Abstract
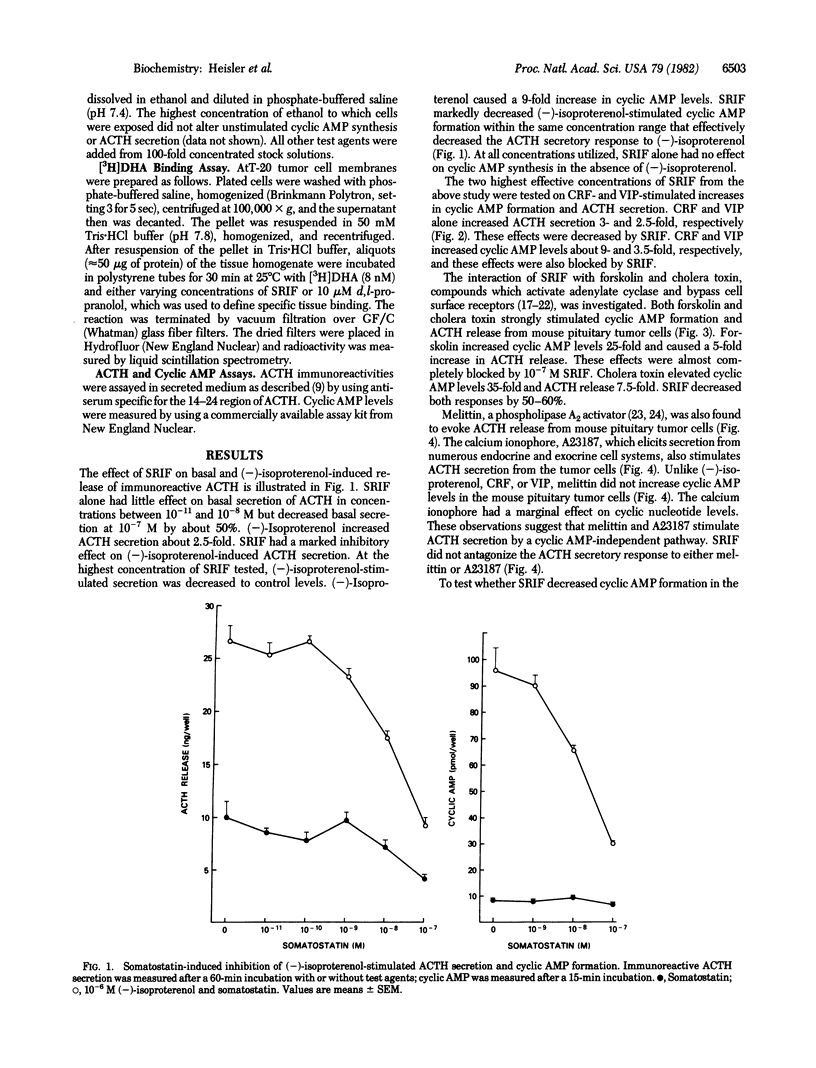
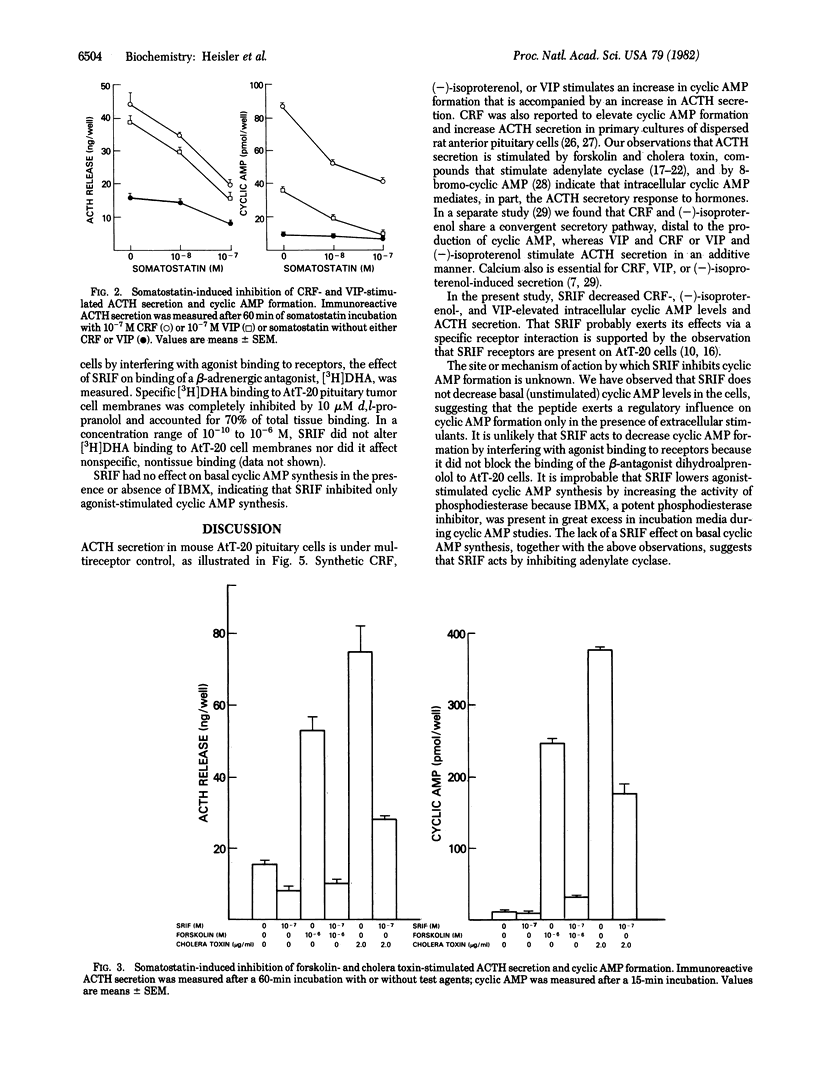
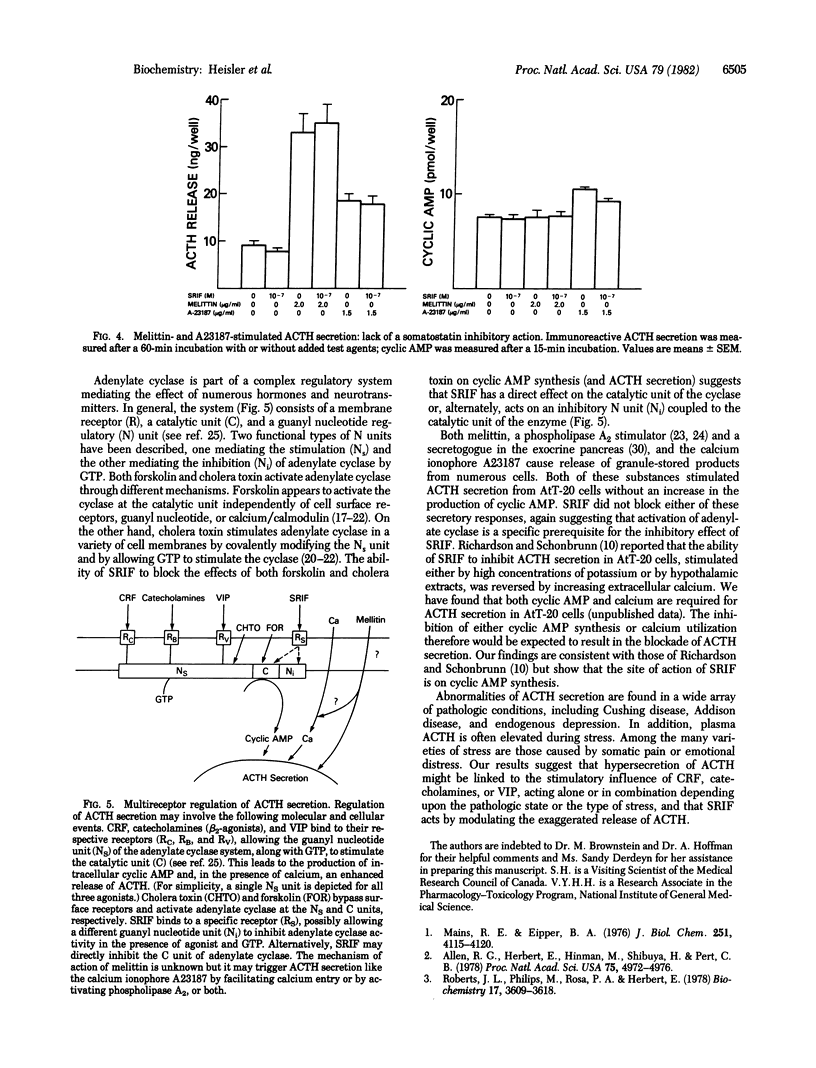
The AtT-20/D16-16 mouse pituitary tumor cell secretes corticotropin (ACTH) in response to corticotropin-releasing factor (CRF), (-)-isoproterenol, and vasoactive intestinal peptide (VIP). These responses are associated with a rapid increase in cyclic AMP formation. Somatostatin (SRIF) markedly decreases the stimulatory effect of CRF, (-)-isoproterenol, and VIP on both cyclic AMP formation and immunoreactive ACTH secretion. Forskolin and cholera toxin, adenylate cyclase activators, also stimulate cyclic AMP formation and ACTH secretion in AtT-20 cells and these responses are all inhibited by SRIF. The ACTH secretory responses to melittin and to the calcium ionophore A23187, neither of which increases cyclic AMP in AtT-20 cells, were not inhibited by SRIF. SRIF did not affect the binding of a tritiated beta-adrenergic receptor antagonist to AtT-20 membranes nor did it decrease basal cyclic AMP formation even in the presence of excess phosphodiesterase inhibitor, indicating that the reduction of cyclic AMP levels by SRIF did not involve either an interference with beta-adrenergic agonist binding to receptors or stimulation of cyclic AMP degradation. These results indicate that the inhibition of CRF-, (-)-isoproterenol-, and VIP-stimulated ACTH secretion by SRIF may be regulated by its inhibitory action on adenylate cyclase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. G., Herbert E., Hinman M., Shibuya H., Pert C. B. Coordinate control of corticotropin, beta-lipotropin, and beta-endorphin release in mouse pituitary cell cultures. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4972–4976. doi: 10.1073/pnas.75.10.4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Mechanism of adenylate cyclase activation by cholera toxin: inhibition of GTP hydrolysis at the regulatory site. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3307–3311. doi: 10.1073/pnas.74.8.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eipper B. A., Mains R. E. Structure and biosynthesis of pro-adrenocorticotropin/endorphin and related peptides. Endocr Rev. 1980 Winter;1(1):1–27. doi: 10.1210/edrv-1-1-1. [DOI] [PubMed] [Google Scholar]
- Enjalbert A., Tapia-Arancibia L., Rieutort M., Brazeau P., Kordon C., Epelbaum J. Somatostatin receptors on rat anterior pituitary membranes. Endocrinology. 1982 May;110(5):1634–1640. doi: 10.1210/endo-110-5-1634. [DOI] [PubMed] [Google Scholar]
- Fehm H. L., Voigt K. H., Lang R., Beinert K. E., Raptis S., Pfeiffer E. F. Somatostatin: a potent inhibitor of ACTH-hypersecretion in adrenal insufficiency. Klin Wochenschr. 1976 Feb 15;54(4):173–175. doi: 10.1007/BF01468882. [DOI] [PubMed] [Google Scholar]
- Giguère V., Labrie F., Côté J., Coy D. H., Sueiras-Diaz J., Schally A. V. Stimulation of cyclic AMP accumulation and corticotropin release by synthetic ovine corticotropin-releasing factor in rat anterior pituitary cells: site of glucocorticoid action. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3466–3469. doi: 10.1073/pnas.79.11.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M., Meren R. ADP-ribosylation of membrane proteins catalyzed by cholera toxin: basis of the activation of adenylate cyclase. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3050–3054. doi: 10.1073/pnas.75.7.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner B., Kelly R. B. Two distinct intracellular pathways transport secretory and membrane glycoproteins to the surface of pituitary tumor cells. Cell. 1982 Jan;28(1):51–59. doi: 10.1016/0092-8674(82)90374-9. [DOI] [PubMed] [Google Scholar]
- Habermann E. Bee and wasp venoms. Science. 1972 Jul 28;177(4046):314–322. doi: 10.1126/science.177.4046.314. [DOI] [PubMed] [Google Scholar]
- Hall R., Besser G. M., Schally A. V., Coy D. H., Evered D., Goldie D. J., Kastin A. J., McNeilly A. S., Mortimer C. H., Phenekos C. Action of growth-hormone-release inhibitory hormone in healthy men and in acromegaly. Lancet. 1973 Sep 15;2(7829):581–584. doi: 10.1016/s0140-6736(73)92413-6. [DOI] [PubMed] [Google Scholar]
- Hook V. Y., Heisler S., Sabol S. L., Axelrod J. Corticotropin releasing factor stimulates adrenocorticotropin and beta-endorphin release from AtT-20 mouse pituitary tumor cells. Biochem Biophys Res Commun. 1982 Jun 30;106(4):1364–1371. doi: 10.1016/0006-291x(82)91264-5. [DOI] [PubMed] [Google Scholar]
- Insel P. A., Stengel D., Ferry N., Hanoune J. Regulation of adenylate cyclase of human platelet membranes by forskolin. J Biol Chem. 1982 Jul 10;257(13):7485–7490. [PubMed] [Google Scholar]
- Johnson G. L., Kaslow H. R., Bourne H. R. Reconstitution of cholera toxin-activated adenylate cyclase. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3113–3117. doi: 10.1073/pnas.75.7.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mains R. E., Eipper B. A. Biosynthesis of adrenocorticotropic hormone in mouse pituitary tumor cells. J Biol Chem. 1976 Jul 10;251(13):4115–4120. [PubMed] [Google Scholar]
- Mains R. E., Eipper B. A. Coordinate, equimolar secretion of smaller peptide products derived from pro-ACTH/endorphin by mouse pituitary tumor cells. J Cell Biol. 1981 Apr;89(1):21–28. doi: 10.1083/jcb.89.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson U. I., Schonbrunn A. Inhibition of adrenocorticotropin secretion by somatostatin in pituitary cells in culture. Endocrinology. 1981 Jan;108(1):281–290. doi: 10.1210/endo-108-1-281. [DOI] [PubMed] [Google Scholar]
- Roberts J. L., Phillips M., Rosa P. A., Herbert E. Steps involved in the processing of common precursor forms of adrenocorticotropin and endorphin in cultures of mouse pituitary cells. Biochemistry. 1978 Aug 22;17(17):3609–3618. doi: 10.1021/bi00610a030. [DOI] [PubMed] [Google Scholar]
- Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980 Mar 6;284(5751):17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- Sabol S. L. Storage and secretion of beta-endorphin and related peptides by mouse pituitary tumor cells: regulation by glucocorticoids. Arch Biochem Biophys. 1980 Aug;203(1):37–48. doi: 10.1016/0003-9861(80)90151-4. [DOI] [PubMed] [Google Scholar]
- Schonbrunn A. Glucocorticoids down-regulate somatostatin receptors on pituitary cells in culture. Endocrinology. 1982 Apr;110(4):1147–1154. doi: 10.1210/endo-110-4-1147. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Padgett W., Daly J. W. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamon K., Daly J. W. Activation of adenylate cyclase by the diterpene forskolin does not require the guanine nucleotide regulatory protein. J Biol Chem. 1981 Oct 10;256(19):9799–9801. [PubMed] [Google Scholar]
- Shier W. T. Activation of high levels of endogenous phospholipase A2 in cultured cells. Proc Natl Acad Sci U S A. 1979 Jan;76(1):195–199. doi: 10.1073/pnas.76.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell J. B., Lorenzi M., Gerich J. E., Forsham P. H. Inhibition by somatostatin of ACTH secretion in Nelson's syndrome. J Clin Endocrinol Metab. 1975 Jun;40(6):1125–1127. doi: 10.1210/jcem-40-6-1125. [DOI] [PubMed] [Google Scholar]
- Vale W., River C. Substances modulating the secretion of ACTH by cultured anterior pituitary cells. Fed Proc. 1977 Jul;36(8):2094–2099. [PubMed] [Google Scholar]


