Abstract
We have developed and implemented a novel mathematical model for simulating transients in surface pH (pHS) and intracellular pH (pHi) caused by the influx of carbon dioxide (CO2) into a Xenopus oocyte. These transients are important tools for studying gas channels. We assume that the oocyte is a sphere surrounded by a thin layer of unstirred fluid, the extracellular unconvected fluid (EUF), which is in turn surrounded by the well-stirred bulk extracellular fluid (BECF) that represents an infinite reservoir for all solutes. Here, we assume that the oocyte plasma membrane is permeable only to CO2. In both the EUF and intracellular space, solute concentrations can change because of diffusion and reactions. The reactions are the slow equilibration of the CO2 hydration-dehydration reactions and competing equilibria among carbonic acid (H2CO3)/bicarbonate ( ) and a multitude of buffers. Mathematically, the model is described by a coupled system of reaction-diffusion equations that—assuming spherical radial symmetry—we solved using the method of lines with appropriate stiff solvers. In agreement with experimental data (Musa-Aziz et al, PNAS 2009, 106:5406–5411), the model predicts that exposing the cell to extracellular 1.5% CO2/10 mM (pH 7.50) causes pHi to fall and pHS to rise rapidly to a peak and then decay. Moreover, the model provides insights into the competition between diffusion and reaction processes when we change the width of the EUF, membrane permeability to CO2, native extra-and intracellular carbonic anhydrase-like activities, the (intrinsic) intracellular buffering power, or mobility of intrinsic intracellular buffers.
Keywords: pH, spherical cell, competing equilibria, multiple buffers
1. Introduction
Extracellular pH (pHo) and intracellular pH (pHi) are of broad interest to biologists because pH perturbations can alter a wide range of processes. Thus, maintaining pHo and pHi in a normal range is of fundamental importance for correct cellular and tissue function. Cells regulate their pH by transporting acids or bases—particularly the hydrogen ion (H+) or the bicarbonate ion ( )—across the plasma membrane. In addition, the movements of dissolved gases such as carbon dioxide (CO2) or ammonia (NH3) also have major effects on pHo and pHi. In fact, fluxes of the above substances generally will have reciprocal effects on pH on opposite sides of the membrane.
The oocyte from the African clawed frog Xenopus laevis is a major experimental model system used extensively in developmental biology, molecular biology, and electrophysiology. Because of their large size (≈1 mm in diameter), these cells can be easily handled and manipulated. For example, they can be injected with RNA from other organisms and then used to study protein expression using biochemical or electrophysiological approaches. In the laboratory of one of the authors, Xenopus laevis oocytes are used to study three categories of proteins that can have a substantial impact on pH: (1) sodium-coupled bicarbonate transporters (NCBTs), which play important roles in pHi regulation and epithelial acid-base transport (Romero et al., 1997; Choi et al., 2000; Grichtchenko et al., 2001); (2) aquaporins (AQPs) and Rhesus (Rh) proteins, which can serve as conduits (i.e., gas channels) for the diffusion of CO2 or NH3 (Nakhoul et al., 1998; Cooper and Boron, 1998; Endeward et al., 2006; Musa-Aziz et al., 2009; Boron, 2010); and (3) carbonic anhydrases (CAs), enzymes that catalyze the interconversion of CO2 and and play important roles in the transport of CO2 and .
Understanding the transport across the plasma membrane of substances such as H+, , or CO2, is not trivial. Cells normally contain and are surrounded by a number of buffers. Thus, acid-base transport triggers: (1) a multitude of interconnected extra- and intracellular buffer reactions that explicitly or implicitly involve H+ and (2) diffusion events—all of which vary in space and time. The result is changes in solute concentrations, including pH, that vary in space and time throughout the system. These changes are likely to be so complex that it would be impossible to understand them in the absence of an appropriate mathematical model.
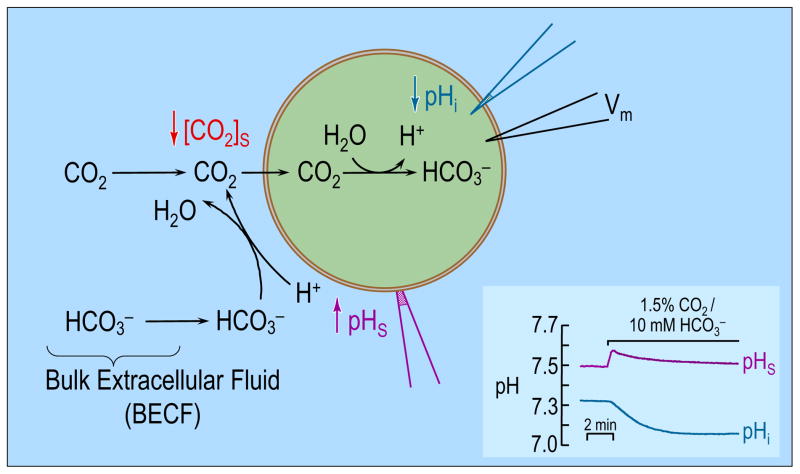
Endeward et al and Musa-Aziz et al exploited a novel technique to assess the CO2 permeability of a Xenopus oocyte (Endeward et al., 2006; Musa-Aziz et al., 2009). Figure 1 illustrates the approach, in which one measures surface pH (pHS) by pushing a liquid-membrane pH-sensitive microelectrode against the oocyte surface, thereby dimpling the oocyte membrane slightly. Subsequently applying in the bulk extracellular fluid (BECF) causes pHS to rise to a peak and then decay (Figure 1 inset, upper record). Simultaneous measurements with an intracellular electrode reveal the familiar fall in pHi (Figure 1 inset, lower record). The events underlying these changes in pHS and pHi are summarized by the diagram in the main part of Figure 1. As CO2 enters the cell, the concentration of CO2, [CO2], at the cell surface decreases. The lost CO2 can be replenished by diffusion from the BECF or by reaction at the cell surface from and H+. The observed rise in pHS reflects the consumption of H+ via this reaction. Conversely, in the intracellular space, the influx of CO2 leads to the generation of H+ (and therefore to a decrease in pHi) via the opposite reaction.
Figure 1.
Model of an oocyte exposed to . As CO2 enters the cell, the concentration of CO2 near the outer surface of the oocyte falls. The lost CO2 can be replenished by 1) diffusion from the bulk extracellular fluid (BECF) and 2) the reaction(s) at the cell surface. This reaction, which consumes H+, causes pHS to rise. Conversely, the entry of CO2 into the cell leads to the generation of intracellular H+ via the reaction(s) , therefore causing a fall in pHi. (Cell model adapted from ref (Boron, 2010). The inset at the bottom right shows experimental pHS and pHi records for a H2O-injected oocyte exposed to 1.5% CO2/10mM . Data kindly provided by Dr. Musa-Aziz).
The magnitude of the pHS spike in Figure 1 ought to be an index of CO2 permeability. Indeed, the expression of AQPs or Rh proteins in oocytes causes the pHS spike to increase (Endeward et al., 2006; Musa-Aziz et al., 2009)—part of the evidence that these proteins are gas channels. With the appropriate mathematical model, one might be able to extract—from the time course of pHS in Figure 1—the permeability of the membrane to CO2. Moreover, one could gain insight into events not directly accessible by experiments (e.g., the time course of intracellular solute concentrations). Finally, an appropriate model would help in the formulation and testing of hypotheses. The requirements of such a model include: (a) a 3-dimensional spherical cell, (b) the transport of CO2 across the plasma membrane, (c) the reactions of a multitude of extra- and intracellular buffers, (d) diffusion of solutes through the extra- and (d′) intracellular spaces, (e) CA activity at specific loci, and (f) temporal and (f′) spatial variations of solute concentrations.
Several authors have presented mathematical models of acid-base transport that have assisted in the interpretation of data collected in their respective laboratories.
In 1976, Boron and De Weer, using a system of two ordinary differential equations, predicted for the first time the time course of pHi when CO2 and —or NH3 and —enter or leave the cell (Boron and De Weer, 1976). Although valuable, this model does not address requirements (a), (c), (d), (d′), (e) or (f′).
In 2002, Vaughan-Jones et al recognized the importance of studying the spatio-temporal behavior of pH and developed a two-dimensional mathematical model to study the free diffusion of intracellular H+ from a constant source in ventricular myocytes (Vaughan-Jones et al., 2002). The model leads to estimates of the apparent intracellular H+ diffusion coefficient. Although it accounts for spatial variability of solutes, it does not address requirements (a), (b), (c), or (e).
More recently, this same group has developed reaction-diffusion models to study the relationship between extracellular CAIX and the spatio-temporal coordination of pHo and pHi in a multicellular cluster of tumor cells (Swietach et al., 2008; Swietach et al., 2009). This model does not consider a single cell but a spherical cluster of cells and does not address requirements (a), (c), or (d′).
Missner et al. developed a one-dimensional reaction-diffusion mathematical model to study the diffusion of CO2 through planar lipid bilayers at steady state (Missner et al., 2008b). This model is also not suitable for our purposes because it does not address requirements (a), (c) or (f).
Gros and coworkers developed a time-dependent mathematical model of six ordinary differential equations to interpret their mass-spectrometric data obtained in a chemically pre-equilibrated system in which they monitored the diffusion of 18O-labeled across the red blood cell (RBC) membrane (Endeward and Gros, 2005). Their approach yielded estimates of the apparent membrane permeabilities to CO2 and . This model does not address requirements (a), (c), (d), (d′), or (f′).
More recently, these same authors extended their model to simulate the diffusion of CO2, , and H2O in one-dimensional space from a stirred bulk solution, through an extracellular unstirred layer, through the cell membrane, and into the RBC (Endeward and Gros, 2009). The model can provide insights into the effect of intra- and extracellular unstirred layers in the estimation of the CO2 permeability of the RBC membrane. Although this model is a reaction-diffusion model and accounts for spatial variation, it does not address requirements (a) and (c).
It is clear from the above discussion that none of the available models is well suited to the interpretation of the aforementioned oocyte data (Figure 1). In the present paper, we develop a three-dimensional mathematical model under the assumption of spherical symmetry of a cell (e.g., an oocyte) and of its surrounding environment (requirement (a), above), with the goal of simulating the observed changes in pHS and pHi caused by the entry of CO2 (requirement (b)). Each species can react with other solutes in both the intra- and extracellular spaces, including a multitude of buffers (requirement (c)). To our knowledge, ours is the first model capable of handling an indefinite number of buffers. Each species also can move via diffusion through the extra- and intracellular spaces (requirements (d) and (d′)). Finally, the model includes CA-catalysis (requirement (e)) as well as temporal (requirement (f)) and spatial variations (requirement (f′)).
Mathematically, the present model consists of a coupled system of reaction-diffusion equations, describing the diffusion of solutes in the intracellular and extracellular space and through the cell membrane, as well as local chemical interactions between the species. Because we use a spherical model of the cell and assume no directional dependency in the boundary conditions, the symmetry allows us to reduce the model to a radial system. However, the reaction rates may vary as a function of the radius, so the system has non-constant coefficients. We use the method of lines, the spatial discretization being based on finite difference approximation, leading to a stiff system which is solved numerically by appropriate stiff solvers.
We hypothesize that the present model will reproduce the essential features of Figure 1, and serve as the foundation for more advanced models that could provide estimates of parameters such as CO2 permeability. The model successfully predicts that, when an oocyte is exposed to extracellular CO2/HCO3−, the influx of CO2 causes pHi to fall monotonically and pHS to rise and then decay. Moreover, the model allows one to extract information on variables (e.g., time and space profiles of solute concentrations) that cannot be measured directly. For example, in typical electrophysiological experiments, one might measure pHS and pHi, but not have access to the concentrations of the other solutes involved in the process. The model also provides reasonable predictions when we change the width of the extracellular unconvected layer surrounding the oocyte, the CO2 membrane permeability, and the extra- and intracellular CA-like activities. The model provides insights into the competition between reaction and diffusion near the outer surface of the cell membrane and makes valuable predictions when we change the intracellular buffering power, or change the ratio of mobile to immobile buffers.
2. Model
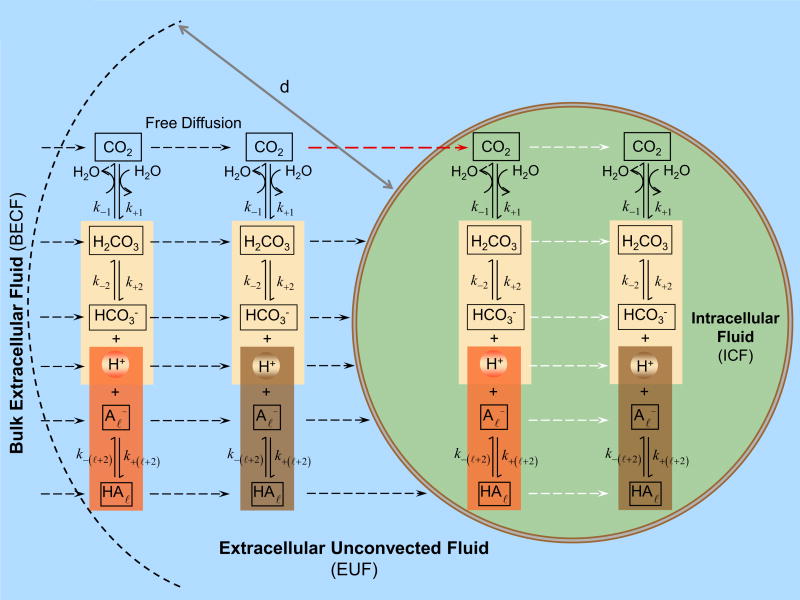
The key components of the model are summarized in the cartoon of Figure 2. The model assumes that the oocyte is surrounded by the extracellular unconvected fluid (EUF) which in turn is surrounded by a volume of bulk extracellular fluid. The EUF is a thin layer adjacent to the surface of the oocyte where no convection occurs, but reactions and diffusion do occur. The bulk extracellular fluid is an infinite reservoir for all solutes where convection (not included in the present model) could occur but not reaction or diffusion. All solutes can diffuse between the BECF and EUF. The plasma membrane may have different permeabilities to different solutes. To avoid the need to specify the thickness of the membrane, in the present implementation, we use a computational model in which we take the limit where the membrane thickness parameter goes to zero, while the permeability is kept constant. Moreover, we assume that the plasma membrane is permeable only to CO2—a reasonable assumption, given the low permeability of the Xenopus oocyte membrane to H+ (Fei et al., 1994; Gunshin et al., 1997; Steel et al., 1997) and (Costa et al., 1989; Toye et al., 2006; Lee et al., 2011). In the EUF and intracellular fluid (ICF), the concentration of solutes can change because of diffusion and reaction processes; each solute has a specific, position-dependent mobility. In both EUF and ICF, the model accounts for the slow equilibration of the CO2 hydration/dehydration reactions, for competing equilibria among the buffer and a multitude of buffers. In Figure 2, the buffers are indicated by .
Figure 2.
Key components of the mathematical model. The oocyte is surrounded by the extracellular unconvected fluid (EUF), which in turn is surrounded by the bulk extracellular fluid (BECF). The BECF is an infinite reservoir for all solutes, where convection (not included in the present model) could occur but no reaction or diffusion. All solutes can diffuse between the BECF and EUF. The EUF is a thin layer of thickness d adjacent to the oocyte surface where no convection occurs, but reaction and diffusion do occur. The plasma membrane is permeable only to CO2. In the intracellular fluid (ICF), both reaction and diffusion occur. In both the EUF and ICF the model accounts for the slow equilibration of the CO2 hydration/dehydration reactions, competing equilibria among the buffers and a multitude of buffers (indicated as ).
The objective is to simulate numerically the dynamics of the concentrations of the solutes of interest, and in particular, the pH on both sides of the cell membrane.
Let Ω ⊂ ℝ3 denote the domain occupied by the oocyte and ∂Ω the boundary membrane. The concentration distribution of the CO2 is denoted by
| (1) |
t being the time. Furthermore, the concentration distribution of the H+ is denoted by
| (2) |
The numbering of the concentrations of carbonic acid (H2CO3), of the L non-H2CO3 weak acids (HAℓ, 1 ≤ ℓ ≤ L ), and of their corresponding conjugate weak bases ( and ), respectively, are written as
| (3) |
and
| (4) |
respectively. Note that we chose to ignore the dissociation of to form plus H+ because the pK of this reaction (≈10.3) is much higher than the pH values in the current simulations.
The reactions of carbonic acid formation and the corresponding rate constants are denoted by
| (5) |
and the reactions of dissociation with the corresponding rate constants are
| (6) |
and
| (7) |
Note that the results of recent metadynamic simulations (Stirling and Papai, 2010) are most consistent with a model in which the reactions involving CO2, H2CO3, , and H+ proceed not as outlined in equations (5) and (6) but in the order . However, the ordering of these reactions would have no effect on the results of the present paper.
The reaction fluxes Φℓ, indexed according to their respective rate constants kℓ, ℓ = ±1, ±2, …, ±(L + 2), are modeled using the law of mass action, that is,
| (8) |
| (9) |
and
| (10) |
As described below, the rate constants for each reaction (i.e., k+ℓ and k−ℓ) respect the equilibrium constant ( Kℓ) that governs that reaction. Moreover, we can assume that the rate constants depend on location, kℓ = kℓ(x). This assumption is relevant, for example, when including a non-homogenous distribution of the enzyme carbonic anhydrase which, when present, increases the rate constants k+1 and k−1.
Consider now a single solute w, whose concentration we denote by cw = cw (t, x), that is, cw = uj or cw = vj for some j. The corresponding reaction-diffusion equation is written as
| (11) |
Here, the first term on the right-hand side of the equation is Fick’s second law of diffusion, where Dw = Dw (x) is the diffusion coefficient of solute w defining its mobility in the medium. The second term describes the chemical reactions among the solutes. The coefficients Sw,ℓ are the stoichiometric coefficients, describing if the ℓth reaction produces the solute w ( Sw,ℓ =1), consumes it ( Sw,ℓ = −1), or does not change its concentration ( Sw,ℓ = 0 ).
The reaction-diffusion equation (11) is assumed to hold inside and outside the boundary membrane ∂Ω. At the membrane, a boundary condition needs to be postulated, reflecting the permeability of the membrane to each one of the solutes. We denote by “ ” the boundary value from inside (−) and outside (+) of the membrane (i.e. in the aqueous phase adjacent to the intracellular and extracellular sides of the plasma membrane), respectively. To find a reasonable boundary condition, assume first that the membrane has a positive thickness h > 0, and assume that the substance moves through the membrane via diffusion. Letting the thickness of the membrane tend to zero while decreasing proportionally the solute mobility at the plasma membrane, the diffusion of solute w through the membrane can be approximated by a transmission boundary condition (see Appendix A). Let D w,+ and D w,− denote the diffusion coefficients in the aqueous phases adjacent to the extracellular and intracellular sides of the membrane, respectively. We posit the transmission conditions, which use Fick’s first law to establish the continuity of fluxes across the membrane for solute w. Denoting by the outer normal derivative at the boundary, the conservation of flux implies that
| (12) |
On the other hand, from the analysis in Appendix A, we have
| (13) |
where P∂Ω,w is the true membrane permeability to solute w.
The boundary condition at the outer boundary ∂Ω∞ of the computational domain is a Dirichlet boundary condition,
| (14) |
where cw,∞ is the assumed constant concentration in the BECF. The computational domain Ω∞ comprises the cell and the surrounding EUF while the outer boundary ∂Ω∞ identifies the BECF.
Finally, we collect the concentrations of all 2L + 4 solutes into a single vector having 2L + 4 components,
| (15) |
The initial-boundary value problem that we consider is the reaction-diffusion equation, the matrix-vector form of equation (11),
| (16) |
where D = D(x) is a diagonal matrix containing the diffusion coefficients, S is the stoichiometric matrix and Φ = Φ(U) is the reaction flux vector. The components of U satisfy the transmission conditions given by equations (12) and (13) at the cell membrane ∂Ω, the specified initial conditions (i.e., concentrations of all solutes at time zero within the domain of integration), and the boundary conditions. Thus we write
| (17) |
where U0( x) ∈ ℝ2L+4 is a vector containing the initial concentrations in the computational domain Ω∞ and U∞ specifies the boundary conditions at the outer boundary ∂Ω∞.
Remark: A natural extension of the model would include a convection term in the exterior domain, i.e., a first order term v(x) · ∇ U(x, t), where v is a velocity field modeling the liquid flow. This addition is not included in the present model.
The implementation of this model in a spherical geometry is discussed in the following section.
3. Computational model
In this section, we consider the governing equations in a spherically symmetric case. We apply the method of lines using a spatial semi-discretization by finite difference approximation. Although this technique is rather standard (Iserles, 1996), for the convenience of the reader not familiar with it, we present the method of lines in the geometry considered.
3.1 Spherically symmetric case
Assume from now on that the oocyte can be modeled as a sphere Ω of radius R concentric with the larger sphere Ω∞ of radius R∞ defining the computational domain. Furthermore, we assume that the diffusion coefficients and initial values depend on the distance from the origin only, and that the boundary condition is direction independent, i.e., U∞ (t, x) = U∞ (t). With these restrictions, the concentration vector is a function of the radial variable r only, U = U (t, r), and the diffusion equation assumes the form
| (18) |
which is a matrix-vector form of equation (11). The transmission conditions, from equations (12) and (13), are
| (19) |
and
| (20) |
Here, P is a diagonal matrix containing the membrane permeability coefficients of each solute in its diagonal. The initial-boundary conditions, analogous to equation (17), are
| (21) |
3.2 Semi-discretization
To obtain a numerically feasible form, we use the method of lines and discretize the system in the spatial direction using an appropriate finite difference scheme.
We start by dividing the space interval [0, R∞] into N subintervals, denoting the grid points as rj, 0 = r0 < r1 < · · ·< rN = R∞, and defining the grid functions Cℓ, 1 ≤ ℓ ≤ 2L + 4,
| (22) |
In Appendix B, we develop for each grid function a finite difference approximation of the diffusion operator, i.e., we write a semi-discretized system,
| (23) |
where Lℓ ∈ ℝN ×N is a second order finite-difference matrix that takes into account the sampled values of the diffusion coefficient and transport across the cell membrane, cℓ,∞(t) ∈ ℝ is the boundary (scalar) value of the concentration at r = R∞, and Ẽ∞ is a constant vector (see Appendix B). Finally, Φℓ′ = Φℓ′ ({Cℓ}) ∈ ℝN is a vector representing the reaction rates of the ℓ′ th reaction at the grid points, and Sℓ, ℓ′ is the stoichiometric matrix.
Finally, by stacking all the vectors Cℓ into a single vector C ∈ ℝN (2L+4), and likewise the reaction fluxes, and by organizing the matrices into single block matrices, we can write the system compactly as
| (24) |
where the boundary condition vector is c∞ (t) ∈ ℝ2L+4. This is a stiff system of differential equations, whose numerical solution requires a specialized solver.
4. Results and Discussion
4.1 Equilibration of buffers
Before describing the computational experiments in detail in the next section, we address the issue of competing equilibria among buffers (see Figure 2), and how we implement them in the model.
A critical reaction—though not strictly a buffer reaction because it does not involve H+—is the CO2 hydration - dehydration shown in equation (5). In the absence of one of the carbonic anhydrase enzymes, this reaction is extremely slow. We simulate CA-like activity by multiplying the rate constants of reaction (5), k+1 and k−;1, by an acceleration factor A (Ao for extracellular CA-like activity and Ai for intracellular CA-like activity). Note that CA does not change the equilibrium state, only the rate at which it is achieved.
In addition to the above reaction, the model contains 1+L buffer reactions, one for the pair plus L reactions for the buffers—all of which compete for a common pool of H+, and all of which are virtually instantaneous (i.e., limited only by diffusion) (Roughton, 1941; Harned and Owen, 1958). For the pair, we denote the equilibrium constant associated with reaction (6) by
| (25) |
To ensure instantaneous equilibrium of reaction (6) we write an approximation
| (26) |
and choose the parameter εb > 0 (Table 1) so small that, in the time scale of the diffusion and reaction (6), this reaction virtually takes place immediately. This approximation adds to the stiffness of the system (24). However, because the diffusion equation is already stiff, the stiffness depending on the discretization, specialized solvers need to be employed anyway.
Table 1.
Parameter values used in the “standard in-silico experiment”.
| Bulk ECF | Bulk ICF | Reference | |||
|---|---|---|---|---|---|
|
| |||||
| Sphere diameter | 0.15 cm (d = 100 μm) | 0.13 cm | |||
|
| |||||
| # of subintervals | 100 | 80 | |||
|
| |||||
| Mobility | CO2 | 1.71× 10−5 cm2/sec | 1.71× 10−5 cm2/sec | (Gros and Moll, 1971) | |
| H2CO3 | 1.11× 10−5 cm2/sec | 1.11× 10−5 cm2/sec | computed* using data from refs. (Vanysek, 2011) and (Swietach et al., 2003) | ||
| HCO3− | 1.11× 10−5 cm2/sec | 1.11× 10−5 cm2/sec | computed* using data from refs. (Vanysek, 2011) and (Swietach et al., 2003) | ||
| H+ | 8.69× 10−5 cm2/sec | 8.69× 10−5 cm2/sec | computed* using data from refs. (Vanysek, 2011) and (Swietach et al., 2003) | ||
| HA1 | 1.56× 10−5 cm2/sec | 1.56× 10−5 cm2/sec | (Gutknecht and Tosteson, 1973) | ||
|
|
1.56× 10−5 cm2/sec | 1.56× 10−5 cm2/sec | calculated** from (Gutknecht and Tosteson, 1973) | ||
|
| |||||
| Concentration | CO2 | 0.4720 mM | 0 mM | ||
| H2CO3 | 0.0013 mM | 0 mM | |||
| HCO3− | 9.9 mM | 0 mM | |||
| H+ | 3.1623× 10−5 mM | 6.3096 × 10−5 mM | |||
| HA | 2.5 mM | 12.0910 mM | |||
| A− | 2.5 mM | 15.2216 mM | |||
| Total CO2 | 10.3733 mM | 0 mM | |||
|
| |||||
| pH | 7.50 | 7.20 | |||
| pKHA | 7.50 | 7.10 | |||
|
| |||||
| H2CO3 acid formation: | |||||
| Rate constants | k1 | 0.0302 sec−1 | 0.0302 sec−1 | computed* using data from refs. (Gibbons and Edsall, 1963) and (Zhao et al., 2003) | |
| k−1 | 10.9631 sec−1 | 10.9631 sec−1 | computed* using data from refs. (Gibbons and Edsall, 1963) and (Zhao et al., 2003) | ||
| Equilibrium constants | K1 (pK1) | 0.0028 (2.5599) | 0.0028 (2.5599) | calculated as k1/k−1 | |
|
| |||||
| H2CO3 dissociation: | |||||
| Rate constants | k2 | 1 × 1016 sec−1 | 1 × 1016 sec−1 | chosen to achieve equilibrium | |
| k−2 | 4.1530 × 1016 mM −1sec−1 | 4.1530 × 1016 mM−1sec−1 | (calculated as k−2= k2/K2) | ||
| Equilibrium constants | K2 (pK2) | 0.2408 (3.6184) mM | 0.2408 (3.6184) mM | calculated | |
|
| |||||
|
|
|||||
| Rate constants | k3 | 1 × 1010 sec−1 | 1 × 1010 sec−1 | chosen to achieve equilibrium | |
| k−3 | 3.1623 × 1014 mM−1sec−1 | 1.2589 × 1014 mM−1sec−1 | (calculated as k−3= k3/KHA) | ||
| Equilibrium constants | KHA (pKHA) | 3.1623 × 10−5 (7.5) mM | 7.9433 × 10−5 (7.1) mM | assumed | |
|
| |||||
| CA-like activity | A-factor | 20 | 20 | chosen | |
See supplementary data for how we used the Arrhenius equation to compute this value.
See supplementary data for an explanation on how we calculated this value.
When we add a buffer pair , we similarly assume instantaneous equilibrium of the buffering reaction (7) by expressing the corresponding rate constants in terms of the equilibrium constant
| (27) |
with a sufficiently small time constant εℓ > 0 (Table 1).
4.2 Numerical Experiments
All computations are performed in MATLAB. The systems of ODEs arising from the method of lines (24) are solved using the built-in variable-accuracy stiff solver ODE15S. To ensure that the system achieves equilibrium—in time and in space—we not only assigned sufficiently large rate constants as noted above (see Table 1), but also chose a value of 1 × 10−12 for both the relative tolerance (RelTol) and absolute tolerance (AbsTol). The number of discretization points was a compromise between ensuring that the system achieves equilibrium and a reasonable computational time, which was on the order of a couple of minutes in a standard dual-core PC.
4.2.1 The “standard in-silico experiment”
Setting up the model
We start by describing the computational model used in a “standard in-silico experiment”. Consider a control oocyte (i.e., not injected with or expressing a heterologous protein). Before the beginning of the experiment, we assume that the plasma membrane is impermeable to all solutes. We assume that the EUF has thickness d, d being the distance between the plasma membrane and the BECF.
Prior to the beginning of the experiment, the BECF and EUF both contain 1.5% CO2/9.9 mM 7.50 plus a single buffer with a pK of 7.50 (e.g., HEPES) and a total concentration of 5 mM (i.e., [TA1]BECF = [HA1]BECF + [A−1]BECF = 5 mM). Because the membrane is totally impermeable, all solute concentrations throughout the EUF are the same as in the BECF, and all reactions are at equilibrium.
Regarding the intracellular composition before the beginning of the experiment, we assume an initial intracellular pH of 7.20, and that the concentrations of CO2, H2CO3, and are all zero. We also assume that the intracellular fluid contains only a single buffer pair, the properties of which we determined as follows. Consistent with experimental data, we assume that exposing an oocyte to 1.5% CO2 will cause pHi to fall from 7.20 to 7.00. From the Davenport diagram (Davenport, 1958; Boron, 2009)—a plot of [ ] vs. pH for CO2 values, in this case, of 0% and 1.5%—we can conclude that, over the pHi interval (pH1, pH2) = (7.00, 7.20), the mean (or “intrinsic”) intracellular buffering power is
| (28) |
We arbitrarily assume that the buffer pK is 7.10 (i.e., halfway between the initial and final pH values). To determine the total concentration [TA1]i of this single, hypothetical, intracellular buffer, we used an approach similar to one described previously (Boron, 1977). By definition, β̄ over the relevant pHi range is
| (29) |
where [TA1]i·Z is the number of moles per liter of H+ being buffered in the reaction . Expressing β̄ in the form
| (30) |
where the integrand function β(pH )i is the buffering power of a buffer in a “closed system” (i.e., [HA1]i + [A1−]i = constant) (Koppel and Spiro, 1914; Roos and Boron, 1980; Van Slyke, 1922),
| (31) |
where K = 10−pK, we can identify [TA 1]i·Z with the value of the integral.
Integrating the expression for β(pHi) yields
| (32) |
from which it follows that [TA1]i ≈ 27.3126 mM. We assume that, before the beginning of the experiment, the pH and the buffer components are uniformly distributed throughout the cell and are in equilibrium.
We assume that the BECF, EUF and ICF have the properties of pure water. Wherever possible, we choose parameter values from the literature at 22°C. Otherwise, we compute parameter values from published values at nearby temperatures (see Table 1 and Table 2).
Table 2.
More Parameters*
| Membrane permeability | CO2 | 34.20 cm/sec | calculated, assuming that the diffusion coefficient in the membrane is the same as an equivalent thickness of water. |
| H2CO3 | 0 | assumed | |
| HCO3− | 0 | assumed | |
| H+ | 0 | assumed | |
| HA1 | 0 | assumed | |
|
|
0 | assumed | |
|
| |||
| Temperature | 22°C | (room temperature) | |
|
| |||
| Solubility | CO2 | 0.0434 mM/mmHg | calculated** |
In the computer code, we represent zero as 1 × 10−20 cm/sec
See supplementary data for an explanation on how we calculated this value.
Starting “the standard in-silico experiment”
We begin the experiment—at time zero—by switching the diffusion constant for CO2 in the membrane (DM,CO2) from zero to the value that it would have in water (i.e., Dwater,CO2 = 1.71× 10−5 cm2 s−1). Thus, we assume that the membrane is a film of water of the same thickness hM as the membrane. The true membrane permeability PM,CO2 would be DM,CO2 divided by hM, which we assume to be 5 nm. Thus, PM,CO2 = 34.20 cm/sec.
During the experiment, all solutes are allowed to move freely between the BECF and EUF, while the dynamics in the EUF and ICF are governed by the reaction-diffusion equations. Only CO2 is allowed to diffuse across the membrane. We also assume that all extracellular and intracellular buffers are mobile, and that the mobilities of solutes are constant over the entire computational domain. We choose an EUF thickness of d = 100 μm, which corresponds to a computational domain of radius R∞ = 0.15/2 cm (R∞ = R + d). Further, we assume that CA-like enzymatic activity is uniformly distributed inside the oocyte and on the extracellular surface of the membrane.
In summary, in a “standard in-silico experiment” we implement:
A mobile buffer in the extracellular space, and a separate mobile buffer in the intracellular space.
The mobile buffer system in both the extracellular and intracellular spaces. A membrane permeable to only CO2.
CA-like activity everywhere inside the oocyte as well as on the extracellular surface of the plasma membrane.
An extracellular unconvected fluid with a thickness d = 100 μm.
Table 1 and Table 2 list the parameters used in a “standard in-silico experiment”. When we change a parameter from a value reported in Table 1 and Table 2, we will state this explicitly.
Testing the model: multiple intracellular buffers
In the standard experiment, we implement one mobile, buffer (see equation (7)) with different total buffer concentrations and pK values in the extra- vs intracellular fluid. To verify that the model can, as intended, handle multiple buffers , we introduced a second intracellular buffer . Knowing that the total buffering power of a mixture of buffers is the sum of the individual buffering powers (Boron, 2009), we considered two oocytes, one representing the “standard in-silico experiment” and thus having a single intracellular buffer ([TA1]i = 27.3126, pK = 7.1), and an otherwise identical oocyte with two intracellular buffers, each at half the standard concentration ([TA1]i = [TA2]i = 27.3126/2, pK = 7.1). As expected, the model yielded identical results for the two oocytes (data not shown).
Simulations of “the standard in-silico experiment”
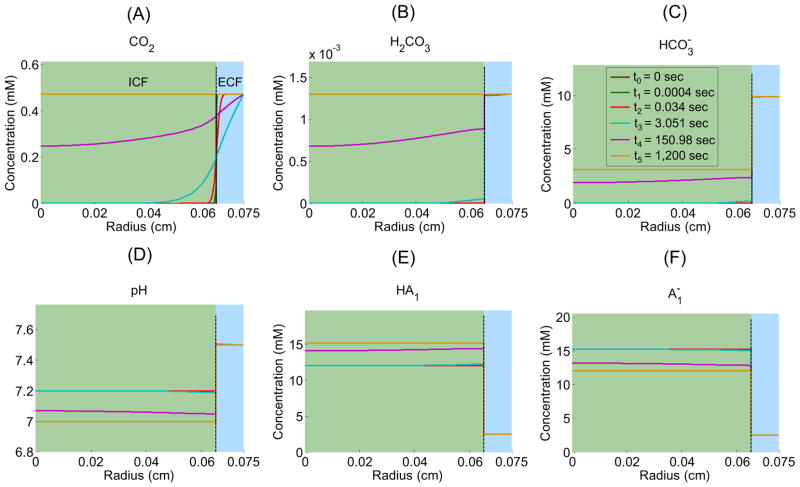
Figure 3 shows the time profiles of each extracellular solute (CO2, H2CO3, , H+ as pH, HA1, ) in the EUF at five radial distances from the center of the oocyte: 651 μm (i.e., 1 μm away from the outer surface of the membrane), 670 μm, 690μm, 710 μm, and 730 μm as well as in the BECF (i.e., beyond 750 μm). Similarly, Figure 4 shows the time profiles of each intracellular solute at five radial distances from the oocyte center: ≈ 8 μm (i.e., close to the center), ≈ 160 μm, ≈ 320 μm, ≈ 480 μm, and ≈ 640 μm as well as at 650 μm (i.e., the inner surface of the plasma membrane). Figure 5 illustrates how solute concentrations change as a function of the radial distance from the center of the oocyte at six time instances.
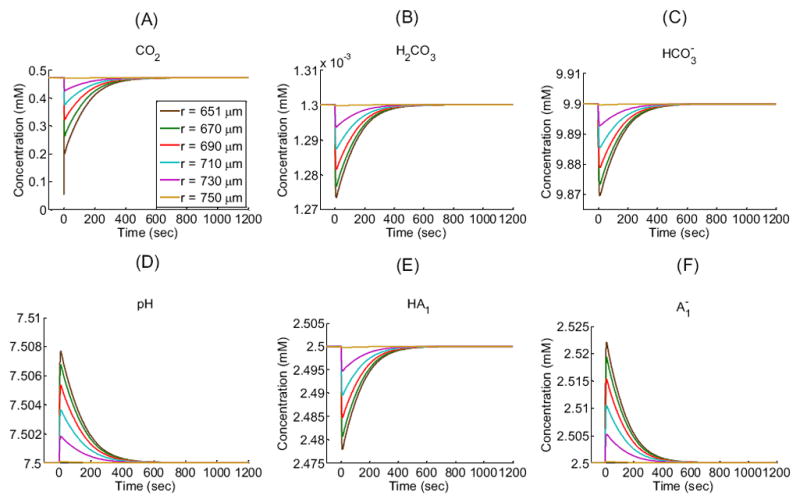
Figure 3.
Extracellular concentration-time profiles for solutes (CO2, H2CO3, HCO3−, H+ as pH, HA1, ) in a “standard in-silico experiment” at five exterior radial distances (r) from the center of an oocyte of radius R = 650 μm: 651 μm (i.e., 1 μm away from the outer surface of the membrane), 670 μm, 690 μm, 710 μm, and 730 μm in the extracellular unconvected fluid as well as 750 μm at the border with the bulk extracellular fluid. These data are the extracellular counterpart of the data in Figure 4. See Table 1 and Table 2 for parameter values.
Figure 4.
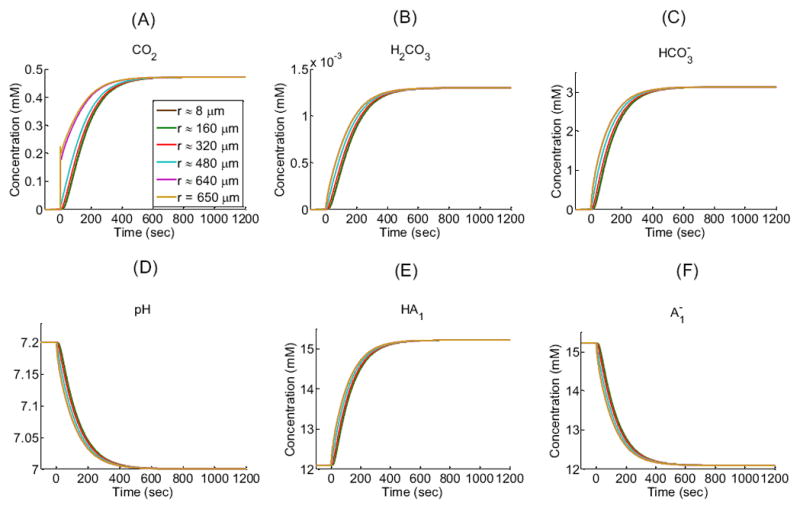
Intracellular concentration-time profiles for solutes (CO2, H2CO3, HCO3−, H+ as pH, HA1, ) in a “standard in-silico experiment” for five interior radial distances (r) from the center of an oocyte of radius R = 650 μm: ≈ 8 μm (i.e., close to the center), ≈ 160 μm, ≈ 320 μm, ≈ 480 μm, ≈ 640 μm and at 650 μm (i.e., the cytosolic surface of the plasma membrane). Note that the green curve for r ≈160 μm almost completely overlies the brown curve for r ≈ 8 μm. See Table 1 and Table 2 for parameter values.
Figure 5.
Extra- and intracellular concentration-distance profiles for solutes (CO2, H2CO3, HCO3−, H+ as pH, HA1, ) in a “standard in-silico experiment” at six time instances: t0 = 0 sec, t1 ≈ 0.0004 sec, t2 ≈ 0.034 sec, t3 ≈ 3.051 sec, t4 ≈ 150.98 sec, and t5 ≈ 1,200 sec. The dashed black vertical line indicates the oocyte membrane.
The simulation shows that, after the plasma membrane becomes permeable to CO2 at time t = 0, the CO2 concentration difference across the membrane (i.e., 0.4720 mM – 0 mM = 0.4720 mM; see Table 1) causes the diffusion of CO2 into the cell. This influx perturbs the pre-existing equilibria in the regions on opposite sides of the membrane for the buffer system and for the buffers. Moreover, this perturbation propagates in time away from the membrane in both directions, causing the following events to occur:
In the bulk extracellular fluid, the influx of CO2 from the EUF to the intracellular space produces an initial decrease in [CO2] (see Figure 3A)—largest and most rapidly in the extracellular fluid closest to the oocyte surface. This decrease in [CO2] promotes the diffusion of CO2 from the BECF toward the membrane, thereby in part replenishing the depleted CO2. The decreases in [CO2] throughout the EUF also perturb the equilibria, causing the reactions to replenish the depleted CO2, in the process, consuming first H2CO3 (Figure 3B) and then (Figure 3C) and H+, thereby causing pHo to rise (Figure 3D). The resulting decrease of [H+] throughout the EUF simultaneously perturbs the equilibria, causing the reactions to replenish the depleted H+, consuming [HA1] (Figure 3E) and producing (Figure 3F). Notice that the diffusion of CO2 to the outer surface of the cell membrane and the reaction forming CO2 from at the outer surface compete to replenish surface CO2. That is, to the extent that diffusion replenishes CO2, the contribution of the reaction falls. We quantify this competition by introducing the diffusion/reaction (DRR) ratio. The DRR is calculated in the volume of extracellular fluid that extends out to 1 μm from the plasma membrane, and is defined as the ratio of the rate of CO2 replenishment (μmoles/sec) by diffusion divided by the rate of CO2 produced by reaction (5). In the “standard in-silico experiment”, DRR spikes to a value of ≈ 2800 right at t = 0 sec and then rapidly falls to a value of ≈ 35 in approximately 10 sec as the slow reaction catches up. The changes in all solute concentrations become smaller and slower (e.g., the time to maximal change becomes greater) as we move away from the oocyte surface. Moreover, these changes dissipate in time—approaching their respective values in the BECF—as the CO2 equilibrates across the cell membrane and thus throughout the EUF and ICF (Figure 5A, gold line).
In the intracellular fluid, initially no CO2 is present. As CO2 enters the cell and diffuses towards its center, the increase in [CO2] throughout the ICF (Figure 4A) shifts the equilibria of the buffer ( ) towards the right, producing first H2CO3 (Figure 4B) and then (Figure 4C) and H+, thereby causing pHi to decrease (Figure 4D). The resulting increase of [H+] throughout the intracellular domain simultaneously perturbs the equilibria, causing the reactions to buffer the newly produced H+, consuming (Figure 4F) and producing [HA1] (Figure 4E). As we move from the surface towards the center of the oocyte, the changes in solute concentrations begin later and proceed more slowly, reflecting in part the time that the CO2 takes to diffuse towards the center of the cell. For example, pH requires only 0.12 sec to reach its maximal rate of decline just beneath the membrane, but requires about 46 sec to reach its maximum rate near the cell center. Note that at time t = 0+, the [CO2] at the inner side of the plasma membrane (Figure 4A, gold line) exhibits an overshoot because of the sudden, large influx of CO2. As the CO2 equilibrates across the cell membrane, all solute concentrations approach the same respective values throughout the cell. Note that CO2 and H2CO3 are the only solutes whose concentrations—at equilibrium— are the same from the BECF to the cell center (Figure 5A, gold line).
Remark: Our model, simulating a “standard in-silico experiment”, does a reasonably good job predicting the observed shape of the pH profile on the surface of the oocyte (see Figure 3D, r = 651 μm). However, the maximum height of the pH spike at that location is less than 20% as large as the value measured by a surface-pH electrode (Endeward et al., 2006; Musa-Aziz et al., 2009). This difference probably has at least two major causes: (a) The “vitelline membrane” that surrounds the entire oocyte creates an additional unconvected space, in series with the cell membrane. (b) The blunt surface-pH electrode—pushed up against the vitelline membrane—creates yet another unconvected space that, although it represents an extremely small fraction of the surface area of the oocyte, could have a major impact on what the electrode senses. Each of these effects reduces the contribution of diffusion from the BECF and therefore emphasizes the contribution of the reaction in replenishing the depleted CO2 at the cell surface. The result is an unusually small DRR.
Remark: Our model, simulating a “standard in-silico experiment”, also does a reasonably good job predicting the shape and magnitude of the observed pHi trajectory (Figure 4D).
4.2.2 Effect of changing d, the width of the EUF
Unstirred layers (ULs) are thin, diffuse layers of fluid, always present near the surface of solid bodies immersed in fluid, where—because of the slow laminar flow of liquid parallel to the surface of the solid—molecules move predominantly via diffusion (Dainty and House, 1966; Korjamo et al., 2009). The presence of unstirred layers on the outer cellular surface as well as within the intracellular space represents a resistance to diffusion that is in series with the resistance of the membrane. As the unstirred layers become thicker, the fractional contribution of the membrane to overall resistance becomes smaller, and may eventually become insignificant (Gutknecht and Tosteson, 1973; Gutknecht et al., 1977; Missner et al., 2008b; Endeward and Gros, 2009; Boron, 2010; Boron et al., 2011).
The width of the unstirred layer (δ) is generally regarded as a steady-state concept. For a particular solute, the thickness of the effective unstirred layer around the membrane is defined as
| (33) |
where D is the diffusion constant and P is the empirically measured permeability. Note that δ is solute-dependent (Levich, 1962). Finally, the definition of δ does not take into account the effects of chemical reactions—particularly important when working with weak acids and bases—on the size of the UL (Endeward and Gros, 2009). Because (a) our system is dynamic—approaching a steady state (i.e., an equilibrium) only near the end of a simulation, (b) involves multiple solutes, and (c) these solutes react in the “unstirred layer”, it would be virtually impossible to build our model around δ values. Therefore, we chose to focus on the width (d) of the EUF (see Figure 2). Note that EUF has the advantage of being readily applicable to a dynamic system, has the same thickness for all solutes, and is amenable to complex reactions occurring within the layer. Thus, EUF is a generalization of the concept of UL.
The motivation for the studies in this section is two-fold. First, because in real experiments it is difficult to estimate d (Korjamo et al., 2009) or to alter d with precision in order to explore how d influences pHS and pHi trajectories, which, in turn, are important tools for studying gas channels. Therefore, we use the model to investigate how changes in d affect the computed profiles of pHS and pHi. Second, changing d should give insights into the competition between diffusion and reaction in replenishing the lost CO2 at the cell surface.
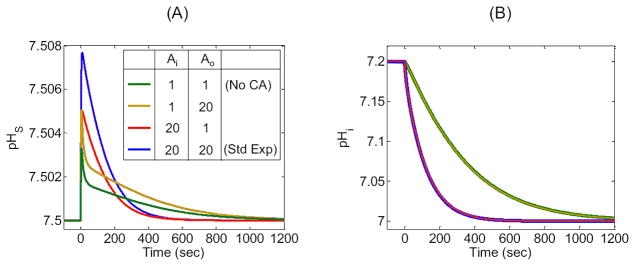
In addition to the standard d = 100 μm, we examine six other cases (Table 3) differing only in the size of the computational domain (i.e., R∞). Figure 6 displays the results of the simulations for pHS and pHi, the two parameters for which experimental data exist.
Table 3.
Values for d and corresponding number of subintervals used in the simulations where we explore the role of the width of the EUF*
| Extracellular Unconvected fluid (EUF)Width d (μm) | Number of Subintervals nout |
|---|---|
|
| |
| 150 | 150 |
| 100** | 100 |
| 50 | 50 |
| 25 | 25 |
| 10 | 10 |
| 5 | 5 |
| 1 | 5 |
The intracellular discretizations are the same as for the “standard in-silico experiment”, 80 subintervals.
Standard in-silico experiment.
Figure 6.
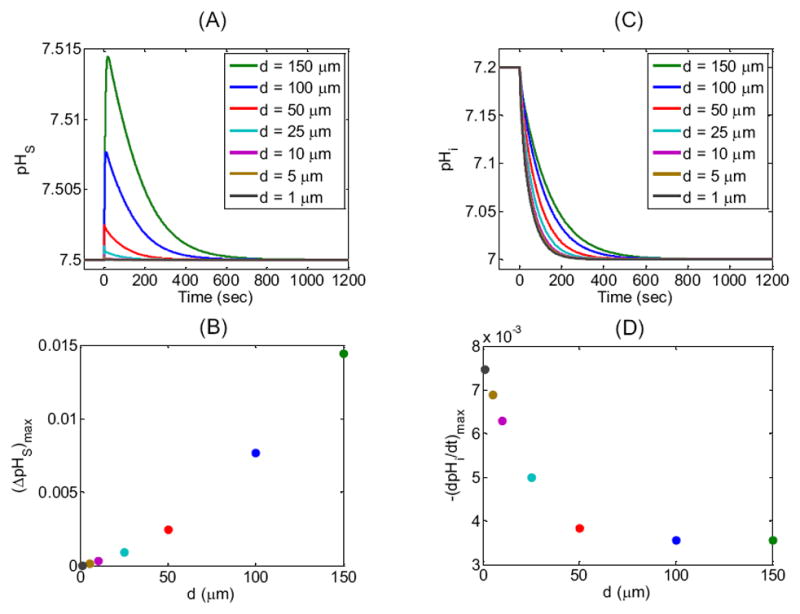
Effect of changing the width d of the extracellular unconvected fluid on the surface (pHS) and intracellular pH (pHi) time profiles. (A) pHS transients for seven simulations corresponding to seven values of d (see legend and Table 3 for parameter values). (B) Maximum height of the pHS spike—(ΔpHS)max—as a function of d for the seven simulations reported in (A). (C) Corresponding pHi transients at a depth of ≈ 50 μm for the simulations in (A). (D) Maximal rate of pHi change—(dpHi/dt)max—as a function of d for the seven simulations reported in (C).
We define pHS as the value just outside the cell membrane. ΔpHS (the difference between the actual and initial values of pHS) mainly reflects the velocity of H+ consumption at the cell surface, which in turn depends on the CO2 influx as part of a complex cascade of reactions and diffusion events indicated in Figure 2. Figure 6A and B show that, as the thickness of the EUF decreases from 150 μm to 1 μm, the maximum height of the pHS spike, (ΔpHS)max, decreases markedly. The height of the pHs spike declines because—as d falls, thereby reducing the overall resistance to the diffusion of CO2—the diffusive flux of CO2 from the BECF to the external surface of cell membrane becomes increasingly more dominant than the reactions in replenishing CO2 near the outer surface of the plasma membrane. Thus, DRR rises.
In Figure 6C and D, the intracellular pH corresponds to a depth of 50 μm into the oocyte, which is not unreasonable for the depth of penetration of a microelectrode into an oocyte. The maximal rate of pHi change, (dpHi/dt)max, mainly reflects the velocity of H+ production at this intracellular depth, which in turn depends on the CO2 influx and the complex events outlined in Figure 2. Figure 6C and D show that, as the thickness of the EUF decreases from 150 μm to 1 μm, pHi declines more rapidly. The magnitude of (dpHi/dt)max rises because, as d decreases, the diffusive flux of CO2 from the BECF increases, causing a rise in [CO2] on the outer surface of the cell membrane, thereby increasing the CO2 influx.
4.2.3 Effects of decreasing CO2 membrane permeability, PM,CO2
The motivation for the studies in this section is that—in electrophysiology experiments on Xenopus oocytes—both (ΔpHS)max (Endeward et al., 2006; Musa-Aziz et al., 2009) and (−dpHi/dt)max (Cooper and Boron, 1997; Nakhoul et al., 1998) have provided semi-quantitative indices of the CO2 permeability of the cell membrane, leading to the initial description of AQP1 as a gas channel. Moreover, pHS trajectories remain a valuable experimental tool for studying gas channels. Although the present model is not sufficiently sophisticated to permit the extraction of meaningful PM,CO2 estimates from experimental data, it ought to provide valuable insights into the sensitivity of (ΔpHS)max and (−dpHi/dt)max to changes in PM,CO2, and thus the likelihood of eventually being able to extract PM,CO2 values.
In our “standard in-silico experiment”, we assume that the permeability of the cell membrane corresponds to a film of water (i.e., DM,CO2 = Dwater,CO2 = 1.71× 10−5 cm2/sec) with the thickness (i.e., hM = 5 nm) of a typical cell membrane. Thus, in the “standard in-silico experiment” PM,CO2 is 34.20 cm/sec. In Figure 7, we show the effects of decreasing PM,CO2 on pHS and pHi. The blue curves replicate the “standard in-silico experiment”. Virtually identical curves ensue when we reduce PM,CO2 by factors of 101, 102, and 103, whereas reducing PM,CO2 by factors of 104, 2.5·104, 5.0·104, 7.5·104 and 105 has visible and increasingly large effects.
Figure 7.
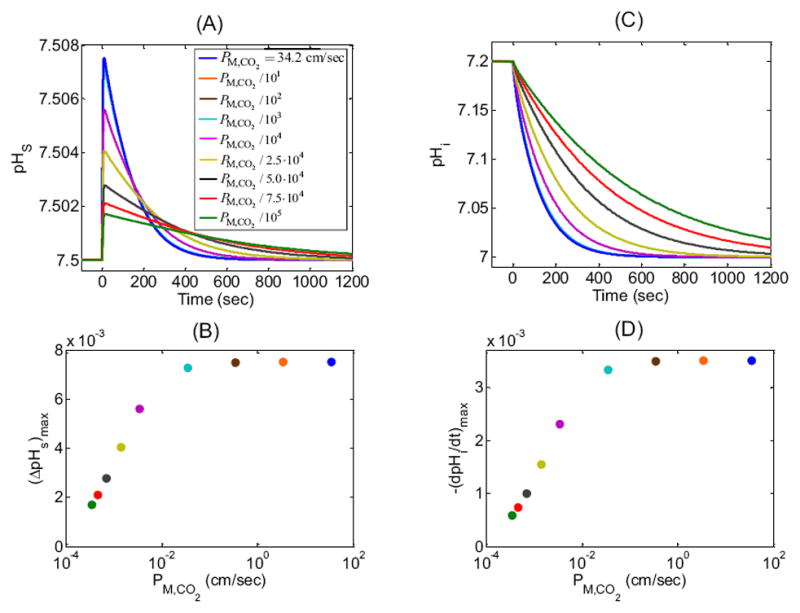
Effect of changing CO2 membrane permeability (PM,CO2) on the surface (pHS) and intracellular pH (pHi) time profiles. We show results of nine simulations for PM,CO2 = 34.20 cm/sec (blue, “standard in-silico experiment”, which corresponds to the mobility of CO2 in H2O) and for PM,CO2 reduced by factors of 101, 102, 103, 104, 2.5·104, 5.0·104, 7.5·104 and 105. (A) pHS transients for the nine simulations. Note that virtually superimposable on the blue curve are the ones for which PM,CO2 is reduced by factors of 101, 102, and 103. (B) Maximum height of the pHS spike—(ΔpHS)max—as a function of PM,CO2 for the nine simulations reported in (A). (C) pHi transients at a depth of ≈ 50 μm for the simulations in (A). Note that virtually superimposable on the blue curve are the ones for which PM,CO2 is reduced by factors of 101, 102, and 103. (D) Maximal rate of pHi change—(dpHi/dt)max—as a function of PM,CO2 for the nine simulations reported in (C).
Focusing first on the extracellular cell surface (Figure 7A), we see that, for the PM,CO2 values examined, the first visible effect on the pHS trajectory appears when we reduce PM,CO2 by a factor of 104 (magenta curve): (ΔpHS)max is smaller and the decay from the peak to the equilibrium state is slower. Further reductions in PM,CO2 cause further decreases in both (ΔpHS)max and the speed of pHS equilibration (Figure 7A). Note that the maximal sensitivity of (ΔpHS)max to changes in PM,CO2 occurs near fractional PM,CO2 decreases of 2.5·104 (Figure 7B).
As is the case for pHS, the first substantial effects on pHi trajectories (Figure 7C) occur when we reduce PM,CO2 by a factor of 104 (magenta curve): (dpHi/dt)max is smaller and the decay to the equilibrium state is slower. As PM,CO2 is decreased further, so do both (dpHi/dt)max and the speed of pHi decline. As for (ΔpHS)max, the maximal sensitivity of (−dpHi/dt)max to changes in PM,CO2 occurs near fractional PM,CO2 decreases of 2.5·104 (Figure 7D).
An explanation for the effects in Figure 7A is that as PM,CO2 falls, the velocities of both the diffusion and reaction processes also fall, the latter effect reducing the height of the pHS spike. As the system approaches equilibrium, the total amount of CO2 that enters the cell is the same, regardless of the speed of CO2 entry. Thus, decreases in PM,CO2, which lengthen the time required for a fixed amount of CO2 to enter the cell, necessarily broaden the descending phase of the pHS trajectory (Figure 7A) and the entire pHi (Figure 7C) trajectory. The plots of (ΔpHS)max vs PM,CO2 (Figure 7B) and of (−dpHi/dt)max vs PM,CO2 (Figure 7D) are similar because both (ΔpHS)max and (−dpHi/dt)max reflect the same fundamental process: the influx of CO2. The simulations in Figure 7 imply that, for PM,CO2 values below a certain threshold, one could in principle use either (ΔpHS)max or (−dpHi/dt)max to estimate PM,CO2. For this set of simulations, the PM,CO2 threshold is ≈10−2 cm/sec, above which the EUF layer becomes rate limiting.
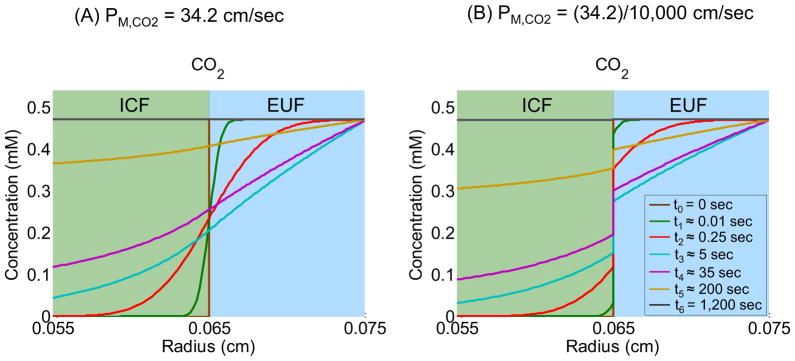
Figure 8 shows how [CO2] varies with radial distance from the center of the cell at seven time instances, including the initial time t0 = 0 sec (brown line) and the final time t6 = 1,200 sec (black line). To the right of the cell membrane (i.e., r > R = 0.065 cm), the figure shows the profiles of [CO2] in the EUF (d = 100 μm), up to the border with the BECF (i.e., r = R∞ = 0.075 cm). To the left of the cell membrane (i.e., r < R = 0.065 cm), the figure shows the profiles of [CO2] inside the oocyte up to a depth of 100 μm. Figure 8A corresponds to the “standard in-silico experiment” (PM,CO2 = 34.2 cm/sec) and Figure 8B corresponds to the simulation where PM,CO2 is decreased by a factor of 104. The colors of the curves in Figure 8A correspond to the same time instances as those in Figure 8B (see figure legend). Comparing Figure 8A and Figure 8B, we observe that, at any radial distance, reducing PM,CO2 increases the time required for [CO2] to fall or rise to a particular value. Moreover, reducing PM,CO2 increases, at any given time instance, the discontinuity between the extra- and intracellular [CO2] trajectories. This discontinuity reflects a smaller CO2 influx, due to a larger barrier to CO2 diffusion exerted by the plasma membrane. See Supplementary data for the seven other plots, comparable to those in Figure 8A and Figure 8B, for the PM,CO2 values introduced in Figure 7.
Figure 8.
Effects of reducing the CO2 membrane permeability (PM,CO2) on [CO2]-distance profiles at seven time instances: t0 = 0 sec, t1 ≈ 0.01 sec, t2 ≈ 0.25 sec, t3 ≈ 5 sec, t4 ≈ 35 sec, t5 ≈ 200 sec and t6 ≈ 1,200 sec. (A) Simulation corresponding to the “standard in-silico experiment” (PM,CO2 = 34.2 cm/sec). (B) Simulation corresponding to the experiment in which PM,CO2 is reduced by a factor of 104.
4.2.4 Effects of changing Ao and Ai, the extra- and intracellular CA-like activities, to mimic native oocytes
The motivation for the studies in this section is that we hypothesized that carbonic-anhydrase activities both at the outer surface of the cell membrane and in the cytosol are important for shaping pHS and pHi trajectories. Because the only published oocyte experiments on CO2 permeability deal with cells having relatively low, native CA activities, our goal is to investigate Ao and Ai values in the native range for the purpose of gaining insight into PM,CO2.
In our “standard in-silico experiment”, we assume that a CA-like activity amplifies the reaction rates k1 and k−1 by a factor Ai everywhere inside the cell and by a factor Ao outside. The CA-like activity on the outside, however, is limited to the immediate vicinity of the oocyte surface. In our “standard in-silico experiment” we use Ao = Ai = 20. For comparison, Ai in a human erythrocyte is typically 10–25,000 (Geers and Gros, 2000; Endeward et al., 2008). The blue curves in Figure 9A and B replicate the “standard in-silico experiment” for pHS and pHi, respectively. The figure also illustrates the results of additional simulations in which we reduced the CA-like activity to Ao = 1, or Ai = 1, or Ao = Ai = 1, that is, no enzymatic activity on the oocyte surface, or throughout the cytoplasm, or both.
Figure 9.
Effects of changing the extracellular CA-like activity (Ao) and intracellular CA-like activity (Ai) on the surface (pHS) and intracellular pH (pHi) time profiles. (A) pHS transients for four simulations corresponding to all combinations of Ao and Ai acceleration factors of 1 or 20. (B) Corresponding pHi-time profiles at a depth of ≈ 50 μm. Note that, in this panel, the green and gold curves overlap, as do the blue and red curves. The blue curves reproduce the “standard in-silico experiment”.
Focusing first on the extracellular cell surface, we see that with Ao = Ai = 1 (Figure 9A, green curve), pHS has an unnaturally sharp spike. Raising Ao to 20 while leaving Ai = 1 increases the spike height, making the pHS spike even sharper (gold curve). On the other hand, raising Ai to 20 while leaving Ao = 1 produces a naturally shaped pHS trajectory, though (ΔpHS)max is still small (red curve). Comparing the blue curve (Ao = Ai = 20) with the red curve (Ao = 1, Ai = 20), we see that implementing modest CA activity on the oocyte surface increases (ΔpHS)max and retains a natural trajectory. An explanation for the sharp spikes predicted for the two simulations in which Ai = 1 is that the production of CO2 on the outer surface of the membrane initially promotes a large CO2 influx that quickly wanes because of the inability of the cell to dispose of the incoming CO2. Raising Ai to 20 overcomes this problem.
Turning our attention to pHi, we see that lowering both Ao and Ai to 1 (Figure 9B, green curve) produces an unnaturally slow fall in pHi, though to the proper equilibrium level. Raising Ao to 20 while leaving Ai = 1 has virtually no effect, yielding an unnaturally slow pHi decline (gold curve). Raising Ai to 20 while leaving Ao = 1 produces a reasonably natural pHi trajectory (red curve), which is nearly indistinguishable from the “standard in-silico experiment” (blue curve). An explanation for these results is that, from the perspective of pHi and considering only modest levels of CA activity (i.e., Ao = 20 or Ai = 20), the only important factor is the ability of intracellular CA to generate the H+ that the intracellular pH microelectrode detects. In the process, the intracellular CA also disposes of incoming CO2, thereby magnifying the out-to-in CO2 gradient and also promoting CO2 influx per se (Musa-Aziz et al., 2005).
4.2.5 Effect of altering intracellular, , closed-system buffers
The motivation for the studies in this section is that the magnitude of intracellular buffering power ought to have a major influence on the trajectory of pHi and, by extension, pHS—both of which are used to assess PM,CO2 and thus the properties of gas channels. Moreover, before the development of the present model—with its ability to handle multiple buffers—it has been impractical to ask whether reciprocal changes in mobile vs. immobile intracellular buffers (at fixed buffering power) influence pHS or pHi trajectories. This question is important because the mobility profile of intracellular buffers is not known with any precision, and because the pHS and pHi trajectories are critical for assessing PM,CO2 in the study of gas channels.
Effect of changing the buffering power
In our “standard in-silico experiment”, we chose the buffering power of the single intracellular, , closed-system buffer to be β = βstd = 15.6533mM/pH, so that an exposure to 1.5% CO2 would cause pH to fall from 7.20 to 7.00. In the simulations summarized in Figure 10, we explored β values of 1000βstd (magenta) as an approximation of ∞, 5βstd (cyan), 2βstd (red), βstd (blue), βstd/2 (green), and 0 (black). We achieved these values in the simulation by altering the concentration of total buffer [TA1]i = [HA1]i + [A1−]i.
Figure 10.
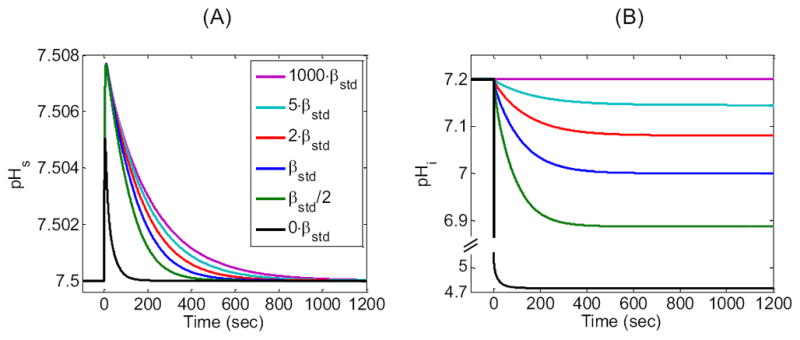
Effect of changing the closed-system intracellular buffering power β on the surface (pHS) and intracellular pH (pHi) time profiles. We show the results of six simulations corresponding to β values of 1000·βstd, 5·βstd, 2·βstd, βstd, βstd/2, and 0.·βstd is the buffering power used for the “standard in-silico experiment” (i.e., the β for a single buffer with pK = 7.1 and concentration ≈ 27.3126 mM). The curve for β = 1000·βstd is an approximation of an infinite β. (A) Time course of simulated surface pH. (B) Time course of simulated intracellular pH at a depth of ≈ 50 μm. Note the break in the ordinate, in this panel, which is necessary for showing the large pHi decrease that would occur with a β of 0.
Figure 10A shows that (ΔpHS)max is little affected as we lower β from 1000βstd to βstd/2, but falls off at a β of 0. The rate of pHS relaxation from the peak pHS progressively rises as β falls.
Figure 10B shows that, as expected, pHi does not fall visibly at a β of 1000βstd. At lower values of β, the (−dpHi/dt)max progressively increases and the time to reach equilibrium progressively decreases. At the same time, the equilibrium pH progressively falls, reaching, for β = 0, a value of ≈ 4.75 (i.e., the pH that we would achieve if we equilibrated a beaker of pure water with 1.5% CO2).
The reason that the pHS relaxation and the pHi decline are most rapid for β = 0 is that all the H+ produced by the intracellular reaction remains free, so that the flux through the reaction is limited by the equal buildup of the two products, and H+. In fact, the reaction causes [ ] and [H+] to increase by very little: . In other words, the consumption of CO2—equivalent to or Δ[H+]—is extremely low (i.e., ≈ 0.016 mM). Thus, free intracellular CO2 builds up very quickly, and the CO2 equilibrates rapidly throughout the system. Conversely, at β = ∞, where [H+] cannot change from its initial value of 10−7.2 M, the reaction is limited only by the build-up of . Our simulation (and the Henderson-Hasselbalch equation) show that [ ] rises from 0 before the CO2 exposure to ≈ 5 mM at equilibrium. In other words, for β = ∞, the consumption of CO2, equivalent to , is extremely high (i.e., ≈ 5 mM). In fact, the consumption of CO2 for β = ∞ is 5/0.016 mM ≈ 300-fold greater than the consumption of CO2 for β = 0. Because ≈ 300-fold more CO2 must enter the cell, the equilibration requires a much longer time.
Effect of switching to immobile intracellular buffers
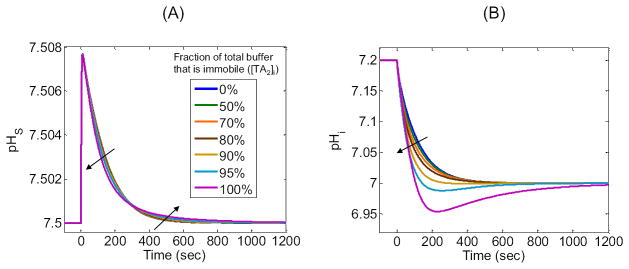
So far, we have implemented a single intracellular, , closed-system buffer that is mobile. However, real cells have a multitude of , closed-system buffers that differ in pK, concentration, and mobility. Here we investigate the importance of buffer mobility by splitting our original buffer, , with [TA1]i equal to a constant (i.e., ≈ 27.3126 mM) that we will now define as [TA]i, into two buffers and . These two buffers have the same pK, and their concentrations sum to [TA]i (i.e., [TA1]i + [TA2]i = [TA]i). We assign the same mobilities as in our “standard in-silico experiment”, and assign mobilities of zero. For the seven simulations summarized in Figure 11, we allow [TA2]i to range from 0% of [TA]i, that is, the “standard in-silico experiment” with all-mobile buffer (blue curves) to 100% of [TA]i, that is, all-immobile buffer (magenta curves), as summarized in the legends. For example, if 70% of the buffer is immobile, [TA2]i = 70%·[TA]i, then the remaining 30% is mobile, [TA1]i = 30%·[TA]i.
Figure 11.
Mobile versus immobile intracellular buffers. We show the results of simulations in which we split our original intracellular buffer HA/A− into two distinct intracellular buffers: (assumed mobile) and (assumed immobile). (A) pHS time profiles for different ratios of immobile to mobile buffers, expressed as the fractions (%) of total buffer ([TA2]i) that are immobile. The blue curve reproduces the “standard in-silico experiment.” (B) Corresponding pHi time profiles calculated at a depth of ≈ 50 μm. The directions of the arrows in panels A and B show the effects of graded increases in the % of immobile buffer.
Figure 11A shows that, as the percentage of immobile buffer increases from 0% (i.e., “standard in-silico experiment”) to 100%, the pHS profiles have approximately the same (ΔpHS)max, but the initial phase of decay becomes progressively faster as the later phase becomes progressively slower.
Figure 11B shows that, as the percentage of immobile buffer increases from 0% to 100%, (−dpHi/dt)max becomes progressively greater, the pHi values achieved at 200–300 sec become progressively lower (producing a minimum or undershoot at 95% and 100% immobile buffer), and the time to achieve true equilibrium gradually increases.
The reason why 0% immobile buffer corresponds to the smallest (−dpHi/dt)max—or the slowest decline in pHi—is that, as CO2 enters the cell and leads to the formation of H+, which is buffered in the reaction H+ + A− → HA, the fully mobile buffer can maximally replenish the consumed A− and dispose via diffusion of the newly formed HA. This counter-diffusion of A− and HA effectively raises the local buffering power, slowing the descent of pHi. On the other hand, with 100% immobile buffer, the above reaction is limited by the availability of A− and the buildup of HA. Thus, the local buffering power is relatively low, speeding the descent of pHi. In fact, the local build-up of H+ causes pHi to fall substantially below the equilibrium value reached at infinite time. The slow diffusion of H+ away from the membrane, which is the rate-limiting factor for attaining equilibrium, produces the slow increase of pHi at times greater than ≈ 250 sec.
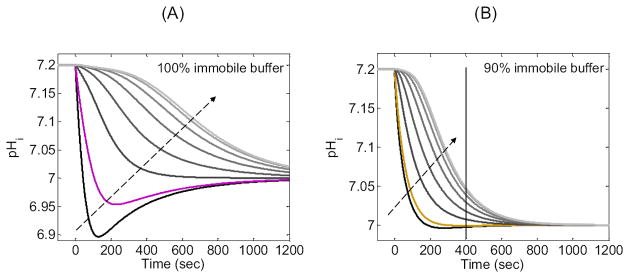
The “pHi” in Figure 11B is at a depth of ≈ 50 μm below the membrane. Figure 12A is a family of pHi trajectories from the 100% immobile simulation in Figure 11B, but in Figure 12A we examine a wide range of depths below the membrane. Note that as we move deeper into the cell, the minimum pH value becomes larger and occurs later in time. Eventually, the minimum disappears entirely, and pH falls monotonically, more slowly as we approach the center of the cell. Note also that the delay between the start of the simulation and the time of steepest pH descent increases as we approach the center of the cell.
Figure 12.
Exploring the effects of 100% or 90% immobile buffer on the pHi time profile at eight depths into the cell. (A) Family of pH time profiles based on the simulation in Figure 11B for 100% immobile buffer. The black curve represents a depth of ≈ 10 μm. The magenta curve is the same as in Figure 11B, and represents a depth of ≈ 50 μm. The gray curves, in the direction of the arrow, represent increasing depths beneath the membrane of ≈ 150 μm, ≈ 250 μm, ≈ 350 μm, ≈ 450 μm, ≈ 550 μm and ≈ 650 μm (i.e., near the center of the cell). (B) Family of pH time profiles based on the simulation in Figure 11B for 90% immobile buffer. The gold curve is the same as in Figure 11B, and represents a depth of ≈ 50 μm. The other colors and the arrow have the same meaning as in panel A.
Of particular interest in Figure 12A is the slow pH increase (i.e., “pH recovery” in the physiological literature) at the positions closest to the membrane, and at times after the pH undershoot. This recovery could be mistaken for the “active”, energy-requiring extrusion of acid equivalents that was first described in experiments on squid giant axons, and which provided the first direct evidence for pHi regulation (Boron and De Weer, 1976; Roos and Boron, 1981). However, the pHi recoveries in Figure 11B and Figure 12A reflect a purely passive process, the diffusion of H+ away from the membrane. Note that in the squid-axon experiments (Boron and De Weer, 1976; Roos and Boron, 1981), the pH electrode was positioned along the axis of the cylindrical axon, analogous to the center of the spherical cell in our simulation in Figure 12A. The pH trajectory at the center of our sphere decreases monotonically, confirming the interpretation in squid-axon study that the pHi recovery is a reflection of active transport (Boron and De Weer, 1976; Roos and Boron, 1981).
Our last observation begins with the gold curve in Figure 11B. In Figure 11B, all simulations correspond to a depth of ≈ 50 μm. The gold curve represents 90% immobile buffer, the condition in which pHi appears to reach the equilibrium value of 7.00 most rapidly without an undershoot. However, this apparent rapid equilibration is an illusion. In Figure 12B, the gold curve reproduces the one in Figure 11B. The other curves in Figure 12B (90% immobile buffer) represent the same range of depths presented in Figure 12A (100% immobile buffer). We see in Figure 12B that, even though pH at a depth of ≈ 50 μm appears to be approaching its equilibrium value of 7.00 at a time of ≈ 400 sec (vertical line), pH at greater depths has not yet reached its equilibrium value. The greater the depth, the more distant is pHi from its equilibrium value at t = 400 sec. Thus, although data obtained at a depth of 50 μm makes it appear as if the system has reached equilibrium within 400 sec, in fact, the system remains far out of equilibrium at this time instance.
4.2.6 Conclusions
We have developed and implemented a three-dimensional reaction-diffusion model for CO2 influx into a spherical cell with radial symmetry (e.g., a Xenopus oocyte). As hypothesized, this model reproduces the essential features of Figure 1. Namely, the application of CO2 causes pHS to rise rapidly to a peak and then decay, and simultaneously causes pHi to fall monotonically with approximately the same time constant as the decay in pHS.
The model is flexible because, when we alter parameters as discussed above in Sections 4.2.2 through 4.2.5, the model yields results that either qualitatively match physiological data, or that make intuitive sense to a physiologist:
Geometric parameters, including the thickness of the extracellular unconvected fluid. Simulations in which we change d give insight into how altering the width of the EUF impacts both the height of the pHS spike (Figure 6B) and the maximal rate of intracellular acidification (Figure 6D). The importance of d for the empirically measured membrane permeability to CO2 is the object of continuing discussion (Boron et al., 2011). Pohl and colleagues argued that the combination of a large d (e.g., 100 μm) and a large true membrane permeability (i.e., the permeability of the lipid phase of a membrane) makes gas channels irrelevant (Missner et al., 2008a; Missner et al., 2008b; Missner and Pohl, 2009). However, the Pohl, Gros, and Boron groups later agreed that, given a sufficiently small PM,CO2, gas channels could measurably contribute to CO2 permeability, even with a large d (Boron et al., 2011). Indeed, our simulations show that, even with the relatively large d characteristic of our “standard in-silico experiment” (i.e, 100 μm), both (ΔpHS)max and (−dpHi/dt)max rise more or less linearly with log(PM,CO2)—provided PM,CO2 is below ~10−2 cm/sec (see Figure 7B and D, respectively). Thus, our model provides an important new physiological insight.
Mobilities of solutes in space, including the permeability of the membrane to CO2. Based on the discussion in (a), as well as the observation that expressing AQPs or Rh proteins in oocytes cause a substantial increase in (ΔpHS)max (Endeward et al., 2006; Musa-Aziz et al., 2009), we can conclude that d and PM,CO2 must be low enough for gas channels to make a significant contribution in oocytes. Moreover, in principle it would be possible to extract, from experimental data, estimates of the CO2 permeability of gas channels.
Activity and localization of carbonic-anhydrase activity. Our simulations lead to the novel conclusion that native Xenopus oocytes must have a small amount of cytosolic CA activity (Figure 9A) in order to account for the general shape of pHS vs. time in actual recordings from oocytes.
Number, concentration, mobility, and pK value of each closed-system buffer. A novel insight from our simulations is that at least a small fraction of intracellular buffers must be mobile in order to account for the observed monotonic fall in pHi that is induced by CO2 (Figure 11B).
In addition, the model provides interesting new insights into the competition between diffusion and reaction processes near the outer surface of the plasma membrane. First, the DRR changes dynamically during the CO2 exposure, with diffusion dominating early. Second, (ΔpHS)max—a measure of the reaction rate—increases in response to raising d (which reduces the contribution of diffusion, so that the reaction makes a greater contribution by default) or raising Ao (which speeds the reaction).
The model is capable of reproducing qualitatively the shape of the pHS and pHi transients in Figure 1. Quantitative agreement with experimental data will require refinements to make the model more realistic. Enhancements might include (a) a vitelline membrane, (b) a surface amplification factor to account for microvilli, (c) a more realistic (i.e., lower) value for DM,CO2, (d) a reduction of intracellular mobility to reflect the presence of cytoplasm and intracellular vesicles, (e) recognition that an oocyte is not entirely aqueous, as well as the introduction of (f) a discrete surface-pH electrode and (g) convection of the extracellular fluid.
Finally, the model is easily expandable. For example, it would be straightforward to implement additional solutes (e.g., NH3 and ), allow solutes other than CO2 to diffuse across the membrane (e.g., NH3), examine the effects of gas channels (e.g., AQPs and Rh proteins), and to introduce transporter proteins (e.g., a Na/HCO3 cotransporter) into the plasma membrane.
Supplementary Material
Highlights.
Mathematical model of CO2 influx into a spherical cell
The model accounts for competing equilibria among multiple buffers
We simulate the transients in surface and intracellular pH caused by CO2 influx
Acknowledgments
We thank Drs. Raif Musa-Aziz and Mark Parker for valuable discussions. Supported by grants from the Office of Naval Research (N00014-08-10532) and the National Institutes of Health (DK30344 and NS18400) to W.F.B. The work of R.O. was supported by the fellowship 11POST7670015 from the American Heart Association. Dr. W.F. Boron gratefully acknowledges the support of the Myers/Scarpa endowed chair.
Appendix A: Membrane crossing and boundary conditions
We derive the membrane boundary conditions through a limiting process. Consider a substance w with concentration cw,+ and denote by cw,+ and cw,− its concentrations outside and inside the membrane, respectively. Similarly, let D w,+ and D w,− denote the diffusion coefficients outside and inside the membrane. To find a reasonable boundary condition, assume first that the membrane has a positive thickness hM > 0, and assume that the substance traffic through the membrane happens via diffusion. Let Dw,* denote the diffusion coefficient of the membrane, and denote by cw,* the concentration within the membrane. Without limiting the generality, let us assume that the membrane is locally a spherical surface, the inner and outer boundaries being at r = R and r = R + hM, respectively. To have the diffusion equation hold in the weak sense, we impose first the boundary condition at the inner membrane boundary, requiring that
| (A.1) |
Similarly, at the outer boundary, we require that
| (A.2) |
From the Taylor expansion at r = R,
| (A.3) |
and upon substituting (A.1), we have that
| (A.4) |
By letting the thickness of the membrane go to zero simultaneously with the diffusion constant in such a way that
| (A.5) |
we arrive at the mixed transmission boundary condition,
| (A.6) |
We observe that the limit (A.5) is not a physiological limit but rather a computational approximation. The idea is that we take the the computational membrane thickness to zero in such a way that the permeability of the membrane is not changed.
Similarly, by using the Taylor expansion at the outer surface and the boundary conditions (A.2), we find through the similar limiting process that
| (A.7) |
which amount to the effective transmission conditions.
Observing that α = 1/Pw, with Pw the true membrane permeability to solute w, we can rewrite equations (A.6) and (A.7) in the forms
| (A.8) |
and
| (A.9) |
Appendix B: Details of the finite difference approximation
In this appendix, we give details concerning the finite difference approximation of the diffusion operator for a single solute w with concentration cw = cw (t, r) and diffusion coefficient Dw(r).
Consider first the interior of the cell, r < R. We divide it in nin spherical shells by the grid points
| (B.1) |
Introduce the notation cw (t, rj ) = cw,j (t) and rj±1/2 = rj±h/2, where h = R/nin, and Dw(rj±1/2) = Dw, j± 1/2, 1 ≤ j ≤ nin − 1. We use the finite difference approximation
| (B.2) |
By introducing the notation
| (B.3) |
the formula (B.2) can be written more concisely as
| (B.4) |
For r < r1, we assume that Dw (r) = Dw,0 is constant. The solution must be smooth and symmetric through the origin, so we can write for r < r1,
| (B.5) |
where the second term must vanish due to symmetry, and by using symmetric finite differences,
| (B.6) |
To find a discretization at the outer boundary of the cell, we use the transmission condition (13)
| (B.7) |
and write an approximation
| (B.8) |
We collect the elements of the grid function cw, j to a vector,
| (B.9) |
and define a vector
| (B.10) |
which will provide coupling of the solutions in the interior domain to the exterior. Furthermore, define
| (B.11) |
With these notations, the diffusion equation inside can be approximated as
| (B.12) |
We treat in a similar way the exterior domain, dividing the interval [R, R∞] in nout equal intervals. The interior boundary r = R is treated analogously to the exterior boundary of the inner domain. The outer boundary needs to be treated separately. At j = nout − 1, we approximate
| (B.13) |
which, after organizing the terms appropriately in arrays, leads to a matrix equation
| (B.14) |
where the arrays are given by
| (B.15) |
Here, the elements are defined analogously to the interior domain, and
| (B.16) |
Finally, we put the single substance equations together by stacking the vectors as
| (B.17) |
and, after assembling the matrices,
| (B.18) |
| (B.19) |
we arrive at the system
| (B.20) |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Boron WF. Intracellular pH transients in giant barnacle muscle fibers. Am J Physiol. 1977;233:C61–C73. doi: 10.1152/ajpcell.1977.233.3.C61. [DOI] [PubMed] [Google Scholar]
- Boron WF. Acid-base physiology. In: Boron WF, Boulpaep EL, editors. Medical Physiology. A Cellular and Molecular Approach. Saunders Elsevier; 2009. pp. 652–671. [Google Scholar]
- Boron WF. The Sharpey-Schafer Lecture: Gas channels. Exp Physiol. 2010;95:1107–1130. doi: 10.1113/expphysiol.2010.055244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron WF, De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3 and metabolic inhibitors. J Gen Physiol. 1976;67:91–112. doi: 10.1085/jgp.67.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron WF, Endeward V, Gros G, Musa-Aziz R, Pohl P. Intrinsic CO2 permeability of cell membranes and potential biological relevance of CO2 channels. Chemphyschem. 2011;12:1017–1019. doi: 10.1002/cphc.201100034. [DOI] [PubMed] [Google Scholar]
- Choi I, Aalkjær C, Boulpaep EL, Boron WF. An electroneutral sodium/bicarbonate cotransporter NBCn1 and associated sodium channel. Nature. 2000;405:571–575. doi: 10.1038/35014615. [DOI] [PubMed] [Google Scholar]
- Cooper GJ, Boron WF. The CO2 permeability of the AQP1 water channel, expressed in Xenopus oocytes. J Am Soc Nephrol. 1997;8:16A. [Google Scholar]
- Cooper GJ, Boron WF. Effect of PCMBS on CO2 permeability of Xenopus oocytes expressing aquaporin 1 or its C189S mutant. Am J Physiol. 1998;275:C1481–C1486. doi: 10.1152/ajpcell.1998.275.6.C1481. [DOI] [PubMed] [Google Scholar]
- Costa PF, Emilio MG, Fernandes PL, Ferreira HG, Ferreira KG. Determination of ionic permeability coefficients of the plasma membrane of Xenopus laevis oocytes under voltage clamp. J Physiol. 1989;413:199–211. doi: 10.1113/jphysiol.1989.sp017649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dainty J, House CR. Unstirred layers in frog skin. J Physiol. 1966;182:66–78. doi: 10.1113/jphysiol.1966.sp007809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport HW. The ABC of Acid-Base Chemistry. 4. University of Chicago Press; Chicago: 1958. [Google Scholar]
- Endeward V, Cartron JP, Ripoche P, Gros G. RhAG protein of the Rhesus complex is a CO2 channel in the human red cell membrane. FASEB J. 2008;22:64–73. doi: 10.1096/fj.07-9097com. [DOI] [PubMed] [Google Scholar]
- Endeward V, Gros G. Low carbon dioxide permeability of the apical epithelial membrane of guinea-pig colon. J Physiol. 2005;567:253–265. doi: 10.1113/jphysiol.2005.085761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endeward V, Gros G. Extra- and intracellular unstirred layer effects in measurements of CO2 diffusion across membranes - a novel approach applied to the mass spectrometric 18O technique for red blood cells. J Physiol. 2009 doi: 10.1113/jphysiol.2008.165027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endeward V, Musa-Aziz R, Cooper GJ, Chen L, Pelletier MF, Virkki LV, Supuran CT, King LS, Boron WF, Gros G. Evidence that Aquaporin 1 is a major pathway for CO2 transport across the human erythrocyte membrane. FASEB J. 2006;20:1974–1981. doi: 10.1096/fj.04-3300com. [DOI] [PubMed] [Google Scholar]
- Fei YJ, Kanai Y, Nussberger S, Ganapathy V, Leibach FH, Romero MF, Singh SK, Boron WF, Hediger MA. Expression cloning of a mammalian proton-coupled oligopeptide transporter. Nature. 1994;368:563–566. doi: 10.1038/368563a0. [DOI] [PubMed] [Google Scholar]
- Geers C, Gros G. Carbon dioxide transport and carbonic anhydrase in blood and muscle. Physiol Rev. 2000;80:681–715. doi: 10.1152/physrev.2000.80.2.681. [DOI] [PubMed] [Google Scholar]
- Gibbons BH, Edsall JT. Rate of hydration of carbon dioxide and dehydration of carbonic acid at 25°. J Biol Chem. 1963;238:3502–3507. [PubMed] [Google Scholar]
- Grichtchenko II, Choi I, Zhong X, Bray-Ward P, Russell JM, Boron WF. Cloning, characterization, and chromosomal mapping of a human electroneutral Na+-driven Cl-HCO3 exchanger. J Biol Chem. 2001;276:8358–8363. doi: 10.1074/jbc.C000716200. [DOI] [PubMed] [Google Scholar]
- Gros G, Moll W. The diffusion of carbon dioxide in erythrocytes and hemoglobin solutions. Pflugers Arch. 1971;324:249–266. doi: 10.1007/BF00586422. [DOI] [PubMed] [Google Scholar]
- Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- Gutknecht J, Bisson MA, Tosteson FC. Diffusion of carbon dioxide through lipid bilayer membranes. J Gen Physiol. 1977;69:779–794. doi: 10.1085/jgp.69.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutknecht J, Tosteson DC. Diffusion of weak acids across lipid bilayer membranes: effects of chemical reactions in the unstirred layers. Science. 1973;182:1258–1261. doi: 10.1126/science.182.4118.1258. [DOI] [PubMed] [Google Scholar]
- Harned HS, Owen BB. The physical chemistry of electrolytic solutions. Reinhold Pub. Corp; New York: 1958. [Google Scholar]
- Iserles A. A first course in the numerical analysis of differential equations. Cambridge University Press; 1996. [Google Scholar]
- Koppel M, Spiro K. Uber die Wirkung von Moderatoren (Puffern) bei der Verschiebung des Saure-Basengleichgewichtes in biologischen Flussigkeiten. Biochem Z. 1914;65:409–439. [Google Scholar]
- Korjamo T, Heikkinen AT, Monkkonen J. Analysis of unstirred water layer in in vitro permeability experiments. J Pharm Sci. 2009;98:4469–4479. doi: 10.1002/jps.21762. [DOI] [PubMed] [Google Scholar]
- Lee SK, Boron WF, Parker MD. Relief of autoinhibition of the electrogenic Na/HCO3 cotransporter NBCe1-B: role of IRBIT versus amino-terminal truncation. Am J Physiol Cell Physiol. 2011;302:C518–26. doi: 10.1152/ajpcell.00352.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levich VJ. Physicochemical Hydrodynamics. Prentice-Hall; Englewood Cliffs, NJ: 1962. [Google Scholar]
- Missner A, Kugler P, Antonenko YN, Pohl P. Passive transport across bilayer lipid membranes: Overton continues to rule. Proc Natl Acad Sci U S A. 2008a;105:E123. doi: 10.1073/pnas.0809606106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missner A, Kugler P, Saparov SM, Sommer K, Mathai JC, Zeidel ML, Pohl P. Carbon dioxide transport through membranes. J Biol Chem. 2008b;283:25340–25347. doi: 10.1074/jbc.M800096200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missner A, Pohl P. 110 years of the Meyer-Overton rule: predicting membrane permeability of gases and other small compounds. Chemphyschem. 2009;10:1405–1414. doi: 10.1002/cphc.200900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musa-Aziz R, Chen L, Pelletier MF, Boron WF. Relative CO2/NH3 selectivities of AQP1, AQP4, AQP5, AmtB, and RhAG. Proc Natl Acad Sci, USA. 2009;106:5406–5411. doi: 10.1073/pnas.0813231106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musa-Aziz R, Grichtchenko II, Boron WF. Evidence from surface-pH transients that CA IV & CAII enhances CO2 influx into Xenopus oocytes. Journal of the American Society of Nephrology. 2005;16:TH-PO016. Abstract. [Google Scholar]
- Nakhoul NL, Davis BA, Romero MF, Boron WF. Effect of expressing the water channel aquaporin-1 on the CO2 permeability of Xenopus oocytes. Am J Physiol. 1998;274:C543–C548. doi: 10.1152/ajpcell.1998.274.2.C543. [DOI] [PubMed] [Google Scholar]
- Romero MF, Hediger MA, Boulpaep EL, Boron WF. Expression cloning and characterization of a renal electrogenic Na+/HCO3− cotransporter. Nature. 1997;387:409–413. doi: 10.1038/387409a0. [DOI] [PubMed] [Google Scholar]
- Roos A, Boron WF. The buffer value of weak acids and bases: origin of the concept, and first mathematical derivation and application of physico-chemical systems. The work of M. Koppel and K. Spiro (1914) Respir Physiol. 1980;40:1–32. doi: 10.1016/0034-5687(80)90002-x. [DOI] [PubMed] [Google Scholar]
- Roos A, Boron WF. Intracellular pH. Physiol Rev. 1981;61:296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Roughton FJW. The kinetics and rapid thermochemistry of carbonic acid. J Am Chem Soc. 1941;63:29–30. [Google Scholar]
- Steel A, Nussberger S, Romero MF, Boron WF, Boyd CAR, Hediger MA. Stoichiometry and pH-dependence of the mammalian proton-dependent oligopeptide transporter PepT1. J Physiol (Lond ) 1997;498:563–569. doi: 10.1113/jphysiol.1997.sp021883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling A, Papai I. H2CO3 forms via HCO3− in water. J Phys Chem B. 2010;114:16854–16859. doi: 10.1021/jp1099909. [DOI] [PubMed] [Google Scholar]
- Swietach P, Patiar S, Supuran CT, Harris AL, Vaughan-Jones RD. The role of carbonic anhydrase 9 in regulating extracellular and intracellular pH in three-dimensional tumor cell growths. J Biol Chem. 2009;284:20299–20310. doi: 10.1074/jbc.M109.006478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swietach P, Wigfield S, Cobden P, Supuran CT, Harris AL, Vaughan-Jones RD. Tumor-associated carbonic anhydrase 9 spatially co-ordinates intracellular pH in three-dimensional multicellular growths. J Biol Chem. 2008;283:20473–83. doi: 10.1074/jbc.M801330200. [DOI] [PubMed] [Google Scholar]
- Swietach P, Zaniboni M, Stewart AK, Rossini A, Spitzer KW, Vaughan-Jones RD. Modelling intracellular H+ ion diffusion. Prog Biophys Mol Biol. 2003;83:69–100. doi: 10.1016/s0079-6107(03)00027-0. [DOI] [PubMed] [Google Scholar]
- Toye AM, Parker MD, Daly CM, Lu J, Virkki LV, Pelletier MF, Boron WF. The human NBCe1-A mutant R881C, associated with proximal renal tubular acidosis, retains function but is mistargeted in polarized renal epithelia. Am J Physiol Cell Physiol. 2006;291:C788–C801. doi: 10.1152/ajpcell.00094.2006. [DOI] [PubMed] [Google Scholar]
- Van Slyke DD. On the measurement of buffer values and on the relationship of buffer value to the dissociation constant of the buffer and the concentration and the reaction of the buffer solution. J Biol Chem. 1922;52:525–570. [Google Scholar]
- Vanysek P. Ionic conductivity and diffusion at infinite dilution. In: Haynes WM, editor. CRC Handbook of Chemistry and Physics. CRC Press/Taylor and Francis; 2011. pp. 76–78. [Google Scholar]
- Vaughan-Jones RD, Peercy BE, Keener JP, Spitzer KW. Intrinsic H+ ion mobility in the rabbit ventricular myocyte. J Physiol. 2002;541:139–158. doi: 10.1113/jphysiol.2001.013267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Zhou Y, Boron WF. Effect of isolated removal of either basolateral HCO3− or basolateral CO2 on HCO3− reabsorption by rabbit S2 proximal tubule. Am J Physiol Renal Physiol. 2003;285:F359–F369. doi: 10.1152/ajprenal.00013.2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.