Abstract
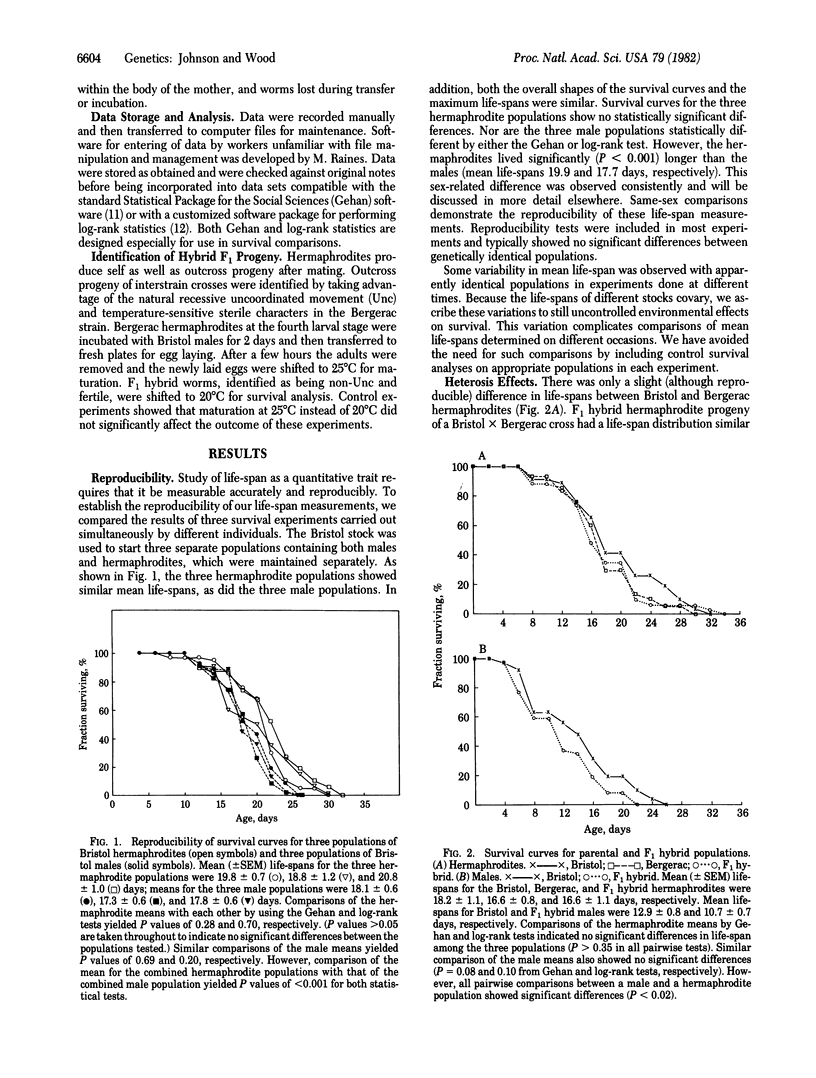
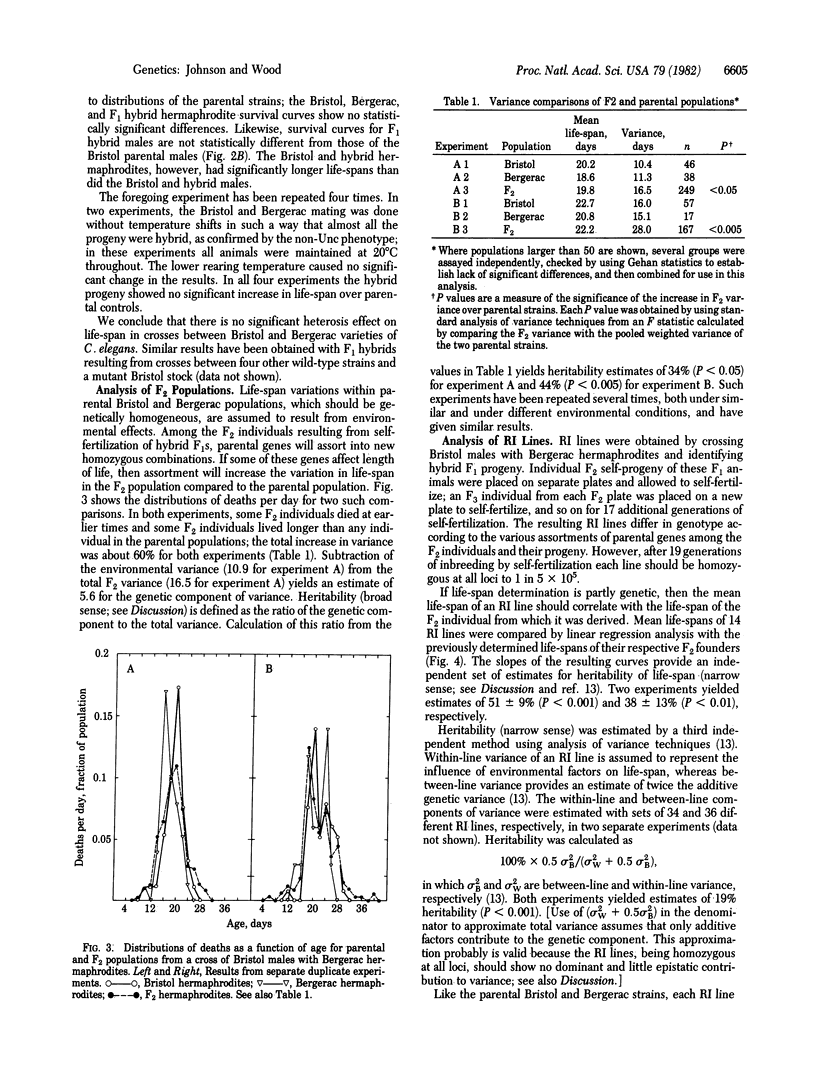
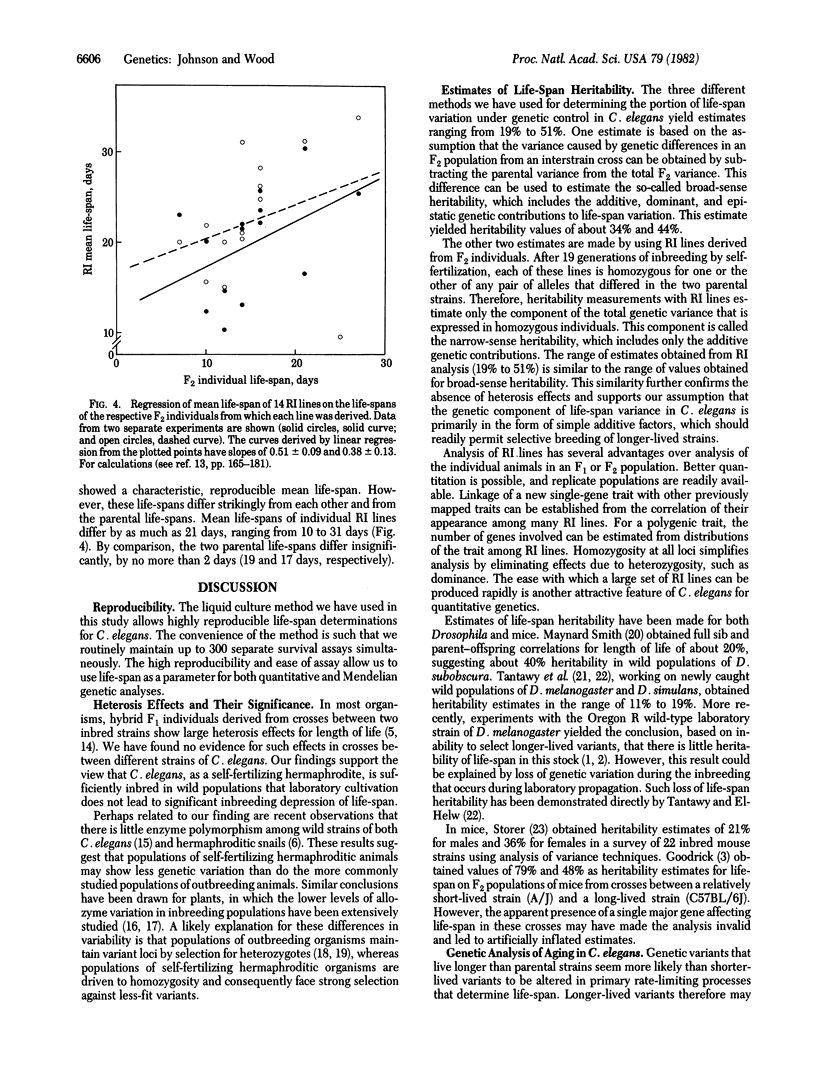
Crosses between Bristol and Bergerac strains of the self-fertilizing hermaphroditic nematode Caenorhabditis elegans do not show the heterosis effects for life-span that complicate analysis of interstrain crosses with Drosophila or mice. Instead they yield F1 progeny with life-spans similar to those of the parent strains. By analysis of life-span variation among progeny F2 populations from such crosses and by two independent analyses of life-spans among recombinant inbred lines derived from F2 individuals by 18 rounds of self-fertilization, we estimate that the heritability of life-span in C. elegans is between 20% and 50%. Recombinant inbred lines show a range in mean life-spans of 10 days to 31 days compared to life-spans of about 18 days for each of the two parental strains. We conclude that life-span variation in C. elegans has a substantial genetic component and that this organism offers promising opportunities for selective breeding of longer-lived strains and genetic analysis of senescence.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974 May;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler M. H., Wall S. M., Luehrsen K. R., Fox G. E., Hecht R. M. Molecular relationships between closely related strains and species of nematodes. J Mol Evol. 1981;18(1):18–23. doi: 10.1007/BF01733207. [DOI] [PubMed] [Google Scholar]
- Flanagan J. R. Detecting early-life components in the determination of the age of death. Mech Ageing Dev. 1980 May;13(1):41–62. doi: 10.1016/0047-6374(80)90129-3. [DOI] [PubMed] [Google Scholar]
- Goodrick C. L. Life-span and the inheritance of longevity of inbred mice. J Gerontol. 1975 May;30(3):257–263. doi: 10.1093/geronj/30.3.257. [DOI] [PubMed] [Google Scholar]
- Klass M. R. Aging in the nematode Caenorhabditis elegans: major biological and environmental factors influencing life span. Mech Ageing Dev. 1977 Nov-Dec;6(6):413–429. doi: 10.1016/0047-6374(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Klass M., Hirsh D. Non-ageing developmental variant of Caenorhabditis elegans. Nature. 1976 Apr 8;260(5551):523–525. doi: 10.1038/260523a0. [DOI] [PubMed] [Google Scholar]
- Lints F. A., Stoll J., Gruwez G., Lints C. V. An attempt to select for increased longevity in Drosophila melanogaster. Gerontology. 1979;25(4):192–204. doi: 10.1159/000212340. [DOI] [PubMed] [Google Scholar]
- McCracken G. F., Selander R. K. Self-fertilization and monogenic strains in natural populations of terrestrial slugs. Proc Natl Acad Sci U S A. 1980 Jan;77(1):684–688. doi: 10.1073/pnas.77.1.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peto R., Pike M. C., Armitage P., Breslow N. E., Cox D. R., Howard S. V., Mantel N., McPherson K., Peto J., Smith P. G. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977 Jan;35(1):1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons M. J., Crow J. F. Mutations affecting fitness in Drosophila populations. Annu Rev Genet. 1977;11:49–78. doi: 10.1146/annurev.ge.11.120177.000405. [DOI] [PubMed] [Google Scholar]
- Storer J. B. Longevity and gross pathology at death in 22 inbred mouse strains. J Gerontol. 1966 Jul;21(3):404–409. doi: 10.1093/geronj/21.3.404. [DOI] [PubMed] [Google Scholar]
- TANTAWY A. O., RAKHA F. A. STUDIES ON NATURAL POPULATIONS OF DROSOPHILA. IV. GENETIC VARIANCES OF AND CORRELATIONS BETWEEN FOUR CHARACTERS IN D. MELANOGASTER AND D. SIMULANS. Genetics. 1964 Dec;50:1349–1355. doi: 10.1093/genetics/50.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantawy A. O., el-Helw M. R. Studies on natural populations of Drosophila. V. Correlated response to selection in Drosophila melanogaster. Genetics. 1966 Jan;53(1):97–110. doi: 10.1093/genetics/53.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey M. L., Ayala F. J. Genetic load in natural populations: is it compatible with the hypothesis that many polymorphisms are maintained by natural selection? Genetics. 1974 Jul;77(3):569–589. doi: 10.1093/genetics/77.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. B., Hecht R., Carr S., Vanderslice R., Wolf N., Hirsh D. Parental effects and phenotypic characterization of mutations that affect early development in Caenorhabditis elegans. Dev Biol. 1980 Feb;74(2):446–469. doi: 10.1016/0012-1606(80)90445-5. [DOI] [PubMed] [Google Scholar]


