Abstract
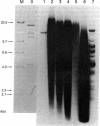
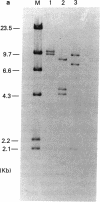
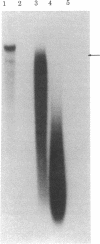
By Southern blotting and hybridization analysis using 32P-labeled poly(dT-dG) . poly(dC-dA) as a probe, we have found, in eukaryotic genomes, a huge number of stretches of dT-dG alternating sequence, a sequence that has been shown to adopt the Z-DNA conformation under some conditions. This sequence was found in all eukaryotic genomes examined from yeast to human, indicating extraordinary evolutionary conservation. The number of the sequence ranged from about 100 in yeast to tens of thousands in higher eukaryotes. Comparison of nucleotide sequences of dT-dG alternating regions and its flanking regions in several cloned genes showed that the repeated element [the Z(T-G) element]] consists only of dT-dG alternating sequence with variable length. The presence of another purine-pyrimidine alternating sequence was also surveyed in eukaryotic genomes by Southern blot hybridization using 32P-labeled poly(dG-dC) . poly(dG-dC) as the probe. The stretches of dC-dG alternating sequence [the Z(C-G) element] were found to be moderately repetitive in human, mouse, and salmon genomes. However, a few and no copies of the Z(C-G) element were found in yeast and calf genomes, respectively. These results provide evidence for the abundance of potential Z-DNA-forming sequences in nature.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. W., Kaufman R. E., Kretschmer P. J., Harrison M., Nienhuis A. W. A family of long reiterated DNA sequences, one copy of which is next to the human beta globin gene. Nucleic Acids Res. 1980 Dec 20;8(24):6113–6128. doi: 10.1093/nar/8.24.6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnott S., Chandrasekaran R., Birdsall D. L., Leslie A. G., Ratliff R. L. Left-handed DNA helices. Nature. 1980 Feb 21;283(5749):743–745. doi: 10.1038/283743a0. [DOI] [PubMed] [Google Scholar]
- Behe M., Felsenfeld G. Effects of methylation on a synthetic polynucleotide: the B--Z transition in poly(dG-m5dC).poly(dG-m5dC). Proc Natl Acad Sci U S A. 1981 Mar;78(3):1619–1623. doi: 10.1073/pnas.78.3.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Crawford J. L., Kolpak F. J., Wang A. H., Quigley G. J., van Boom J. H., van der Marel G., Rich A. The tetramer d(CpGpCpG) crystallizes as a left-handed double helix. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4016–4020. doi: 10.1073/pnas.77.7.4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew H., Takano T., Tanaka S., Itakura K., Dickerson R. E. High-salt d(CpGpCpG), a left-handed Z' DNA double helix. Nature. 1980 Aug 7;286(5773):567–573. doi: 10.1038/286567a0. [DOI] [PubMed] [Google Scholar]
- Epplen J. T., McCarrey J. R., Sutou S., Ohno S. Base sequence of a cloned snake W-chromosome DNA fragment and identification of a male-specific putative mRNA in the mouse. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3798–3802. doi: 10.1073/pnas.79.12.3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch E. F., Lawn R. M., Maniatis T. Molecular cloning and characterization of the human beta-like globin gene cluster. Cell. 1980 Apr;19(4):959–972. doi: 10.1016/0092-8674(80)90087-2. [DOI] [PubMed] [Google Scholar]
- Hamada H., Kakunaga T. Potential Z-DNA forming sequences are highly dispersed in the human genome. Nature. 1982 Jul 22;298(5872):396–398. doi: 10.1038/298396a0. [DOI] [PubMed] [Google Scholar]
- Hamada H., Leavitt J., Kakunaga T. Mutated beta-actin gene: coexpression with an unmutated allele in a chemically transformed human fibroblast cell line. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3634–3638. doi: 10.1073/pnas.78.6.3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H., Muramatsu M., Urano Y., Onishi T., Kominami R. In vitro synthesis of a 5S RNA precursor by isolated nuclei of rat liver and HeLa cells. Cell. 1979 May;17(1):163–173. doi: 10.1016/0092-8674(79)90304-0. [DOI] [PubMed] [Google Scholar]
- Hamada H., Petrino M. G., Kakunaga T. Molecular structure and evolutionary origin of human cardiac muscle actin gene. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5901–5905. doi: 10.1073/pnas.79.19.5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakunaga T. Neoplastic transformation of human diploid fibroblast cells by chemical carcinogens. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1334–1338. doi: 10.1073/pnas.75.3.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krayev A. S., Kramerov D. A., Skryabin K. G., Ryskov A. P., Bayev A. A., Georgiev G. P. The nucleotide sequence of the ubiquitous repetitive DNA sequence B1 complementary to the most abundant class of mouse fold-back RNA. Nucleic Acids Res. 1980 Mar 25;8(6):1201–1215. doi: 10.1093/nar/8.6.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafer E. M., Möller A., Nordheim A., Stollar B. D., Rich A. Antibodies specific for left-handed Z-DNA. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3546–3550. doi: 10.1073/pnas.78.6.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown M., Firtel R. A. Evidence for sub-families of actin genes in Dictyostelium as determined by comparisons of 3' end sequences. J Mol Biol. 1981 Oct 5;151(4):593–606. doi: 10.1016/0022-2836(81)90425-3. [DOI] [PubMed] [Google Scholar]
- Miesfeld R., Krystal M., Arnheim N. A member of a new repeated sequence family which is conserved throughout eucaryotic evolution is found between the human delta and beta globin genes. Nucleic Acids Res. 1981 Nov 25;9(22):5931–5947. doi: 10.1093/nar/9.22.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka Y., Leder P. Organization and complete sequence of identical embryonic and plasmacytoma kappa V-region genes. J Biol Chem. 1980 Apr 25;255(8):3691–3694. [PubMed] [Google Scholar]
- Nordheim A., Pardue M. L., Lafer E. M., Möller A., Stollar B. D., Rich A. Antibodies to left-handed Z-DNA bind to interband regions of Drosophila polytene chromosomes. Nature. 1981 Dec 3;294(5840):417–422. doi: 10.1038/294417a0. [DOI] [PubMed] [Google Scholar]
- Rubin C. M., Houck C. M., Deininger P. L., Friedmann T., Schmid C. W. Partial nucleotide sequence of the 300-nucleotide interspersed repeated human DNA sequences. Nature. 1980 Mar 27;284(5754):372–374. doi: 10.1038/284372a0. [DOI] [PubMed] [Google Scholar]
- Santella R. M., Grunberger D., Weinstein I. B., Rich A. Induction of the Z conformation in poly(dG-dC).poly(dG-dC) by binding of N-2-acetylaminofluorene to guanine residues. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1451–1455. doi: 10.1073/pnas.78.3.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sures I., Lowry J., Kedes L. H. The DNA sequence of sea urchin (S. purpuratus) H2A, H2B and H3 histone coding and spacer regions. Cell. 1978 Nov;15(3):1033–1044. doi: 10.1016/0092-8674(78)90287-8. [DOI] [PubMed] [Google Scholar]
- Vanyushin B. F., Tkacheva S. G., Belozersky A. N. Rare bases in animal DNA. Nature. 1970 Mar 7;225(5236):948–949. doi: 10.1038/225948a0. [DOI] [PubMed] [Google Scholar]
- Vorlíckovă M., Kypr J., Stokrová S., Sponar J. A Z-like form of poly(dA-dC).poly(dG-dT) in solution? Nucleic Acids Res. 1982 Feb 11;10(3):1071–1080. doi: 10.1093/nar/10.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]
- Zimmer C., Tymen S., Marck C., Guschlbauer W. Conformational transitions of poly(dA-dC).poly(dG-dT) induced by high salt or in ethanolic solution. Nucleic Acids Res. 1982 Feb 11;10(3):1081–1091. doi: 10.1093/nar/10.3.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]