Abstract
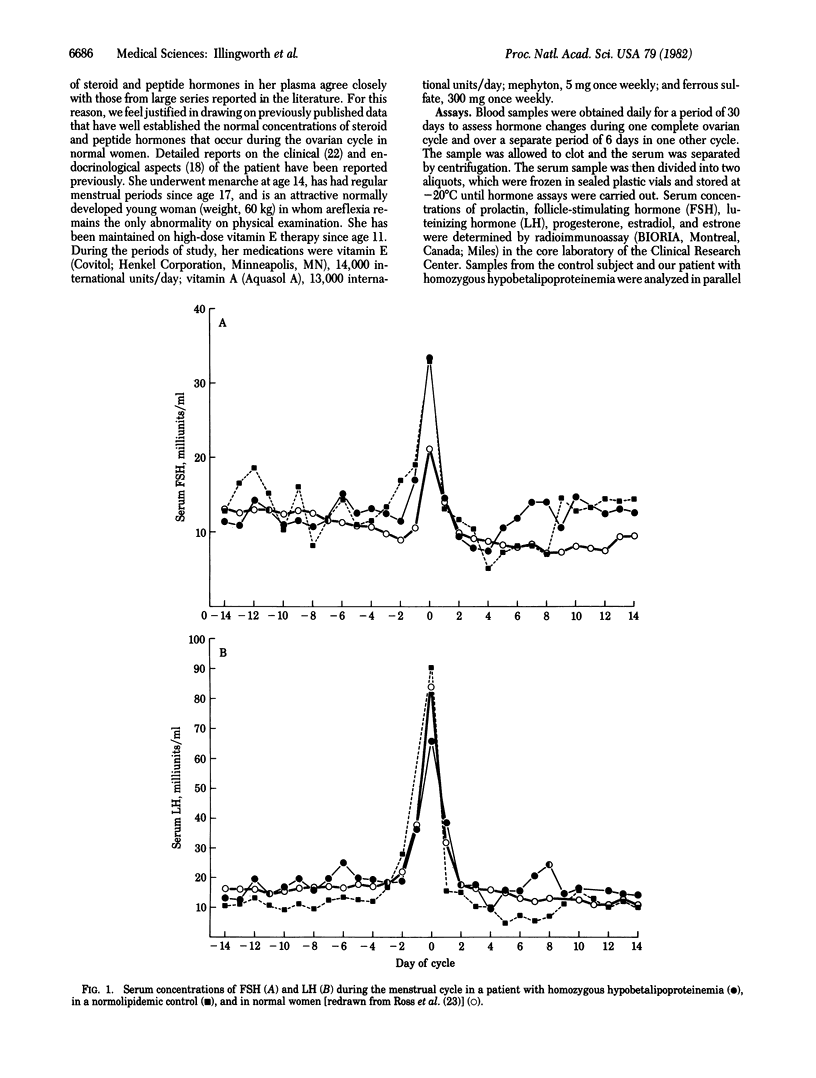
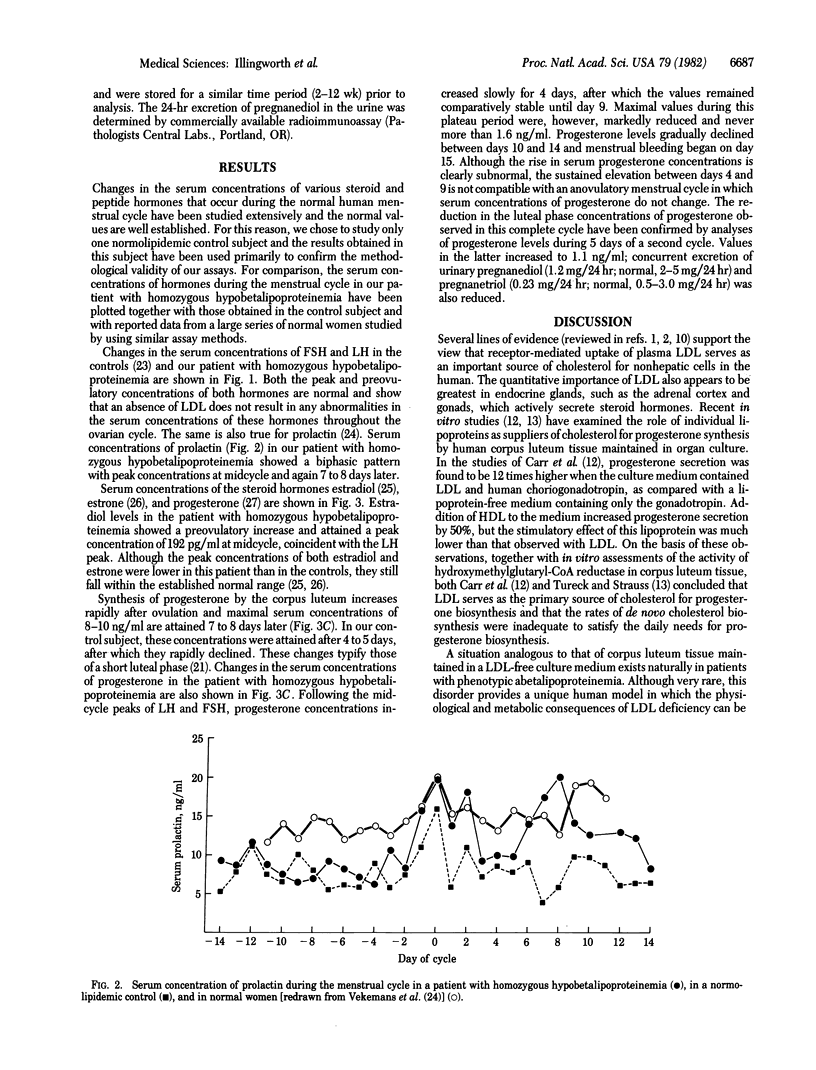
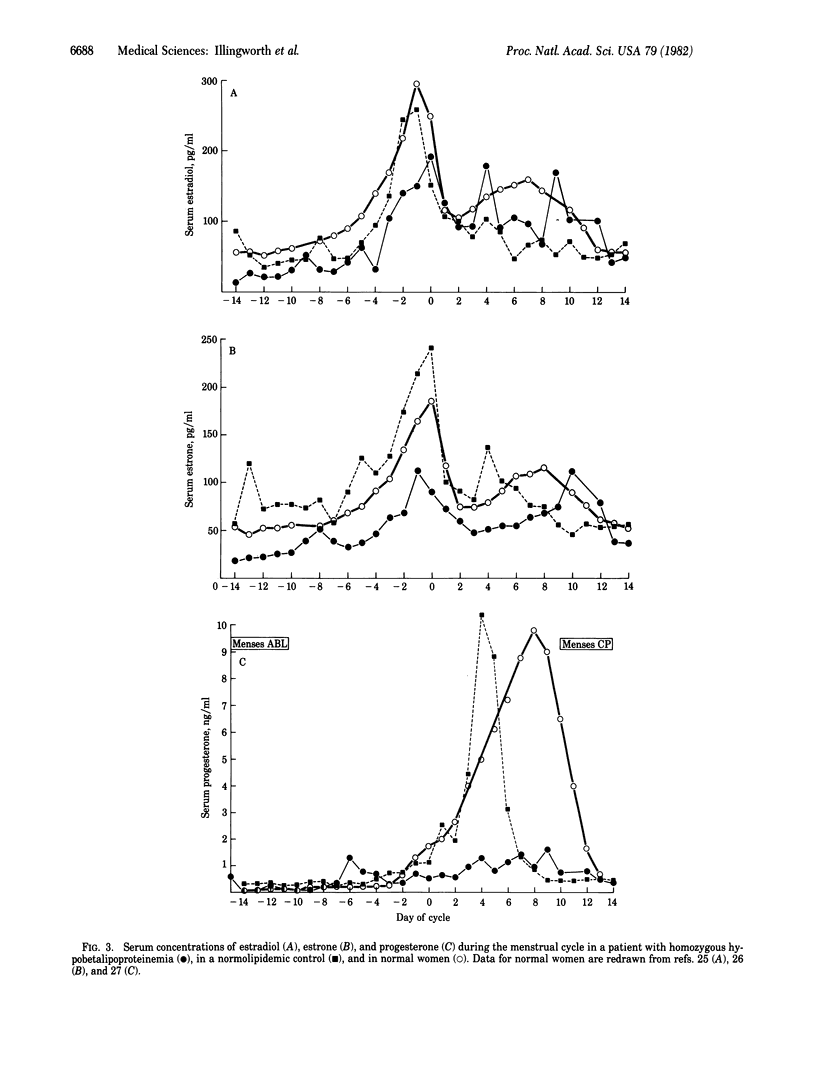
Progesterone synthesis by the human corpus luteum requires a source of cholesterol, which can be derived from both local synthesis and uptake of low density lipoproteins (LDL). When the corpus luteum is maintained in organ culture, progesterone synthesis is primarily dependent on LDL and the rate of progesterone production during growth in a LDL-free media is suboptimal. An in vivo situation analogous to that of corpus luteum grown in LDL-depleted media exists naturally in patients with abetalipoproteinemia. To determine whether a complete deficiency of plasma LDL affects serum concentrations of progesterone (particularly during the luteal phase) or those of other hormones, we have measured the serum concentrations of luteinizing hormone, follicle-stimulating hormone, prolactin, estradiol, estrone, and progesterone during the menstrual cycle in a patient with phenotypic abetalipoproteinemia (on the basis of homozygous hypobetalipoproteinemia). Our results show a normal cyclical pattern with midcycle increases in the concentrations of luteinizing and follicle-stimulating hormones, prolactin, and estrogens but a distinctly subnormal increase in the luteal phase concentrations of progesterone. These results suggest that, in patients with phenotypic abetalipoproteinemia, the absence of LDL leads to an impairment in the maximal rates of production of progesterone by the corpus luteum.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen J. M., Dietschy J. M. Relative importance of high and low density lipoproteins in the regulation of cholesterol synthesis in the adrenal gland, ovary, and testis of the rat. J Biol Chem. 1978 Dec 25;253(24):9024–9032. [PubMed] [Google Scholar]
- Azhar S., Menon K. M. Receptor-mediated gonadotropin action in the ovary. Rat luteal cells preferentially utilize and are acutely dependent upon the plasma lipoprotein-supplied sterols in gonadotropin-stimulated steroid production. J Biol Chem. 1981 Jul 10;256(13):6548–6555. [PubMed] [Google Scholar]
- Biemer J. J., McCammon R. E. The genetic relationship of abetalipoproteinemia and hypobetalipoproteinemia: a report of the occurence of both diseases within the same family. J Lab Clin Med. 1975 Apr;85(4):556–565. [PubMed] [Google Scholar]
- Brown M. S., Kovanen P. T., Goldstein J. L. Receptor-mediated uptake of lipoprotein-cholesterol and its utilization for steroid synthesis in the adrenal cortex. Recent Prog Horm Res. 1979;35:215–257. doi: 10.1016/b978-0-12-571135-7.50009-6. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Kovanen P. T., Goldstein J. L. Regulation of plasma cholesterol by lipoprotein receptors. Science. 1981 May 8;212(4495):628–635. doi: 10.1126/science.6261329. [DOI] [PubMed] [Google Scholar]
- Carr B. R., Parker C. R., Jr, Milewich L., Porter J. C., MacDonald P. C., Simpson E. R. The role of low density, high density, and very low density lipoproteins in steroidogenesis by the human fetal adrenal gland. Endocrinology. 1980 Jun;106(6):1854–1860. doi: 10.1210/endo-106-6-1854. [DOI] [PubMed] [Google Scholar]
- Carr B. R., Sadler R. K., Rochelle D. B., Stalmach M. A., MacDonald P. C., Simpson E. R. Plasma lipoprotein regulation of progesterone biosynthesis by human corpus luteum tissue in organ culture. J Clin Endocrinol Metab. 1981 May;52(5):875–881. doi: 10.1210/jcem-52-5-875. [DOI] [PubMed] [Google Scholar]
- Carr B. R., Simpson E. R. Lipoprotein utilization and cholesterol synthesis by the human fetal adrenal gland. Endocr Rev. 1981 Summer;2(3):306–326. doi: 10.1210/edrv-2-3-306. [DOI] [PubMed] [Google Scholar]
- DRUEZ G. Un nouveau cas d'acanthocytose: dysmorphie érythrocytaire congénitale avec rétinite, troubles nerveux et stigmates dégénératifs. Rev Hematol. 1959 Jan-Mar;14(1):3–11. [PubMed] [Google Scholar]
- Faust J. R., Goldstein J. L., Brown M. S. Receptor-mediated uptake of low density lipoprotein and utilization of its cholesterol for steroid synthesis in cultured mouse adrenal cells. J Biol Chem. 1977 Jul 25;252(14):4861–4871. [PubMed] [Google Scholar]
- Illingworth D. R., Connor W. E., Buist N. R., Jhaveri B. M., Lin D. S., McMurry M. P. Sterol balance in abetalipoproteinemia: studies in a patient with homozygous familial hypobetalipoproteinemia. Metabolism. 1979 Nov;28(11):1152–1160. doi: 10.1016/0026-0495(79)90155-0. [DOI] [PubMed] [Google Scholar]
- Illingworth D. R., Kenny T. A., Orwoll E. S. Adrenal function in heterozygous and homozygous hypobetalipoproteinemia. J Clin Endocrinol Metab. 1982 Jan;54(1):27–33. doi: 10.1210/jcem-54-1-27. [DOI] [PubMed] [Google Scholar]
- Illingworth D. R., Orwoll E. S., Connor W. E. Impaired cortisol secretion in abetalipoproteinemia. J Clin Endocrinol Metab. 1980 May;50(5):977–979. doi: 10.1210/jcem-50-5-977. [DOI] [PubMed] [Google Scholar]
- Is essential fatty acid deficiency part of the syndrome of abetalipoproteinemia? Nutr Rev. 1980 Jul;38(7):244–246. doi: 10.1111/j.1753-4887.1980.tb05915.x. [DOI] [PubMed] [Google Scholar]
- Kovanen P. T., Faust J. R., Brown M. S., Goldstein J. L. Low density lipoprotein receptors in bovine adrenal cortex. I. Receptor-mediated uptake of low density lipoprotein and utilization of its cholesterol for steroid synthesis in cultured adrenocortical cells. Endocrinology. 1979 Mar;104(3):599–609. doi: 10.1210/endo-104-3-599. [DOI] [PubMed] [Google Scholar]
- Landgren B. M., Undén A. L., Diczfalusy E. Hormonal profile of the cycle in 68 normally menstruating women. Acta Endocrinol (Copenh) 1980 May;94(1):89–98. doi: 10.1530/acta.0.0940089. [DOI] [PubMed] [Google Scholar]
- Nuñez M., Aedo A. R., Landgren B. M., Cekan S. Z., Diczfalusy E. Studies on the pattern of circulating steroids in the normal menstrual cycle. 6. Levels of oestrone sulphate and oestradiol sulphate. Acta Endocrinol (Copenh) 1977 Nov;86(3):621–633. doi: 10.1530/acta.0.0860621. [DOI] [PubMed] [Google Scholar]
- Pittman R. C., Attie A. D., Carew T. E., Steinberg D. Tissue sites of degradation of low density lipoprotein: application of a method for determining the fate of plasma proteins. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5345–5349. doi: 10.1073/pnas.76.10.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross G. T., Cargille C. M., Lipsett M. B., Rayford P. L., Marshall J. R., Strott C. A., Rodbard D. Pituitary and gonadal hormones in women during spontaneous and induced ovulatory cycles. Recent Prog Horm Res. 1970;26:1–62. doi: 10.1016/b978-0-12-571126-5.50005-4. [DOI] [PubMed] [Google Scholar]
- Savion N., Laherty R., Cohen D., Lui G. M., Gospodarowicz D. Role of lipoproteins and 3-hydroxy-3-methylglutaryl coenzyme A reductase in progesterone production by cultured bovine granulosa cells. Endocrinology. 1982 Jan;110(1):13–22. doi: 10.1210/endo-110-1-13. [DOI] [PubMed] [Google Scholar]
- Sherman B. M., Korenman S. G. Measurement of plasma LH, FSH, estradiol and progesterone in disorders of the human menstrual cycle: the short luteal phase. J Clin Endocrinol Metab. 1974 Jan;38(1):89–93. doi: 10.1210/jcem-38-1-89. [DOI] [PubMed] [Google Scholar]
- Simpson E. R., Bilheimer D. W., MacDonald P. C., Porter J. C. Uptake and degradation of plasma lipoproteins by human choriocarcinoma cells in culture. Endocrinology. 1979 Jan;104(1):8–16. doi: 10.1210/endo-104-1-8. [DOI] [PubMed] [Google Scholar]
- Simpson E. R., Porter J. C., Milewich L., Bilheimer D. W., MacDonald P. C. Regulation by plasma lipoproteins of progesterone biosynthesis and 3-hydroxy-3-methyl glutaryl coenzyme a reductase activity in cultured human choriocarcinoma cells. J Clin Endocrinol Metab. 1978 Nov;47(5):1099–1105. doi: 10.1210/jcem-47-5-1099. [DOI] [PubMed] [Google Scholar]
- Speroff L., Vande Wiele R. L. Regulation of the human menstrual cycle. Am J Obstet Gynecol. 1971 Jan 15;109(2):234–247. doi: 10.1016/0002-9378(71)90872-6. [DOI] [PubMed] [Google Scholar]
- Tureck R. W., Strauss J. F., 3rd Progesterone synthesis by luteinized human granulosa cells in culture: the role of de novo sterol synthesis and lipoprotein-carried sterol. J Clin Endocrinol Metab. 1982 Feb;54(2):367–373. doi: 10.1210/jcem-54-2-367. [DOI] [PubMed] [Google Scholar]
- Vekemans M., Delvoye P., L'Hermite M., Robyn C. Serum prolactin levels during the menstrual cycle. J Clin Endocrinol Metab. 1977 May;44(5):989–993. doi: 10.1210/jcem-44-5-989. [DOI] [PubMed] [Google Scholar]
- Winkel C. A., Gilmore J., MacDonald P. C., Simpson E. R. Uptake and degradation of lipoproteins by human trophoblastic cells in primary culture. Endocrinology. 1980 Dec;107(6):1892–1898. doi: 10.1210/endo-107-6-1892. [DOI] [PubMed] [Google Scholar]
- Winkel C. A., Snyder J. M., MacDonald P. C., Simpson E. R. Regulation of cholesterol and progesterone synthesis in human placental cells in culture by serum lipoproteins. Endocrinology. 1980 Apr;106(4):1054–1060. doi: 10.1210/endo-106-4-1054. [DOI] [PubMed] [Google Scholar]


