Abstract
OBJECTIVE
The objective of this study was to examine similarities and differences in Caucasian and African-American patients with Bicuspid Aortic Valve (BAV) with respect to morphology, severity of aortic stenosis/insufficiency, and aortic dilatation.
BACKGROUND
Bicuspid aortic valve is a common congenital valve abnormality accounting for a large number of valve replacements.
METHODS
229 patients with the diagnostic code “BAV” were identified retrospectively from our computerized adult echocardiographic database consisting of 91,896 studies performed at the University of Chicago Medical Center from 1998–2009, entailing 40.878 patients. Of those, 183 patients with BAV were included in this retrospective BAV single center cohort study, and reanalyzed with a comprehensive assessment of aortic dimensions, aortic valve morphology and function, clinical cardiovascular risk factors and patient characteristics.
RESULTS
Of the 183 patients with BAV, 138 were Caucasians (C) and 45 were African Americans (AA). Our Echo database encompasses approximately 65% AA, 31% C and 4% other races, for an estimated frequency of BAV in AA of 0.17 % and a frequency of BAV in C of 1.1 % (p=0.001). There were no significant inter-racial differences regarding gender, height, weight, hyperlipidemia, diabetes, tobacco use, cardiac medications, and left ventricular ejection fraction. The AA cohort was older (50±17 vs. 43±17, p<0.05) and had a higher prevalence of hypertension (51% vs. 24%, p <0.05). After adjusting for comorbidities, aortic dimensions were larger in C (C vs. AA: annulus 2.4±0.4 vs. 2.1±0.4, sinuses of Valsalva 3.4±0.7 vs. 3.1±0.6, sino-tubular junction 3.0±0.6 vs. 2.6±0.5, and ascending aorta 3.5±0.7 vs. 3.2±0.5, all p<0.05).
CONCLUSIONS
This is the first study to report racial differences among patient with BAV with reduced aortic dimensions in AA despite the presence of more risk factors, suggestive of marked heterogeneity in the BAV population, and indicating race as a potential disease modifier in BAV.
Keywords: Bicuspid aortic valve, aortopathy, thoracic aortic aneurysm and dissection
Introduction
Bicuspid aortic valve (BAV) is a common congenital heart valve abnormality accounting for a large number of valve replacements in the United States. Although still incompletely understood, the natural history of BAV disease often results in severe aortic stenosis or insufficiency and is associated with ascending aortic dilatation. The population frequency of BAV has been reported at 0.5 to 1.36%; commonly stated as 1–2% (1–3). BAV has a high heritability with a predilection for males of approximately 3:1, supporting the previous recommendation to screen first-degree relatives of patients with BAV (4). Patients with bicuspid compared to those with tri-leaflet aortic valves have larger aortic root dimensions and increased risk of ascending aortic dilation during their lifetime. The aortopathy and increased susceptibility to valvular stenosis and regurgitation places the patient, especially in adulthood, at higher risk for surgical intervention (5, 6). While there is an increased probability of aortic dilation and/or dissection in BAV patients, the optimal way to assess and characterize patients at risk have been a subject of debate in the medical literature. Most previously reported BAV studies included non-racially diverse populations consisting primarily of Caucasians and to a much lesser extent African-Americans. To our knowledge, there are no studies specifically addressing potential racial differences in BAV disease, which could yield important insight into genetic contributions to prevalence and disease phenotype in BAV disease. We, therefore, sought to characterize similarities and differences in Caucasian and African-Americans with BAV with respect to (i) overall BAV frequency, (ii) type of BAV morphology, (iii) presence of aortic stenosis/insufficiency and (iv) aortic dilatation.
Methods
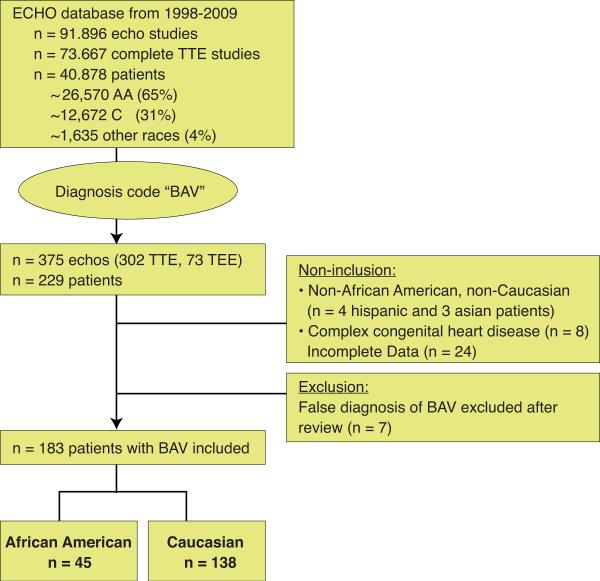
We retrospectively reviewed 229 patients with an echocardiographic diagnosis of BAV who underwent clinically indicated transthoracic studies from 1998 to 2009 at the University of Chicago. The University of Chicago Medical Center is the main tertiary referral center for the south side of Chicago serving a predominantly African American population. We used “BAV” as a diagnosis code to query our computerized echocardiographic database, which consisted of 91,896 trans-thoracic studies performed during the stated time span. Of these, 73,667 studies were complete trans-thoracic studies representing 40,878 individual patients. The inclusion criteria were documentation of BAV on transthoracic echocardiography. Of the 229 patients with diagnosed BAV, we excluded 46 patients because of race other than Caucasian (C) or African American (AA) (4 Hispanic and 3 Asian patients), supra-or sub-valvular stenosis, presence of other complex congenital cardiac defects (5-C, 2-AA), Marfan's disease (1-C), incomplete medical record data and/or poor visualization of aortic valve or ascending aorta (n=24), and lack of confirmation of bicuspid status by two independent echocardiographers (5-C, 2-AA). Accordingly, 183 patients were included in the final analysis, of these 138 were Caucasians and 45 were African Americans (Figure 1).
Figure 1. Study design.
229 patients with bicuspid aortic valve were screened and 183 patients of African American or Caucasian race with bicuspid aortic valve were included.
Echocardiographic data
All echocardiographic studies were performed using the same commercially available equipment in a single adult cardiac imaging center, accredited by the Institutional Commission for the Accreditation of Echocardiography Laboratories (ICAEL). Initially, the echocardiographic studies of all 229 patients with diagnosis code “BAV” were utilized for the purpose of this retrospective study and re-analyzed by two independent and trained observers. A third observer (RML) blinded to previous performed analyses resolved any discrepancies. Aortic valve morphology was identified in multiple parasternal short-axis views and transthoracic studies when available and classified as Type 1 (=congenital fusion of right and left coronary cusp resulting in an anterior-posterior oriented bileaflet morphology; A-P); Type 2 (=congenital fusion of the right and non-coronary cusp resulting in right-left oriented bileaflet morphology; R-L); and Type 3 (=congenital fusion of the non-coronary and left coronary cusps) using a phenotypic classification of leaflet morphology (7). The presence or absence of a raphe was noted, but not used in the final analysis. Using the leading edge technique, aortic diameters were prospectively measured at end-diastole in the parasternal view perpendicular to the long axis at several locations, including: Annulus, Sinuses of Valsalva (SV), sino-tubular junction (STJ) and when possible at least 2 cm distal from the STJ in the ascending aorta or the largest of multiple measurements performed above the ST-junction (8).
Echo-board certified cardiologists read all echocardiograms. The severity of aortic valve stenosis, aortic regurgitation and left ventricular ejection fraction was defined as per practice guidelines (9, 10).
Covariate measurements and definitions
For each patient, medically pertinent data was obtained from medical records, outpatient clinic visits, in-patient hospitalization including age, gender, United States Postal Services codes (ZIP-code) of the home address, height and weight (at time of echocardiographic study), presence of vascular risk factors including hypertension, dyslipidemia, diabetes and smoking history, and medication usage. Hypertension was defined by Joint National Committee VII guidelines (systolic BP of 140 mm Hg, diastolic BP of 90 mm Hg, or reported use of antihypertensive medications within 6 months before or after the echocardiographic evaluation). Body mass index (BMI) was calculated as weight divided by the height squared (kg/m2). Body surface area (BSA) was calculated as height (in) times weight (lbs) divided by 3131 (m2). The race of the 229 patients with BAV was classified as AA, C, Hispanic, or Asian based on information available to us either from the physician order entry for the echocardiographic study, or available in the physician's note. In the case of no available data on race in the BAV-patients, we prospectively performed telephone interviews to obtain this information. Of the 40,878 patients that were initially screened for the diagnostic code “BAV” we had information regarding physician-determined race in 18,475 patients, representing 45.2% of the study cohort. Of these, 65% were AA, 31% were C and 4% were of other race. We estimated, that of the 40,878 patients we studied, approximately 26,570 were AA, 12,672 were C and 1,635 were of other races. The study protocol was approved by the institutional review board including access to health records, imaging data, and telephone contact with patients, in which cases verbal consent was obtained.
Statistical analysis
Data is presented for each race separately and is expressed as mean±SD for continuous variables and as percentages for categorical variables. Independent Wilcoxon test `was used for continuous variables, Fisher exact test were performed to describe different outcome in categorical variables. The Spearman rank correlation was used for calculation of intra and inter-observer variability. Multivariate linear regression analyses for the endpoint of aortic dimension were conducted to determine which clinical and echocardiographic variables were significantly associated with aortic dimensions. We tested age, gender, hypertension status, BSA, Left ventricular (LV) ejection fraction (abnormal defined as an ejection fraction <50%), aortic valve hemodynamics, and use of medications as covariates in this multivariate linear regression model. Intra-observer variability of the aortic measurements was assessed using repeated measurements performed by the same observer on 50 patients (randomly selected 30 C, 20 AA) two months later. Inter-observer variability was evaluated by repeating the analysis on 50 patients (previously randomly selected 30 C, 20 AA) by a second independent observer, blinded to the results of all prior measurements. Variability was defined as the absolute difference between repeated measurements expressed as a percentage of their mean.
Results
1. Clinical characteristics
Our final study population consisted of 183 patients (126 males, 57 females) with BAV. The patients with BAV were divided into 2 groups according to their race; of these 138 (77%) patients were Caucasians, and 45 (25%) were African-Americans. Mean age of these patients was 45 (C 43±17 vs. AA 50±17years, p=0.03). The baseline characteristics are shown in Table 1. There were no significant inter-racial differences regarding gender, height, weight, hyperlipidemia, diabetes, tobacco use, cardiac medications including beta blockers, angiotensin receptor blockers and ace-inhibitors; left ventricular cavity dimensions, and left ventricular ejection fraction. Therapy with diuretics was significantly higher in the AA group (AA 34 vs. 19%, p<0.05). The AA group was not only significantly older, but also had a higher prevalence of hypertension (AA 49 vs. C 24%, p =0.001) with more frequent use of diuretic medications.
Table 1.
Baseline characteristics of the 183 patients with bicuspid aortic valve. For categorical variables the numbers of subjects are shown with the percent in parenthesis, and a Fisher's exact test was used to compare AA and C.
| All patients (n=183) | African-American (n=45) | Caucasian (n=138) | p value | |
|---|---|---|---|---|
| Male, n (%) | 126 (69) | 29 (64) | 97 (70.8) | 0.4 |
| # Age (year) | 44.8 (17) | 49.9 (17.1) | 43.2 (16.8) | 0.03 |
| # Height (in) | 69.4 (4.8) | 68.9 (5.7) | 69.6 (4.5) | 0.6 |
| # Weight (lbs) | 177.9 (41.1) | 183.9 (54.5) | 176.1 (36.3) | 0.5 |
| Hypertension (%) | 55 (30) | 22 (49) | 33 (24.1) | 0.001 |
| Hyperlipemia (%) | 32 (18) | 8 (18) | 24 (17.6) | 0.8 |
| Beta-blocker (%) | 39 (21) | 12 (27) | 30 (22) | 0.3 |
| ACE-I/ARB (%) | 41 (22) | 12 (29) | 32 (23) | 0.3 |
| Type of BAV(%) | 0.11 | |||
| Type 1 | 145 (79) | 32 (71) | 113 (82) | |
| Type 2 | 37 (20) | 13 (29) | 24 (17) | |
| Type 3 | 1 (1) | 0 | 1 (1) | |
| Aortic stenosis (%) (%) | 0.72 | |||
| None | 133 (73) | 34 (76) | 99 (72) | |
| Mild | 24 (13) | 5 (11) | 20 (14) | |
| Moderate | 14 (7) | 4 (9) | 10 (7) | |
| Severe | 11 (6) | 2 (5) | 9 (7) | |
| Aortic insufficiency (%) | 0.48 | |||
| None | 86 (47) | 25 (56) | 61 (45) | |
| Mild | 74 (41) | 15 (33) | 59 (43) | |
| Moderate | 15 (8) | 3 (7) | 12 (9) | |
| Severe | 7 (4) | 2 (4) | 5 (4) | |
| No AS and No AI (%) | 57 (32) | 18 (44) | 39 (29) | 0.07 |
| Coarctation (%) | 9 (5) | 2 (5) | 7 (5) | 0.95 |
| # Mean LVEDD (cm) | 4.9 (0.8) | 4.8 (0.8) | 4.9 (0.7) | 0.46 |
| # Mean LVESD (cm) | 3.2 (0.5) | 3.2 (0.4) | 3.4 (0.5) | 0.88 |
Continuous variables are shown as mean and standard deviation in parenthesis, and AA and C were compared by independent Wilcoxon test.
2. Frequency of BAV in our cohort
We found 229 patients diagnosed with BAV in our cohort of 40,878 patients with an estimated prevalence of 0.56%. After reviewing all cases, we excluded 7 cases, because either expert review or additional available TEE images showed a trileaflet valve. We did not include BAV patients with 1) complex congenital heart disease (n=8), 2) race other than AA or C (n=7), or 3) patients with incomplete datasets (n=24) thereby arriving at the number of 183 well-characterized patients with BAV (Figure 1). Interestingly, we found fewer patients with BAV amongst African Americans; with 45 AA having BAV out of approximately 26,570 screened AA patients represented in our ECHO database. In contrast, 138 C with BAV were identified from approximately 12,672 C patients represented in our ECHO database. From our cohort, we calculated the prevalence of BAV for AA to be 0.17 %, and at 1.1% for C (p=0.001). More than 90% of Caucasians and African Americans with BAV lived within 15 miles from the Medical Center based on their ZIP code, suggesting that this cohort reflects an urban population without specific referral pattern. There was no racial difference in the BAV morphology between AA and Caucasian. Both races had predominantly Type 1 BAV (AA 71%, C 82%; p =0.71) and less often Type 2 BAV (AA 29%, C 18%, p=0.68). Type 3 morphology was only found in one caucasian patient (Table 1).
Interestingly, our data showed a trend toward increased prevalence of functionally normal bicuspid valve in African Americans i.e. lacking any discernible aortic valve stenosis or insufficiency compared to Caucasians, however this finding did not reach statistical significance (AA 44% vs. C 29%, p=0.067). Moreover, there was no statistically significant difference in the prevalence of mild to severe bicuspid valve stenosis or insufficiency between races (Table1). African Americans had severe AS in 5% and severe AI in 4% compared to 7% and 4% in Caucasians, respectively (p>0.05).
3. Aortic dimensions
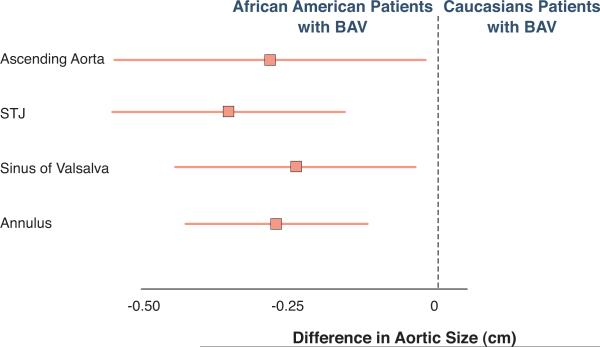
Both, intra and inter-observer variability in the measurement of the aortic dimensions at any site were minimal. The squared correlation coefficients (R2) for intra and inter-observer variability ranged from 0.91 to 0.97 and 0.87 to 0.93 respectively. Most interestingly, there were significant differences in the aortic dimensions between groups (Table 2). The linear dimensions of the aortic annulus, sinuses of Valsalva, STJ, and ascending aorta were significantly larger in Caucasians compared to the African American patients, before and after adjusting for age, gender, hypertension, BSA, presence of moderate-severe aortic stenosis or insufficiency, and medication use including beta blockers, ACE-inhibitors, and angiotensin receptor blockers. The largest difference in diameter occurred at the STJ level (Figure 2).
Table 2.
Aortic measurements shown as mean, (standard deviation), and [95% confidence interval] were compared between AA and C patients with BAV by multivariate linear regression analysis as unadjusted measurements or *adjusted for age, gender, hypertension, moderate-severe aortic stenosis/insufficiency, and use of beta blocker or ACE-inhibitors or ARB's and show significant smaller aortic dimension in AA patients with BAV.
| All patients (n=183) | African-American (n=45) | Caucasian (n=138) | Unadjusted analysis | Adjusted analysis* | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Difference [95% CI] | p value | Difference [95% CI] | p value | ||||
| Annulus (cm) | 2.3 (0.4) | 2.1 (0.4) | 2.4 (0.4) | 0.28 [0.13 ; 0.43] | 0.001 | 0.27 [0.12 ; 0.43] | < 0.0001 |
| Sinus (cm) | 3.3 (0.6) | 3.1 (0.6) | 3.4 (0.7) | 0.23 [−0.01 ; 0.41] | 0.04 | 0.27 [0.03 ; 0.45] | 0.02 |
| STJ (cm) | 2.8 (0.6) | 2.6 (0.5) | 3.0 (0.6) | 0.31 [0.12 ; 0.51] | 0.001 | 0.36 [0.15 ; 0.56] | < 0.0001 |
| Asc. Aorta (cm) | 3.4 (0.7) | 3.2 (0.5) | 3.5 (0.7) | 0.25 [−0.04 ; 0.47] | 0.04 | 0.29 [0.02 ; 0.55] | 0.03 |
Figure 2. African American patients with bicuspid aortic valve have smaller aortic dimension.
Absolute difference in aortic dimensions between African American patients with BAV compared to Caucasian patients with BAV after adjustment for age, gender, hypertension, moderate to severe aortic valve stenosis/insufficiency, use of beta blocker, ACE-inhibitors or ARB's demonstrated smaller aortic dimensions in AA. Data are shown as mean and as 95% confidence interval.
Discussion
Our study is the first to examine racial differences in patients with bicuspid aortic valve in a retrospective cohort from our hospital that represents a large number of African American patients and Caucasian patients undergoing clinically indicated transthoracic echocardiography. Because of a suggested strong genetic contribution for BAV and possibility for progression of BAV-associated complications, such as aortic valve replacement and thoracic aortic aneurysms, we sought to specifically examine BAV-disease characteristics in a community cohort that includes two different races, i.e. African Americans and Caucasians.
Surprisingly, we found an overall lower than reported frequency of BAV in our cohort (0.45%) of 40,878 patients with an even smaller frequency amongst African American patients (0.17%) compared to 1.1% in C. While this is an intriguing finding suggesting a possible lower prevalence of BAV in AA, the retrospective design of our study using a selected cohort of patients undergoing a clinical indicated echocardiographic study precludes such a conclusion. Only a random sample of the entire population could determine the true prevalence of BAV. There is limited information on prevalence of BAV in African Americans in the literature though it has been previously reported in a correspondence as being lower than other populations (11). Moreover, African Americans with BAV trended towards a higher prevalence of preserved aortic valve function, despite being approximately 6 years older, having a higher prevalence of hypertension, and a similar predominance of Type 1 BAV, as previously reported in other cohorts of BAV (7). This suggests that the African American patients could possibly have a milder phenotype of BAV dysfunction consistent with our findings of lesser aortic dilatation in this group. It has been reported that patients with BAV compared to tri-leaflet aortic valve have larger aortic dimensions, increased rate of dilatation, and possibly higher risk of dissection, though the actual reported rate is low at approximately 2–4% (12, 13). There exists considerable variability and uncertainty on extent, definition, and susceptibility to dilatation. Moreover, aortic dilatation prevalence estimations vary depending on the study population, and the specific region of the aorta measured (5, 12, 14, 15).
There is substantial support for genetic factors, for developmental mechanisms related to the shared derivation of the aortic valvular cusps and aortic medial layer from the neural crest cells (16), as well contributing factors related to hemodynamic stress exerted on the aortic wall by the eccentric turbulent flow across the bicuspid valve (17) that collectively contributes to the development of thoracic aortic aneurysms. In our study cohort, the increased aortic dilatation in Caucasians compared to AAs despite similar prevalence in BAV morphology and regurgitant or stenosis severity, raises some important issues regarding the role of genetic or other vascular modifiers. In this context, it is interesting to note, that the multi-Ethnic Study of Atherosclerosis (MESA) found racial differences in aortic physiology among the 1,053 participants without overt clinical cardiovascular disease (and presumably trileaflet aortic valve) demonstrating reduced aortic distensibility and increased aortic stiffness in African Americans. Other risk factors for aortic stiffness in this study included older age, smoking, and hypertension (18).
It is possible that intrinsic factors within the aortic wall, such as increased fibrosis or cross-linking of extracellular matrix, could be mediating the reduced aortic distensibility leading to increased aortic stiffness and potentially prevention of aortic dilatation in African Americans. Indeed, vascular mechanic studies performed on healthy young males post-exertion and in hypertensive, non-medicated African Americans compared to their Caucasian counterparts have demonstrated greater aortic stiffness, augmentation index and reduced wave reflection travel time (19, 20). Indices of aortic stiffness in BAV patients have been found to be abnormal in those with dilated proximal ascending aorta compared to those with BAV without dilated ascending aorta (21, 22). Interestingly, these abnormal load-bearing characteristics of the aorta persist in BAV patients despite making adjustments for vessel dimension and differences in blood pressure (23). There is considerable variability in these parameters in the literature, and it is yet to be established whether aortic stiffness or elasticity differs amongst African Americans and Caucasians with BAV. Amongst all the risk factors, including significant valve disease, age is considered the most important variable associated with dilatation of the ascending aorta (24). Despite the older age, higher incidence of hypertension in our study and similar prevalence of beta-blocker, angiotensin receptor blockers or ACE-inhibitors, the AA group had comparably smaller aortas, supporting the hypothesis of a genetic susceptibility for aortic remodeling.
Currently, thoracic aortic diameter and its progression rate serve as main criteria for risk stratification in BAV patients with or at risk for aortic dilatation and/or dissection, but they do not serve as prerequisites for dissection. It is estimated that 6% of patients with BAV develop a type A thoracic aortic dissection or conversely, BAV is found in 2–9% of patients with this type of dissection (13, 25, 26). Studies stemming from the International Registry of Aortic Dissection (IRAD) have noted that aortic dissections occur at aortic diameters below 5.0 cm, the recommended threshold for elective ascending aortic replacement, albeit the percentage of dissection is small (27--29). Studies from Toronto and Olmstead County on BAV patients indicate a low acute event rate with interventions consisting mostly of surgical procedures involving the aortic valve and aortic root replacements in an older population (13, 30). It is not entirely clear whether dissection rates in these studies were derived from a BAV population that was racially heterogeneous.
Indicative of the low representation of the minority patient populations in these studies is the National Heart, Lung, and Blood Institute funded genetically triggered thoracic aortic conditions (GenTACs) registry which is a very promising start in assessing genetic predisposition to thoracic aortic aneurysms and dissection and is especially interested in recruiting minorities (31). The registry reported that despite representation of clinical centers with large black populations, only 8.9% of the subjects enrolled were self-identified minority, of which 6.6% were of Hispanic origin. There exists significant variation in genetic and racial origins and susceptibilities towards dilatation or dissection in the literature. The reported figures may not account for individuals with thoracic aortic dissection who die undiagnosed outside of hospital settings and without an autopsy. A comparison of the clinical profile between the IRAD population (4% minorities) and autopsy-based study of out-of-hospital deaths from aortic dissection among the more ethnically diverse residents (48%) of Harris County, Texas, yielded interesting findings (32, 33). Compared to IRAD, autopsy results from this study demonstrated that patient with type A aortic dissection were likely to be Hispanic, of lower average age (42 years, 43 years in those with congenital heart disease), and have a higher prevalence of BAV. Our study emphasizes the need to include minorities in BAV-related outcomes studies in order to fully understand and develop risk-stratification and surveillance tools that may better serve this patient population.
Limitations
One of the limitations is the retrospective nature of this study that resulted in exclusion of BAV patients due to incomplete clinical dataset. Another limitation is the possibility of underestimation of BAV since we did not review the images of all other echocardiographic studies in our computerized databank that were not classified as “BAV” during the initial evaluation by an echoboard-certified reader. Moreover, BAV could have been missed in cases of heavily calcified aortic valve disease with severe aortic stenosis. There is a possibility that echocardiography underestimated or missed the presence of BAV and the eccentric remodeling patterns in the ascending aorta that were beyond the imaging capabilities of this 2-dimensional modality. Until cardiac magnetic resonance imaging becomes more accessible, trans-thoracic echocardiography remains the mainstay for screening and interval surveillance of these patients, although full periodic aortic surveillance is warranted in these patients (29). The prevalence of aortic valve or aortic diameter severity in our study may be different from others. Moreover, our study is a single center retrospective study and therefore is limited with regard to samples size. Future studies are needed to confirm our findings of race as a modifier for BAV disease. We cannot exclude the possibility of referral bias, and the influence of socioeconomic factors affecting access to medical care may have influenced our study results; however the zip code analysis of our patient population confirms that it is indeed representative of the South-side of Chicago.
Conclusions
Our finding of lower frequency of BAV and reduced aortic dimensions despite increased presence of risk factors, namely, systemic hypertension and older age in African Americans in our retrospective cohort study calls for further prospective, outcomes-based studies in this minority population to individualize risk stratification and therapy accordingly.
Acknowledgements
The authors are grateful to the patients and their physicians involved in this study.
Funding Source: This work was supported by funding from the National Institute of Health (MAHB: K08 HL090917-02 and by the Doris Duke Charitable Foundation. MAHB is a recipient of the Doris Duke Clinical Scientist Development Award. Dr. Lang is a member of the Philips Healthcare speaker bureau and has received research equipment grants from Philips.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no conflicts of interest related to this study.
References
- 1.Gray GW, Salisbury DA, Gulino AM. Echocardiographic and color flow Doppler findings in military pilot applicants. Aviat Space Environ Med. 1995 Jan;66(1):32–4. [PubMed] [Google Scholar]
- 2.Basso C, Boschello M, Perrone C, Mecenero A, Cera A, Bicego D, et al. An echocardiographic survey of primary school children for bicuspid aortic valve. Am J Cardiol. 2004 Mar 1;93(5):661–3. doi: 10.1016/j.amjcard.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 3.Nistri S, Basso C, Marzari C, Mormino P, Thiene G. Frequency of bicuspid aortic valve in young male conscripts by echocardiogram. Am J Cardiol. 2005 Sep 1;96(5):718–21. doi: 10.1016/j.amjcard.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 4.Cripe L, Andelfinger G, Martin LJ, Shooner K, Benson DW. Bicuspid aortic valve is heritable. J Am Coll Cardiol. 2004 Jul 7;44(1):138–43. doi: 10.1016/j.jacc.2004.03.050. [DOI] [PubMed] [Google Scholar]
- 5.Cecconi M, Manfrin M, Moraca A, Zanoli R, Colonna PL, Bettuzzi MG, et al. Aortic dimensions in patients with bicuspid aortic valve without significant valve dysfunction. Am J Cardiol. 2005 Jan 15;95(2):292–4. doi: 10.1016/j.amjcard.2004.08.098. [DOI] [PubMed] [Google Scholar]
- 6.Novaro GM, Tiong IY, Pearce GL, Grimm RA, Smedira N, Griffin BP. Features and predictors of ascending aortic dilatation in association with a congenital bicuspid aortic valve. Am J Cardiol. 2003 Jul 1;92(1):99–101. doi: 10.1016/s0002-9149(03)00480-6. [DOI] [PubMed] [Google Scholar]
- 7.Schaefer BM, Lewin MB, Stout KK, Gill E, Prueitt A, Byers PH, et al. The bicuspid aortic valve: an integrated phenotypic classification of leaflet morphology and aortic root shape. Heart. 2008 Dec;94(12):1634–8. doi: 10.1136/hrt.2007.132092. [DOI] [PubMed] [Google Scholar]
- 8.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005 Dec;18(12):1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Jr., Faxon DP, Freed MD, et al. Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2008 Oct 7;118(15):e523–661. doi: 10.1161/CIRCULATIONAHA.108.190748. 2008. [DOI] [PubMed] [Google Scholar]
- 10.Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003 Jul;16(7):777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 11.Khan W, Milsevic M, Salciccioli L, Lazar J. Low prevalence of bicuspid aortic valve in African Americans. Am Heart J. 2008 Sep;156(3):e25. doi: 10.1016/j.ahj.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 12.Ferencik M, Pape LA. Changes in size of ascending aorta and aortic valve function with time in patients with congenitally bicuspid aortic valves. Am J Cardiol. 2003 Jul 1;92(1):43–6. doi: 10.1016/s0002-9149(03)00462-4. [DOI] [PubMed] [Google Scholar]
- 13.Tzemos N, Therrien J, Yip J, Thanassoulis G, Tremblay S, Jamorski MT, et al. Outcomes in adults with bicuspid aortic valves. JAMA. 2008 Sep 17;300(11):1317–25. doi: 10.1001/jama.300.11.1317. [DOI] [PubMed] [Google Scholar]
- 14.Nistri S, Sorbo MD, Marin M, Palisi M, Scognamiglio R, Thiene G. Aortic root dilatation in young men with normally functioning bicuspid aortic valves. Heart. 1999 Jul;82(1):19–22. doi: 10.1136/hrt.82.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evangelista A. Bicuspid aortic valve and aortic root disease. Curr Cardiol Rep. 2011 Jun;13(3):234–41. doi: 10.1007/s11886-011-0175-4. [DOI] [PubMed] [Google Scholar]
- 16.Rosenquist TH, Beall AC. Elastogenic cells in the developing cardiovascular system. Smooth muscle, nonmuscle, and cardiac neural crest. Ann N Y Acad Sci. 1990;588:106–19. doi: 10.1111/j.1749-6632.1990.tb13201.x. [DOI] [PubMed] [Google Scholar]
- 17.Girdauskas E, Borger MA, Secknus MA, Girdauskas G, Kuntze T. Is aortopathy in bicuspid aortic valve disease a congenital defect or a result of abnormal hemodynamics? A critical reappraisal of a one-sided argument. Eur J Cardiothorac Surg. 2011 Jun;39(6):809–14. doi: 10.1016/j.ejcts.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Malayeri AA, Natori S, Bahrami H, Bertoni AG, Kronmal R, Lima JA, et al. Relation of aortic wall thickness and distensibility to cardiovascular risk factors (from the Multi-Ethnic Study of Atherosclerosis [MESA]) Am J Cardiol. 2008 Aug 15;102(4):491–6. doi: 10.1016/j.amjcard.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heffernan KS, Jae SY, Wilund KR, Woods JA, Fernhall B. Racial differences in central blood pressure and vascular function in young men. Am J Physiol Heart Circ Physiol. 2008 Dec;295(6):H2380–7. doi: 10.1152/ajpheart.00902.2008. [DOI] [PubMed] [Google Scholar]
- 20.Din-Dzietham R, Couper D, Evans G, Arnett DK, Jones DW. Arterial stiffness is greater in African Americans than in whites: evidence from the Forsyth County, North Carolina, ARIC cohort. Am J Hypertens. 2004 Apr;17(4):304–13. doi: 10.1016/j.amjhyper.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Tzemos N, Lyseggen E, Silversides C, Jamorski M, Tong JH, Harvey P, et al. Endothelial function, carotid-femoral stiffness, and plasma matrix metalloproteinase-2 in men with bicuspid aortic valve and dilated aorta. J Am Coll Cardiol. 2010 Feb 16;55(7):660–8. doi: 10.1016/j.jacc.2009.08.080. [DOI] [PubMed] [Google Scholar]
- 22.La Canna G, Ficarra E, Tsagalau E, Nardi M, Morandini A, Chieffo A, et al. Progression rate of ascending aortic dilation in patients with normally functioning bicuspid and tricuspid aortic valves. Am J Cardiol. 2006 Jul 15;98(2):249–53. doi: 10.1016/j.amjcard.2006.01.096. [DOI] [PubMed] [Google Scholar]
- 23.Nistri S, Grande-Allen J, Noale M, Basso C, Siviero P, Maggi S, et al. Aortic elasticity and size in bicuspid aortic valve syndrome. Eur Heart J. 2008 Feb;29(4):472–9. doi: 10.1093/eurheartj/ehm528. [DOI] [PubMed] [Google Scholar]
- 24.Siu SC, Silversides CK. Bicuspid aortic valve disease. J Am Coll Cardiol. 2010 Jun 22;55(25):2789–800. doi: 10.1016/j.jacc.2009.12.068. [DOI] [PubMed] [Google Scholar]
- 25.Ando M, Okita Y, Morota T, Takamoto S. Thoracic aortic aneurysm associated with congenital bicuspid aortic valve. Cardiovasc Surg. 1998 Dec;6(6):629–34. doi: 10.1016/s0967-2109(98)00094-5. [DOI] [PubMed] [Google Scholar]
- 26.Evangelista A, Mukherjee D, Mehta RH, O'Gara PT, Fattori R, Cooper JV, et al. Acute intramural hematoma of the aorta: a mystery in evolution. Circulation. 2005 Mar 1;111(8):1063–70. doi: 10.1161/01.CIR.0000156444.26393.80. [DOI] [PubMed] [Google Scholar]
- 27.Pape LA, Tsai TT, Isselbacher EM, Oh JK, O'Gara PT, Evangelista A, et al. Aortic diameter >or = 5.5 cm is not a good predictor of type A aortic dissection: observations from the International Registry of Acute Aortic Dissection (IRAD) Circulation. 2007 Sep 4;116(10):1120–7. doi: 10.1161/CIRCULATIONAHA.107.702720. [DOI] [PubMed] [Google Scholar]
- 28.Elefteriades JA, Farkas EA. Thoracic aortic aneurysm clinically pertinent controversies and uncertainties. J Am Coll Cardiol. 2010 Mar 2;55(9):841–57. doi: 10.1016/j.jacc.2009.08.084. [DOI] [PubMed] [Google Scholar]
- 29.Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE, Jr., et al. ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: executive summary. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Catheter Cardiovasc Interv. 2010 Aug 1;76(2):E43–86. doi: 10.1002/ccd.22537. 2010. [DOI] [PubMed] [Google Scholar]
- 30.Michelena HI, Desjardins VA, Avierinos JF, Russo A, Nkomo VT, Sundt TM, et al. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation. 2008 May 27;117(21):2776–84. doi: 10.1161/CIRCULATIONAHA.107.740878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kroner BL, Tolunay HE, Basson CT, Pyeritz RE, Holmes KW, Maslen CL, et al. The National Registry of Genetically Triggered Thoracic Aortic Aneurysms and Cardiovascular Conditions (GenTAC): Results from phase I and scientific opportunities in phase II. Am Heart J. 2011 Oct;162(4):627–32. e1. doi: 10.1016/j.ahj.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prakash SK, Haden-Pinneri K, Milewicz DM. Susceptibility to acute thoracic aortic dissections in patients dying outside the hospital: an autopsy study. Am Heart J. 2011 Sep;162(3):474–9. doi: 10.1016/j.ahj.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 33.Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA. 2000 Feb 16;283(7):897–903. doi: 10.1001/jama.283.7.897. [DOI] [PubMed] [Google Scholar]