Bharadwaj D, Stein MP, Volzer M, Mold C, Du Clos TW. The major receptor for C-reactive protein on leukocytes is fcgamma receptor II. J Exp Med. 1999;190:585–590. doi: 10.1084/jem.190.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu J, Marnell LL, Marjon KD, Mold C, Du Clos TW, Sun PD. Structural recognition and functional activation of FcgammaR by innate pentraxins. Nature. 2008;456:989–992. doi: 10.1038/nature07468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marjon KD, Marnell LL, Mold C, Du Clos TW. Macrophages activated by C-reactive protein through Fc gamma RI transfer suppression of immune thrombocytopenia. J Immunol. 2009;182:1397–1403. doi: 10.4049/jimmunol.182.3.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu J, Marjon KD, Marnell LL, Wang R, Mold C, Du Clos TW, et al. Recognition and functional activation of the human IgA receptor (FcalphaRI) by C-reactive protein. Proc Natl Acad Sci USA. 2011;108:4974–4979. doi: 10.1073/pnas.1018369108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zuniga R, Markowitz GS, Arkachaisri T, Imperatore EA, D'Agati VD, Salmon JE. Identification of IgG subclasses and C-reactive protein in lupus nephritis: the relationship between the composition of immune deposits and FCgamma receptor type IIA alleles. Arthritis Rheum. 2003;48:460–470. doi: 10.1002/art.10930. [DOI] [PubMed] [Google Scholar]
- 46.Shih PB, Manzi S, Shaw P, Kenney M, Kao AH, Bontempo F, et al. Genetic Variation in C-Reactive Protein (CRP) Gene May Be Associated with Risk of Systemic Lupus Erythematosus and CRP Concentrations. J Rheumatol. 2008;35:2171–2178. doi: 10.3899/jrheum.080262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Edberg JC, Wu J, Langefeld CD, Brown EE, Marion MC, McGwin G, Jr., et al. Genetic variation in the CRP promoter: association with systemic lupus erythematosus. Hum Mol Genet. 2008;17:1147–1155. doi: 10.1093/hmg/ddn004. [DOI] [PubMed] [Google Scholar]
- 48.Russell AI, et al. Polymorphism at the C-reactive protein locus influences gene expression and predisposes to systemic lupus erythematosus. Hum Mol Genet. 2004;13:137–147. doi: 10.1093/hmg/ddh021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jonsen A, Gunnarsson I, Gullstrand B, Svenungsson E, Bengtsson AA, Nived O, et al. Association between SLE nephritis and polymorphic variants of the CRP and Fc©RIIIa genes. Rheumatology. 2007;46:1417–1421. doi: 10.1093/rheumatology/kem167. [DOI] [PubMed] [Google Scholar]
- 50.Du Clos TW, Zlock LT, Hicks PS, Mold C. Decreased autoantibody levels and enhanced survival of (NZB × NZW) F1 mice treated with C-reactive protein. Clin Immunol Immunopathol. 1994;70:22–27. doi: 10.1006/clin.1994.1005. [DOI] [PubMed] [Google Scholar]
- 51.Rodriguez W, Mold C, Kataranovski M, Hutt J, Marnell LL, Du Clos TW. Reversal of ongoing proteinuria in autoimmune mice by treatment with C-reactive protein. Arthritis Rheum. 2005;52:642–650. doi: 10.1002/art.20846. [DOI] [PubMed] [Google Scholar]
- 52.Rodriguez W, et al. Prevention and reversal of nephritis in MRL/lpr mice with a single injection of C-reactive protein. Arthritis Rheum. 2006;54:325–335. doi: 10.1002/art.21556. [DOI] [PubMed] [Google Scholar]
- 53.Szalai AJ, et al. Delayed lupus onset in (NZB × NZW)F1 mice expressing a human C-reactive protein transgene. Arthritis Rheum. 2003;48:1602–1611. doi: 10.1002/art.11026. [DOI] [PubMed] [Google Scholar]
- 54.Gershov D, Kim S, Brot N, Elkon KB. C-Reactive protein binds to apoptotic cells, protects the cells from assembly of the terminal complement components, and sustains an antiinflammatory innate immune response: implications for systemic autoimmunity. J Exp Med. 2000;192:1353–1364. doi: 10.1084/jem.192.9.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Szalai AJ, Nataf S, Hu X-Z, Barnum SR. Experimental allergic encephalomyelitis is inhibited in transgenic mice expressing human C-reactive protein. J Immunol. 2002;168:5792–5797. doi: 10.4049/jimmunol.168.11.5792. [DOI] [PubMed] [Google Scholar]
- 56.Hu XZ, et al. Inhibition of Experimental Autoimmune Encephalomyelitis in Human C-Reactive Protein Transgenic Mice Is FcgammaRIIB Dependent. Autoimmune Dis. 2010;2011:484936. doi: 10.4061/2011/484936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jones NR, et al. Collagen-induced arthritis is exacerbated in C-reactive protein deficient mice. Arthritis Rheum. 2011;63:2641–2650. doi: 10.1002/art.30444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mold C, Rodriguez W, Rodic-Polic B, Du Clos TW. C-reactive protein mediates protection from lipopolysaccharide through interactions with Fc©R. J Immunol. 2002;169:7019–7025. doi: 10.4049/jimmunol.169.12.7019. [DOI] [PubMed] [Google Scholar]
- 59.Rodriguez W, Mold C, Kataranovski M, Hutt JA, Marnell LL, Verbeek JS, et al. C-reactive protein-mediated suppression of nephrotoxic nephritis: role of macrophages, complement, and Fcγ receptors. J Immunol. 2007;178:530–538. doi: 10.4049/jimmunol.178.1.530. [DOI] [PubMed] [Google Scholar]
- 60.Samuelsson A, Towers TL, Ravetch JV. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science. 2001;291:484–486. doi: 10.1126/science.291.5503.484. [DOI] [PubMed] [Google Scholar]
- 61.Anthony RM, Ravetch JV. A novel role for the IgG Fc glycan: the anti-inflammatory activity of sialylated IgG Fcs. J Clin Immunol. 2010;30(Suppl):S9–14. doi: 10.1007/s10875-010-9405-6. [DOI] [PubMed] [Google Scholar]
- 62.Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73:209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 63.Bickerstaff MC, Botto M, Hutchinson WL, Herbert J, Tennent GA, Bybee A, et al. Serum amyloid P component controls chromatin degradation and prevents antinuclear autoimmunity. Nat Med. 1999;5:694–697. doi: 10.1038/9544. [DOI] [PubMed] [Google Scholar]
- 64.Zhang W, Wu J, Qiao B, Xu W, Xiong S. Amelioration of lupus nephritis by serum amyloid P component gene therapy with distinct mechanisms varied from different stage of the disease. PloS One. 2011;6:e22659. doi: 10.1371/journal.pone.0022659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Raaz D, et al. FcgammaRIIa genotype is associated with acute coronary syndromes as first manifestation of coronary artery disease. Atherosclerosis. 2009;205:512–516. doi: 10.1016/j.atherosclerosis.2009.01.013. [DOI] [PubMed] [Google Scholar]