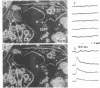
Abstract
The excellent visibility of cultured cells allows the early events during formation of the neuromuscular junction to be suitably studied. It has been shown in various culture systems that synaptic transmission occurs early after nerve-muscle contact. Early synaptic potentials are small in amplitude and slow in time course reflecting a low acetylcholine receptor density at the site of nerve contact. Acetylcholine receptors accumulate later at the contact region. We have examined initial synaptic transmission at the growth cone-muscle contact in Xenopus nerve-muscle cultures. The approaching growth cone was observed under a phase-contrast microscope while the membrane potential of its target muscle cell was continuously monitored by using an intracellular microelectrode. The innervating neuron was stimulated extracellularly at the cell body. No synaptic potential was evoked when the growth cone was contacting the muscle only at the tip of filopodia. However, as soon as the main portion of the growth cone contacted the muscle membrane, nerve-evoked synaptic potentials were detected after stimulation of the nerve. This immediate appearance of synaptic potentials raises the possibility that acetylcholine could be released at the growth cone even prior to contact with muscle cells. As the area of contact enlarged during the observation period the amplitude of end-plate potentials also increased. Spontaneous synaptic potentials (miniature end-plate potentials) were rarely observed in these early growth cone-muscle contacts. Although there were several inherent difficulties, quantal analysis of the end-plate potentials was attempted by using binomial statistics. This analysis suggests that nerve-evoked transmitter release at the growth cone occurs in a quantal fashion.
Full text
PDF
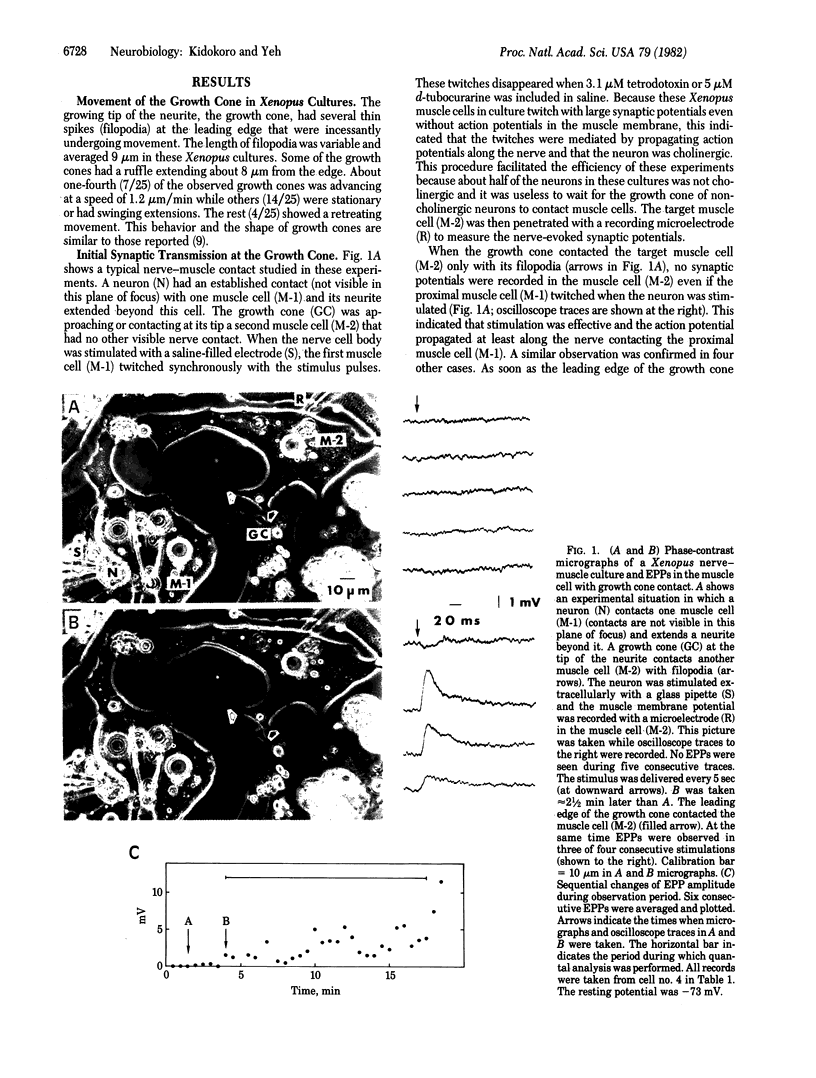
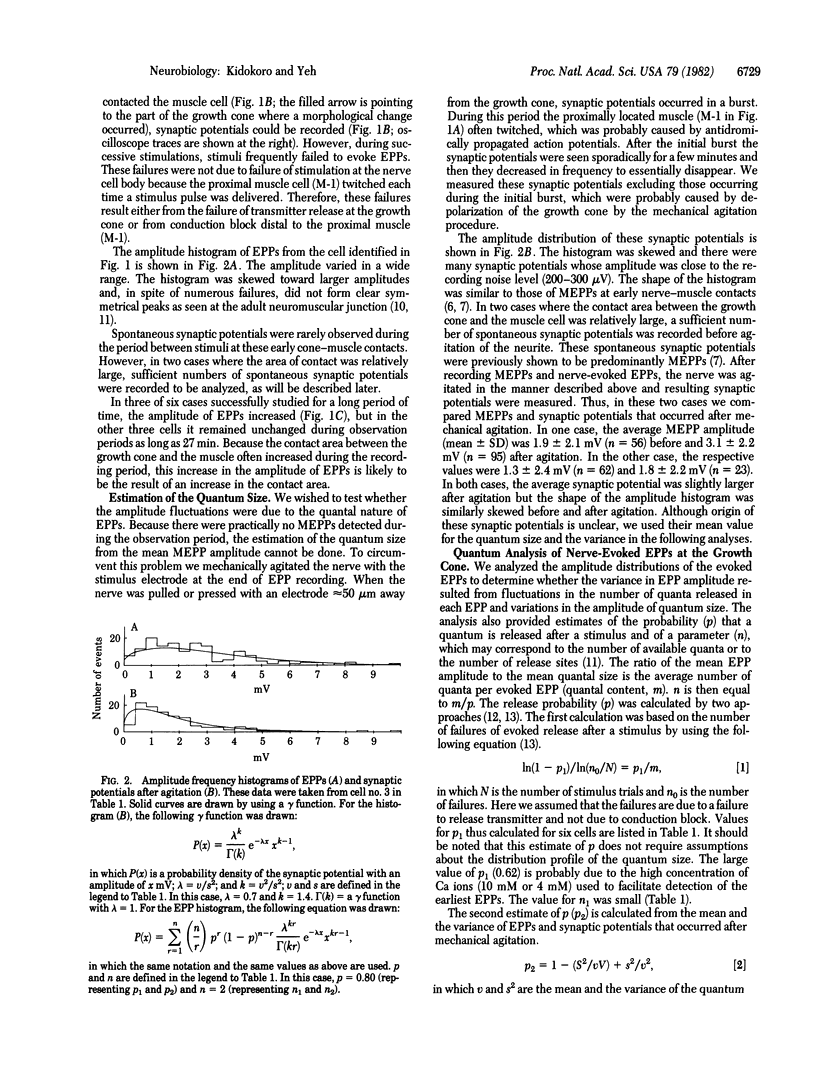


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. J., Kidokoro Y., Gruener R. Correlation between acetylcholine receptor localization and spontaneous synaptic potentials in cultures of nerve and muscle. Brain Res. 1979 Apr 20;166(1):185–190. doi: 10.1016/0006-8993(79)90662-0. [DOI] [PubMed] [Google Scholar]
- BOYD I. A., MARTIN A. R. The end-plate potential in mammalian muscle. J Physiol. 1956 Apr 27;132(1):74–91. doi: 10.1113/jphysiol.1956.sp005503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Florin T., Pettigrew A. G. The effect of calcium ions on the binomial statistic parameters that control acetylcholine release at preganglionic nerve terminals. J Physiol. 1976 Jun;257(3):597–620. doi: 10.1113/jphysiol.1976.sp011387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz W., Osborne M. Effects of innervation on acetylcholine sensitivity of developing muscle in vitro. J Physiol. 1977 Aug;270(1):75–88. doi: 10.1113/jphysiol.1977.sp011939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. A. Early nerve-muscle synapses in vitro release transmitter over postsynaptic membrane having low acetylcholine sensitivity. Proc Natl Acad Sci U S A. 1980 Jan;77(1):644–648. doi: 10.1073/pnas.77.1.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach G. D., Cohen S. A. The distribution of acetylcholine sensitivity over uninnervated and innervated muscle fibers grown in cell culture. Dev Biol. 1973 Mar;31(1):147–162. doi: 10.1016/0012-1606(73)90326-6. [DOI] [PubMed] [Google Scholar]
- Frank E., Fischbach G. D. Early events in neuromuscular junction formation in vitro: induction of acetylcholine receptor clusters in the postsynaptic membrane and morphology of newly formed synapses. J Cell Biol. 1979 Oct;83(1):143–158. doi: 10.1083/jcb.83.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruener R., Kidokoro Y. Acetylcholine sensitivity of innervated and noninnervated Xenopus muscle cells in culture. Dev Biol. 1982 May;91(1):86–92. doi: 10.1016/0012-1606(82)90011-2. [DOI] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S., Dennis M. J., Jan Y., Jan L., Evans L. Synaptic vesicle exocytosis captured by quick freezing and correlated with quantal transmitter release. J Cell Biol. 1979 May;81(2):275–300. doi: 10.1083/jcb.81.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidokoro Y., Anderson M. J., Gruener R. Changes in synaptic potential properties during acetylcholine receptor accumulation and neurospecific interactions in Xenopus nerve-muscle cell culture. Dev Biol. 1980 Aug;78(2):464–483. doi: 10.1016/0012-1606(80)90347-4. [DOI] [PubMed] [Google Scholar]
- Kidokoro Y. Developmental changes of spontaneous synaptic potential properties in the rat neuromuscular contact formed in culture. Dev Biol. 1980 Jul;78(1):231–241. doi: 10.1016/0012-1606(80)90332-2. [DOI] [PubMed] [Google Scholar]
- Kuno M., Turkanis S. A., Weakly J. N. Correlation between nerve terminal size and transmitter release at the neuromuscular junction of the frog. J Physiol. 1971 Mar;213(3):545–556. doi: 10.1113/jphysiol.1971.sp009399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKAI J., KAWASAKI Y. Studies on the mechanism determining the course of nerve fibers in tissue culture. I. The reaction of the growth cone to various obstructions. Z Zellforsch Mikrosk Anat. 1959;51:108–122. doi: 10.1007/BF00345083. [DOI] [PubMed] [Google Scholar]
- NARAHASHI T., MOORE J. W., SCOTT W. R. TETRODOTOXIN BLOCKAGE OF SODIUM CONDUCTANCE INCREASE IN LOBSTER GIANT AXONS. J Gen Physiol. 1964 May;47:965–974. doi: 10.1085/jgp.47.5.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y., Kidokoro Y., Klier F. G. The development of functional neuromuscular junctions in vitro: an ultrastructural and physiological study. Dev Biol. 1980 Jun 1;77(1):52–72. doi: 10.1016/0012-1606(80)90456-x. [DOI] [PubMed] [Google Scholar]
- Obata K. Development of neuromuscular transmission in culture with a variety of neurons and in the presence of cholinergic substances and tetrodotoxin. Brain Res. 1977 Jan 1;119(1):141–153. doi: 10.1016/0006-8993(77)90096-8. [DOI] [PubMed] [Google Scholar]
- Puro D. G., De Mello F. G., Nirenberg M. Synapse turnover: the formation and termination of transient synapses. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4977–4981. doi: 10.1073/pnas.74.11.4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees R. P., Bunge M. B., Bunge R. P. Morphological changes in the neuritic growth cone and target neuron during synaptic junction development in culture. J Cell Biol. 1976 Feb;68(2):240–263. doi: 10.1083/jcb.68.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. Estimation of parameters for a model of transmitter release at synapses. Biometrics. 1976 Mar;32(1):61–68. [PubMed] [Google Scholar]
- Wernig A. Estimates of statistical release parameters from crayfish and frog neuromuscular junctions. J Physiol. 1975 Jan;244(1):207–221. doi: 10.1113/jphysiol.1975.sp010792. [DOI] [PMC free article] [PubMed] [Google Scholar]