Abstract
Macromolecular assemblies can be intrinsically refractive to classical structural analysis, due to their size, complexity, plasticity and dynamic nature. One such assembly is the nuclear pore complex (NPC). The NPC is formed from ~450 copies of 30 different proteins, called nucleoporins, and is the sole mediator of exchange between the nucleus and the cytoplasm in eukaryotic cells. Despite significant progress, it has become increasingly clear that new approaches, integrating different sources of structural and functional data, will be needed to understand the functional biology of the NPC. Here, we discuss the latest approaches trying to address this challenge.
Introduction
The building blocks of cells are a vast set of complex molecular machines, which act coordinately to perform all the processes that define life. These biological machines can be made of many dozens of macromolecular components (as well as accompanying ligands and cofactors), and often can dynamically alter their shape, composition and location in order to perform their functions.
The nuclear pore complex (NPC) is one such intricate and dynamic machine found in all eukaryotes. Each NPC is made from ~450 copies of 30 different proteins (called nucleoporins or Nups), and their overall composition and organization seem conserved [1]. NPCs form selective channels across the double nuclear envelope membrane in eukaryotic cells; as the exclusive gateways connecting the nucleoplasm and the cytoplasm, NPCs support and regulate a huge flow of cellular components, ranging from single proteins to other megadalton-sized molecular machines [2,3]. Transport across the NPC is rapid, regulated, and energy-dependent, involving numerous accessory ‘transport factors’ [4,5]. Many macromolecular cargoes are carried across the NPC by members of a structurally related family of proteins termed Karyopherins (Kaps), which bind to specific import (NLS) or export (NES) signals in their cargos. A GTPase called Ran provides the energy and directionality for Kap-mediated transport. Other, Kap-independent pathways of transport exist, including perhaps the most prominent, the export of mRNAs from the nucleus to the cytoplasm (for more details, see the review from Grunwald and Singer in this issue and [4,5]). The NPC also serves as an anchor for the organization of numerous nuclear processes, and as such can play important roles in organizing nuclear architecture and even the control of gene expression.
As with many of the cell’s biological machines, many different techniques and approaches have over the last few years driven spectacular advances in our understanding of the structure and function of the Nups, transport factors, and other macromolecules that comprise and interact with the NPC. This way, some of the general principles behind nuclear transport have been determined by committed and detailed studies of its individual component players. However, there remains a significant challenge in integrating these insights into an understanding of the nucleocytoplasmic transport mechanism, and in generating a holistic picture of the intricate relationships between the NPC, its surroundings, and its multiple functionalities in the cell.
The situation we are currently in could be compared with the parable of the blind men and the elephant: six blind men were asked to determine what an elephant looked like, just by examining different parts of the elephant’s body. The blind men asserted that the elephant was like a pillar (the one who felt the elephants’ leg), a rope (tail), a tree branch (trunk), a hand fan (ear), a wall (belly) or a pipe (tusk): ‘Each in his own opinion, Exceeding stiff and strong, Though each was partly in the right, And all were in the wrong.’ (The Blind Men and the Elephant’; John Godfrey Saxe). The moral of the story, of course, is that while each had a part of the picture, they needed to come together to agree on a full elephantine structure. Similarly to the parable, while different techniques give us valuable information about specific features of the NPC (Figure 1), only the integration of data coming from different sources, and covering a broad range of resolutions and timescales (to represent the dynamics of the complex) will be able to address all the questions of what this mammoth complex looks like and how it works [6].
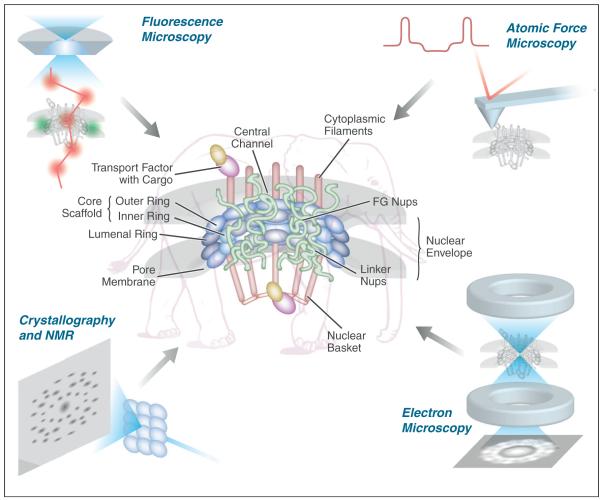
Figure 1.
The NPC, and the traffic through it, has been visualized by many different techniques, representatives of which are shown here: fluorescence microscopy including single molecule techniques, atomic force microscopy, scanning electron microscopy, transmission electron microscopy, molecular modeling, and crystallography and NMR.
Exploring the elephant
Electron microscopists first described the presence of a discrete macromolecular complex residing in pores within the membranous nuclear envelope, and first coined the term ‘nuclear pore complex’. They revealed that the NPC was an octagonally symmetric cylinder, with eight spokes conjoined by coaxial rings, and a central channel through which all cargoes were shown to pass [3]. This overall architecture was also shown to be well conserved in all the eukaryotes analyzed [7,8•,9]. From this ‘core’ structure, eight filaments project from both the nucleoplasmic and the cytoplasmic faces; the nuclear filaments conjoin at a distal ring to form the ‘nuclear basket’. Atomic force microscopy is helping to define the physical and mechanical properties of the NPC, underscoring its extreme flexibility and structural stability [10], and recent advances are opening the possibility of time-lapse analysis of single transport events by this technique [11]. Using immunoelectron microscopy, most Nups were at least roughly localized to portions of the NPC, and importantly such localizations also showed that a particular class of Nups, termed ‘FG Nups’ (because all carry long stretches of Phe-Gly repeats) is found in and around the NPCs central channel [12,13]. Though each FG repeat domain is natively disordered, large numbers of these domains perhaps form a quasi-ordered structure that functions as a ‘virtual gate’, whose dense packing and rapid motion push away nonspecific macromolecules while cargo-carrying transport factors pass across the transporter by jumping from FG docking site to docking site [14–18].
Recently, multiple studies have also generated a wealth of crystallographic and NMR spectroscopy data on numerous Nups and transport factors, opening up the prospect of an atomic-level understanding of nuclear transport. Several fragments from peripheral Nups have been crystallized, either alone or in complexes with transport factors (see [19–21]). But the main targets for crystallographic studies have been the Nups forming the architectural core of the NPC (see below, and the review by Bilokapic and Schwartz published in this same issue). Further advances in atomic resolution techniques will surely give new and exciting insights into the atomic details of Nup arrangements in the near future. However, issues have arisen when piecing these structures back into the NPC; different interpretations of the same homodimeric crystal interfaces, orientation of building blocks, and fitting into the overall NPC structure have led to the proposal of contradictory models for the arrangement of the Nup84 complex building block (the major component of the outer ring; see below and Figure 1) [22,23] and the NPC as a whole [24,25]; moreover, the NPCs from different organisms have species-specific variations in composition and structure that have yet to be understood. We are therefore still a long way from a full architectural understanding of the NPC.
Techniques allowing medium resolution mapping of Nup arrangements and orientations are now adding complementary insights into the NPC structure. Electron microscopy (EM) has provided information about the shape and dimensions of isolated Nups and Nup complexes [26–30]. Fluorescence anisotropy has been used to define in vivo the overall orientation of individual Nups within the NPC [31••]. This way, both yeast Nic96 and human Nup133 were shown to arrange with their long axes approximately parallel to the nuclear envelope plane. These orientations agree with previously published ring arrangements for these Nups within the NPC [28,32,33]. In a classic study, Lutzmann et al. unraveled the overall arrangement of the Nup84 complex using EM and in vitro reconstitution [26]. The same laboratory has recently expanded this approach by sequencing the genome of the thermophilic fungus Chaetomium thermophilum and using the identified fungal Nups for recombinant and ex vivo expression [34••]. The ther-mostable fungal Nups seem to show improved stability for both biochemical and structural characterization, as illustrated by in vitro reconstitution of some core NPC connections and EM analysis of single Nups. By combining these data they were also able to suggest a model for connectivity of some of these Nups [34••], once again underscoring the importance of integration of diverse data sources to piecing together the NPC.
Piecing the elephant together
When considered individually, traditional structural methods, though generating incredibly valuable data, each have their own particular limitations. Thus, crystallography requires that a protein be crystallizable; EM can potentially damage and distort delicate biological structures and often is not high resolution; light microscopy is low resolution on the scale of such assemblies; proteomic methods require interactions to be preserved upon removal from the cellular milieu; and so on. Almost all have difficulties when dealing with flexible or intrinsically disordered regions, which in the NPC represent up to one-third of its mass [13,35]. All approaches also can be challenged by dynamically associating components. While each method gives us one perspective on the NPC, they have been honed by researchers to often provide an exceptional view from that perspective, providing huge amounts of potentially useful data — rather like the very detailed perceptions each of our blind men produces of their part of the elephant. So, how can we piece all these perceptions together into a unified mammoth structure?
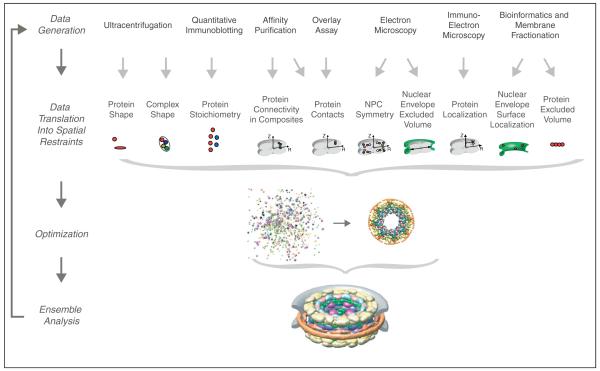
Our laboratory is one of a number that is trying to integrate different kinds of experimental data to generate a holistic view of a macromolecular complex, in our case with a focus on the NPC (Figure 2 and [32,36]). We began by gathering a large and diverse set of proteomic and biophysical data about the stoichiometry, connectivity, shape, and position of each Nup in the NPC. We also determined the dimensions and symmetry of the NPC from our cryoelectron microscopy map [9]. This wealth of complementary structural information was translated into thousands of spatial restraints. Using the computer program MODELLER [37,38] we sought a spatial arrangement of the Nups that optimally fulfilled all these restraints. Our method is similar to the determination of protein structures by NMR spectroscopy, in which a protein’s structure is determined by satisfying distance restraints between pairs of atoms. In our approach, atoms are replaced by Nups, and the size, shape, and relative positions of the Nups are restrained based on a variety of biophysical and biochemical experiments. The resulting best models from tens of thousands of trials were averaged and represented by the corresponding protein density map, which resembled the actual structure of the NPC at ~ 6 nm resolution (or ‘precision’).
Figure 2.
Determining the architecture of the NPC by integrating various types of data. Data from various experiments are translated into ‘spatial restraints’, encoding the information each experiment reveals about the relative position of each component in the NPC. An ensemble of structural solutions that satisfy the data is then obtained by minimizing the violations of the spatial restraints, starting from many different random configurations. This ensemble is analyzed in terms of protein positions, contacts, and configuration [32,36].
The resulting NPC map allowed us to assign particular proteins to previously described NPC structural elements and survey the NPC’s overall design (Figure 1). Several coaxial rings coat the curved surface of the pore membrane in a narrow layer and form the central channel through which macromolecular exchange occurs. The rings are formed by the connection of eight symmetry units called spokes. Each spoke can also be divided into two parallel columns. A history of evolutionary duplication seems reflected in this NPC architecture; every Nup in one column contains a similarly positioned homolog in the adjacent column. There is also evidence for such ancient duplications between the inner and outer rings, suggesting that the bulk of its structure has evolved through extensive gene duplication from a simple precursor set of only a few proteins [1,32].
The NPC structural scaffold, formed by the inner and outer rings, comprises conserved proteins formed almost entirely from either a β-propeller fold, an α-solenoid fold, or a distinctive N-terminal β-propeller/C-terminal α-solenoid (β–α) arrangement [1,39]. Such a β-propeller/ α-solenoid architecture has also been found in the clathrin/adaptin, COPI and COPII vesicle coating complexes, and in membrane tethering and flagellar membrane transport complexes [40–42]. The discovery in NPCs of a membrane-coating scaffold resembling vesicle coating complexes thus allowed us to hypothesize a common evolutionary origin for NPCs and coated vesicles in an early membrane-curving module (the ‘protocoatomer’) that led to the formation of the internal membrane systems in modern eukaryotes [1,32,40,41,43]. Recent studies have highlighted how it appears that upon its innovation, this protocoatomer radiated explosively to generate most of the membrane coating, tethering, and even transporting systems in modern eukaryotes [41]. Atomic structures of exemplar linker [28,44], outer ring [22,23,25,33,45,46,47,48], and inner ring Nups [49] (Figure 1) have upheld these ideas, confirming that indeed β-propellers and topologically variable α-helical solenoid domains are the main structural features of an NPC core with clear similarities to vesicle coating complexes; heterodimeric interaction surfaces unveiled by these studies, as β-propeller invading blades [33,45] and Sec31-like α-solenoid connections [22,23], have further underscored the evolutionary relationship between the NPC and these complexes.
Our first pass demonstrates that a combination of approaches such as the ones described above should be able to generate enough information, from different levels of resolution, to allow their integration into a consensus NPC structure. Moreover, though we used our own data for this, there is nothing preventing the inclusion of many other verified sources of data. But, to avoid inaccuracy, several factors should be carefully considered when trying to apply an integrative approach. First, the initial experimental data should ideally meet the criteria of being ‘correct’ (i.e. free of inaccuracies and false positive inputs), being as comprehensive as possible, originating from multiple sources and describing a single state of the complex [50•]. Second, a very careful — and sometimes conservative — interpretation of the experimental data is required to adequately translate them into spatial restraints. Third, the integration strategy should include checkpoints and assessment steps that ensure the accuracy of the final output; our approach was designed and demonstrated to fail should data be incorrect [32,36]. Finally, one should always consider that machines are only as intelligent as the individual that handles them, so the ultimate checkpoint should always be the scientific insight of the researchers.
What does the elephant do?
A tusk, a tooth, a trunk — individually, each tells only a little of an elephant’s life history. Similarly, structural studies on isolated Nups or small pieces of the NPC can provide only so much insight into the functional roles of the analyzed features. A complementary view, showing how the different structural features support the function of an active NPC, would be needed to address the question of ‘how does an NPC work?’ Conceptual and technical advances are broadening the functional roles of the NPC and opening new ways of gathering such information.
Arguably, the main role of the NPC is to establish this selective barrier between the nucleoplasm and cytoplasm. Kaps bind to their cargos and interact directly with the FG Nups to travel through the NPC. Single molecule imaging techniques are allowing the tracking of these transport events with spatiotemporal resolutions on the order of nanometers and microseconds [51], revealing fascinating features about the biophysical environments within the NPC. High-pressure freezing, combined with low-temperature fixation and immunoelectron microscopy allowed researchers to potentially reveal spatially separated transport routes through the NPC in budding yeast [52], suggesting that import was performed in the radial periphery of the NPC channel, while mRNA export factors travel across the central axis region. Interestingly, deletion of specific FG domains affected certain import pathways but not others, suggesting an even more complex regionalization of the NPC channel. Single-point edge-excitation subdiffraction (SPEED) microscopy analysis performed in permeabilized mammalian cells also suggested separated transport routes through the NPC central channel [53•], indicating that this compartmentalization may be a conserved feature of NPCs. The existence of functionally differentiated environments in the NPC is strengthened by new approaches allowing single molecule detection of in vivo mRNP export kinetics in mammalian cells [54••,55]. Using super registration fluorescence microscopy techniques Grunwald and Singer [54••] were able to define three individual transient steps for mRNPs traveling across the NPC, with a quick translocation event through the central channel and two longer steps spent at both nucleoplasmic and cytoplasmic sides of the NPC. Other studies suggest that import of large size quantum dots-linked cargos in mammalian cells works by reversible substeps through a functionally asymmetric central channel [56•].
Different kinds of functional channels within the NPC have also been revealed by the study of integral membrane protein transport. These proteins could traverse the NPC either by diffusion through adjacent lateral channels or by active transport mediated by Kaps [57]. In both cases, their transmembrane domains have to pass through the pore membrane and, consequently, their soluble domains must move across the NPC core scaffold. Indeed, mutations affecting inner ring Nups show strong integral membrane protein transport defects [58]. In a recent groundbreaking study, Meinema et al. [59••] show that a long intrinsically disordered linker, connecting the transmembrane domain and the soluble Kap-bound domain of the protein, slides through the NPC core scaffold, allowing the transport of the protein into the inner nuclear membrane. This model would imply either the presence of channels across the NPC core scaffold or the transient rearrangement of several core NPC interactions during this transport process.
These different functional environments within the NPC channel and core scaffold must be the result of specific biochemical and structural features, perspectives that lead to questions of how the NPC architecture is defining those functional regions, and how the underlying NPC ‘design’ principles relate to the functional roles of the different regions. Similar questions can be applied to other emerging NPC physiological roles: chromatin association/regulation [60•,61–63], aging [64–67] and transcriptional regulation [68•,69•,70,71]. The challenge remains to, first, tailor functional and structural approaches into sources of data that could be easily integrated into functionally informative maps of the NPC, and second, include a timescale into the integration to show the dynamic behavior of the NPC through its lifespan. Apart from the obvious intellectual challenge of understanding the basics of NPC biology, such integrated structure/function maps would be extremely useful for two key goals of the nuclear transport field: the possibility of designing synthetic devices mimicking key properties of the NPC [72,73] and understanding the role of NPC defects in human aging and disease [74–77].
Conclusions
Cellular life arises from the concerted action of dynamic macromolecular complexes. Understanding the biology of such mammoth assemblies could be made possible by the development of new integrative approaches that, firstly, would elucidate the structure of the assembly, and secondly, would incorporate functional data into the same integrative strategy. The output of these strategies would be functionally informative structures; macromolecular ‘assembly and operation manuals’ that help to understand the functional roles of their different structural features. Ambitious as it sounds, this kind of strategy still does not fully consider a key component of biological systems: dynamics. The incorporation of the temporal dimension remains an additional major challenge for future development of integrative approaches.
Acknowledgements
We wish to acknowledge Andrej Sali and Jeremy Philips for their insights into integrative modeling, and all members of the Rout laboratory for support. We are grateful for the support of the NIH from grants U54 RR022220 (M.R.) and R01 GM62427 (M.R.).
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.DeGrasse JA, DuBois KN, Devos D, Siegel TN, Sali A, Field MC, Rout MP, Chait BT. Evidence for a shared nuclear pore complex architecture that is conserved from the last common eukaryotic ancestor. Mol Cell Proteomics. 2009;8:2119–2130. doi: 10.1074/mcp.M900038-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weis K. Nucleocytoplasmic transport: cargo trafficking across the border. Curr Opin Cell Biol. 2002;14:328–335. doi: 10.1016/s0955-0674(02)00337-x. [DOI] [PubMed] [Google Scholar]
- 3.Wente SR. Gatekeepers of the nucleus. Science. 2000;288:1374–1377. doi: 10.1126/science.288.5470.1374. [DOI] [PubMed] [Google Scholar]
- 4.Peters R. Translocation through the nuclear pore: Kaps pave the way. Bioessays. 2009 doi: 10.1002/bies.200800159. [DOI] [PubMed] [Google Scholar]
- 5.Terry LJ, Shows EB, Wente SR. Crossing the nuclear envelope: hierarchical regulation of nucleocytoplasmic transport. Science. 2007;318:1412–1416. doi: 10.1126/science.1142204. [DOI] [PubMed] [Google Scholar]
- 6.Alber F, Forster F, Korkin D, Topf M, Sali A. Integrating diverse data for structure determination of macromolecular assemblies. Annu Rev Biochem. 2008;77:443–477. doi: 10.1146/annurev.biochem.77.060407.135530. [DOI] [PubMed] [Google Scholar]
- 7.Beck M, Forster F, Ecke M, Plitzko JM, Melchior F, Gerisch G, Baumeister W, Medalia O. Nuclear pore complex structure and dynamics revealed by cryoelectron tomography. Science. 2004;306:1387–1390. doi: 10.1126/science.1104808. [DOI] [PubMed] [Google Scholar]
- 8.Frenkiel-Krispin D, Maco B, Aebi U, Medalia O. Structural analysis of a metazoan nuclear pore complex reveals a fused concentric ring architecture. J Mol Biol. 2010;395:578–586. doi: 10.1016/j.jmb.2009.11.010. • Refinement of the 3D structure of the NPC from Xenopus oocyte achieved by using symmetry-independent averaging of cryo-tomograms containing single asymmetric NPC protomers. The resulting resolution 6.4 nm revealed novel structural features such as the concentric SR rings and supports a remarkable structural plasticity of the NPC.
- 9.Yang Q, Rout MP, Akey CW. Three-dimensional architecture of the isolated yeast nuclear pore complex: functional and evolutionary implications. Mol Cell. 1998;1:223–234. doi: 10.1016/s1097-2765(00)80023-4. [DOI] [PubMed] [Google Scholar]
- 10.Liashkovich I, Meyring A, Kramer A, Shahin V. Exceptional structural and mechanical flexibility of the nuclear pore complex. J Cell Physiol. 2011;226:675–682. doi: 10.1002/jcp.22382. [DOI] [PubMed] [Google Scholar]
- 11.Huang NP, Stubenrauch M, Koser J, Taschner N, Aebi U, Stolz M. Towards monitoring transport of single cargos across individual nuclear pore complexes by time-lapse atomic force microscopy. J Struct Biol. 2010;171:154–162. doi: 10.1016/j.jsb.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Krull S, Thyberg J, Bjorkroth B, Rackwitz HR, Cordes VC. Nucleoporins as components of the nuclear pore complex core structure and Tpr as the architectural element of the nuclear basket. Mol Biol Cell. 2004;15:4261–4277. doi: 10.1091/mbc.E04-03-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rout MP, Aitchison JD, Suprapto A, Hjertaas K, Zhao Y, Chait BT. The yeast nuclear pore complex: composition, architecture, and transport mechanism. J Cell Biol. 2000;148:635–651. doi: 10.1083/jcb.148.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rout MP, Aitchison JD, Magnasco MO, Chait BT. Virtual gating and nuclear transport: the hole picture. Trends Cell Biol. 2003;13:622–628. doi: 10.1016/j.tcb.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Peters R. Translocation through the nuclear pore complex: selectivity and speed by reduction-of-dimensionality. Traffic. 2005;6:421–427. doi: 10.1111/j.1600-0854.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 16.Macara IG. Transport into and out of the nucleus. Microbiol Mol Biol Rev. 2001;65:570–594. doi: 10.1128/MMBR.65.4.570-594.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ribbeck K, Gorlich D. Kinetic analysis of translocation through nuclear pore complexes. EMBO J. 2001;20:1320–1330. doi: 10.1093/emboj/20.6.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel SS, Belmont BJ, Sante JM, Rexach MF. Natively unfolded nucleoporins gate protein diffusion across the nuclear pore complex. Cell. 2007;129:83–96. doi: 10.1016/j.cell.2007.01.044. [DOI] [PubMed] [Google Scholar]
- 19.Brohawn SG, Partridge JR, Whittle JR, Schwartz TU. The nuclear pore complex has entered the atomic age. Structure. 2009;17:1156–1168. doi: 10.1016/j.str.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoelz A, Debler EW, Blobel G. The structure of the nuclear pore complex. Annu Rev Biochem. 2011;80:613–643. doi: 10.1146/annurev-biochem-060109-151030. [DOI] [PubMed] [Google Scholar]
- 21.Suel KE, Cansizoglu AE, Chook YM. Atomic resolution structures in nuclear transport. Methods. 2006;39:342–355. doi: 10.1016/j.ymeth.2006.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brohawn SG, Schwartz TU. Molecular architecture of the Nup84-Nup145C-Sec13 edge element in the nuclear pore complex lattice. Nat Struct Mol Biol. 2009 doi: 10.1038/nsmb.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagy V, Hsia KC, Debler EW, Kampmann M, Davenport AM, Blobel G, Hoelz A. Structure of a trimeric nucleoporin complex reveals alternate oligomerization states. Proc Natl Acad Sci U S A. 2009;106:17693–17698. doi: 10.1073/pnas.0909373106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brohawn SG, Schwartz TU. A lattice model of the nuclear pore complex. Commun Integr Biol. 2009;2:205–207. doi: 10.4161/cib.2.3.7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Debler EW, Ma Y, Seo HS, Hsia KC, Noriega TR, Blobel, Hoelz A. A fence-like coat for the nuclear pore membrane. Mol Cell. 2008;32:815–826. doi: 10.1016/j.molcel.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Lutzmann M, Kunze R, Buerer A, Aebi U, Hurt E. Modular self-assembly of a Y-shaped multiprotein complex from seven nucleoporins. EMBO J. 2002;21:387–397. doi: 10.1093/emboj/21.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lutzmann M, Kunze R, Stangl K, Stelter P, Toth KF, Bottcher B, Hurt E. Reconstitution of Nup157 and Nup145N into the Nup84 complex. J Biol Chem. 2005;280:18442–18451. doi: 10.1074/jbc.M412787200. [DOI] [PubMed] [Google Scholar]
- 28.Schrader N, Stelter P, Flemming D, Kunze R, Hurt E, Vetter IR. Structural basis of the nic96 subcomplex organization in the nuclear pore channel. Mol Cell. 2008;29:46–55. doi: 10.1016/j.molcel.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 29.Siniossoglou S, Lutzmann M, Santos-Rosa H, Leonard K, Mueller S, Aebi U, Hurt E. Structure and assembly of the Nup84p complex. J Cell Biol. 2000;149:41–54. doi: 10.1083/jcb.149.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kampmann M, Blobel G. Three-dimensional structure and flexibility of a membrane-coating module of the nuclear pore complex. Nat Struct Mol Biol. 2009;16:782–788. doi: 10.1038/nsmb.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kampmann M, Atkinson CE, Mattheyses AL, Simon SM. Mapping the orientation of nuclear pore proteins in living cells with polarized fluorescence microscopy. Nat Struct Mol Biol. 2011;18:643–649. doi: 10.1038/nsmb.2056. •• Through a novel strategy, using fluorescence anisotropy to define the orientation of nucleoporins within the NPC, the authors are able to confirm previously suggested arrangements for core NPC building blocks and for the mammalian Nup84 complex.
- 32.Alber F, Dokudovskaya S, Veenhoff L, Zhang W, Kipper J, Devos D, Suprapto A, Karni-Schmidt O, Williams R, Chait B, et al. The molecular architecture of the nuclear pore complex. Nature. 2007;450:695–701. doi: 10.1038/nature06405. [DOI] [PubMed] [Google Scholar]
- 33.Hsia KC, Stavropoulos P, Blobel G, Hoelz A. Architecture of a coat for the nuclear pore membrane. Cell. 2007;131:1313–1326. doi: 10.1016/j.cell.2007.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amlacher S, Sarges P, Flemming D, van Noort V, Kunze R, Devos DP, Arumugam M, Bork P, Hurt E. Insight into structure and assembly of the nuclear pore complex by utilizing the genome of a eukaryotic thermophile. Cell. 2011;146:277–289. doi: 10.1016/j.cell.2011.06.039. •• This paper reports a novel strategy consisting in sequencing the genome of the thermophilic fungus Chaetomium thermophilum and using its biochemically stable nucleoporins to define the connectivity and EM shapes of components of the NPC inner ring complex. A model for the arrangement of this NPC core complex is suggested.
- 35.Cronshaw JM, Krutchinsky AN, Zhang W, Chait BT, Matunis MJ. Proteomic analysis of the mammalian nuclear pore complex. J Cell Biol. 2002;158:915–927. doi: 10.1083/jcb.200206106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alber F, Dokudovskaya S, Veenhoff L, Zhang W, Kipper J, Devos D, Suprapto A, Karni-Schmidt O, Williams R, Chait B, et al. Determining the architectures of macromolecular assemblies. Nature. 2007;450:683–694. doi: 10.1038/nature06404. [DOI] [PubMed] [Google Scholar]
- 37.Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 38.Alber F, Kim M, Sali A. Structural characterization of assemblies from overall shape and subcomplex compositions. Structure. 2005;13:435–445. doi: 10.1016/j.str.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 39.Field MC, Dacks JB. First and last ancestors: reconstructing evolution of the endomembrane system with ESCRTs, vesicle coat proteins, and nuclear pore complexes. Curr Opin Cell Biol. 2009;21:4–13. doi: 10.1016/j.ceb.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 40.Devos D, Dokudovskaya S, Alber F, Williams R, Chait BT, Sali A, Rout MP. Components of coated vesicles and nuclear pore complexes share a common molecular architecture. PLoS Biol. 2004;2:e380. doi: 10.1371/journal.pbio.0020380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Field MC, Sali A, Rout MP. Evolution: on a bender — BARs, ESCRTs, COPs, and finally getting your coat. J Cell Biol. 2011;193:963–972. doi: 10.1083/jcb.201102042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dokudovskaya S, Waharte F, Schlessinger A, Pieper U, Devos DP, Cristea IM, Williams R, Salamero J, Chait BT, Sali A, et al. A conserved coatomer-related complex containing Sec13 and Seh1 dynamically associates with the vacuole in Saccharomyces cerevisiae. Mol Cell Proteomics. 2011;10 doi: 10.1074/mcp.M110.006478. M110.006478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Devos D, Dokudovskaya S, Williams R, Alber F, Eswar N, Chait BT, Rout MP, Sali A. Simple fold composition and modular architecture of the nuclear pore complex. Proc Natl Acad Sci U S A. 2006;103:2172–2177. doi: 10.1073/pnas.0506345103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jeudy S, Schwartz TU. Crystal structure of nucleoporin Nic96 reveals a novel, intricate helical domain architecture. J Biol Chem. 2007;282:34904–34912. doi: 10.1074/jbc.M705479200. [DOI] [PubMed] [Google Scholar]
- 45.Brohawn SG, Leksa NC, Spear ED, Rajashankar KR, Schwartz TU. Structural evidence for common ancestry of the nuclear pore complex and vesicle coats. Science. 2008;322:1369–1373. doi: 10.1126/science.1165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seo HS, Ma Y, Debler EW, Wacker D, Kutik S, Blobel G, Hoelz A. Structural and functional analysis of Nup120 suggests ring formation of the Nup84 complex. Proc Natl Acad Sci U S A. 2009;106:14281–14286. doi: 10.1073/pnas.0907453106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boehmer T, Jeudy S, Berke IC, Schwartz TU. Structural and functional studies of Nup107/Nup133 interaction and its implications for the architecture of the nuclear pore complex. Mol Cell. 2008;30:721–731. doi: 10.1016/j.molcel.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leksa NC, Brohawn SG, Schwartz TU. The structure of the scaffold nucleoporin Nup120 reveals a new and unexpected domain architecture. Structure. 2009;17:1082–1091. doi: 10.1016/j.str.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whittle JR, Schwartz TU. Architectural nucleoporins Nup157/ 170 and Nup133 are structurally related and descend from a second ancestral element. J Biol Chem. 2009 doi: 10.1074/jbc.M109.023580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lasker K, Phillips JL, Russel D, Velazquez-Muriel J, Schneidman-Duhovny D, Tjioe E, Webb B, Schlessinger A, Sali A. Integrative structure modeling of macromolecular assemblies from proteomics data. Mol Cell Proteomics. 2010;9:1689–1702. doi: 10.1074/mcp.R110.000067. • This deep and comprehensive review propose a unified approach for integrative modeling that is able to incorporate any type of information about a macromolecular assembly into the determination of its structure.
- 51.Tu LC, Musser SM. Single molecule studies of nucleocytoplasmic transport. Biochim Biophys Acta. 2011;1813:1607–1618. doi: 10.1016/j.bbamcr.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fiserova J, Richards SA, Wente SR, Goldberg MW. Facilitated transport and diffusion take distinct spatial routes through the nuclear pore complex. J Cell Sci. 2010;123:2773–2780. doi: 10.1242/jcs.070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma J, Yang W. Three-dimensional distribution of transient interactions in the nuclear pore complex obtained from single-moleculesnapshots. ProcNatl Acad Sci USA. 2010;107:7305–7310. doi: 10.1073/pnas.0908269107. • Using SPEED microscopy, the authors were able to map the transient interactions occurring during NPC transport events at a higher spatiotemporal resolution than reached before. The three-dimensional mapping of the detected interactions reveals a series of functional environments within the NPC transport channel.
- 54.Grunwald D, Singer RH. In vivo imaging of labelled endogenous beta-actin mRNA during nucleocytoplasmic transport. Nature. 2010;467:604–607. doi: 10.1038/nature09438. •• This paper describes for the first time the kinetics of a single mRNA on its transit through the NPC with millisecond resolution. Three steps, with different time intervals, are described for the process of translocation through the NPC.
- 55.Mor A, Suliman S, Ben-Yishay R, Yunger S, Brody Y, Shav-Tal Y. Dynamics of single mRNP nucleocytoplasmic transport and export through the nuclear pore in living cells. Nat Cell Biol. 2010;12:543–552. doi: 10.1038/ncb2056. [DOI] [PubMed] [Google Scholar]
- 56.Lowe AR, Siegel JJ, Kalab P, Siu M, Weis K, Liphardt JT. Selectivity mechanism of the nuclear pore complex characterized by single cargo tracking. Nature. 2010;467:600–603. doi: 10.1038/nature09285. • The authors analyze the NPC translocation of large, quantum dot-fused cargos, suggesting how NPC selectivity arises from the combination of reversible and irreversible steps.
- 57.Lusk CP, Blobel G, King MC. Highway to the inner nuclear membrane: rules for the road. Nat Rev Mol Cell Biol. 2007;8:414–420. doi: 10.1038/nrm2165. [DOI] [PubMed] [Google Scholar]
- 58.Theerthagiri G, Eisenhardt N, Schwarz H, Antonin W. The nucleoporin Nup188 controls passage of membrane proteins across the nuclear pore complex. J Cell Biol. 2010;189:1129–1142. doi: 10.1083/jcb.200912045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meinema AC, Laba JK, Hapsari RA, Otten R, Mulder FA, Kralt A, van den Bogaart G, Lusk CP, Poolman B, Veenhoff LM. Long unfolded linkers facilitate membrane protein import through the nuclear pore complex. Science. 2011;333:90–93. doi: 10.1126/science.1205741. •• This study shows how NLS-driven integral nuclear membrane nuclear transport requires natively unfolded linkers connecting the transmembrane domain and the NLS. The length of the linker needed to be enough to reach from the NPC membrane to the central transport channel.
- 60.Ahmed S, Brickner DG, Light WH, Cajigas I, McDonough M, Froyshteter AB, Volpe T, Brickner JH. DNA zip codes control an ancient mechanism for gene targeting to the nuclear periphery. Nat Cell Biol. 2010;12:111–118. doi: 10.1038/ncb2011. • This paper describes the presence of gene recruitment sequences in the promoter of the INO1 gene. This DNA sequences mediate targeting of genes to the nuclear periphery through interactions with NPC components. This gene targeting mechanism seems to be a general and conserved cellular feature.
- 61.Kalverda B, Fornerod M. Characterization of genome–nucleoporin interactions in Drosophila links chromatin insulators to the nuclear pore complex. Cell Cycle. 2010;9:4812–4817. doi: 10.4161/cc.9.24.14328. [DOI] [PubMed] [Google Scholar]
- 62.Liang Y, Hetzer MW. Functional interactions between nucleoporins and chromatin. Curr Opin Cell Biol. 2011;23:65–70. doi: 10.1016/j.ceb.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Light WH, Brickner DG, Brand VR, Brickner JH. Interaction of a DNA zip code with the nuclear pore complex promotes H2A.Z. incorporation and INO1 transcriptional memory. Mol Cell. 2010;40:112–125. doi: 10.1016/j.molcel.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.D’Angelo MA, Raices M, Panowski SH, Hetzer MW. Age-dependent deteriorationofnuclear porecomplexes causes a lossofnuclear integrity in postmitotic cells. Cell. 2009;136:284–295. doi: 10.1016/j.cell.2008.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Khmelinskii A, Keller PJ, Lorenz H, Schiebel E, Knop M. Segregation of yeast nuclear pores. Nature. 2010;466:E1. doi: 10.1038/nature09255. [DOI] [PubMed] [Google Scholar]
- 66.Khmelinskii A, Meurer M, Knop M, Schiebel E. Artificial tethering to nuclear pores promotes partitioning of extrachromosomal DNA during yeast asymmetric cell division. Curr Biol. 2011;21:R17–R18. doi: 10.1016/j.cub.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 67.Shcheprova Z, Baldi S, Frei SB, Gonnet G, Barral Y. A mechanism for asymmetric segregation of age during yeast budding. Nature. 2008;454:728–734. doi: 10.1038/nature07212. [DOI] [PubMed] [Google Scholar]
- 68.Capelson M, Liang Y, Schulte R, Mair W, Wagner U, Hetzer MW. Chromatin-bound nuclear pore components regulate gene expression in higher eukaryotes. Cell. 2010;140:372–383. doi: 10.1016/j.cell.2009.12.054. • Together with Ref. [69 •] these papers show for the first time evidence that certain Drosophila nups are able to act separately from the NPC, directly interacting with non-NPC tethered chromatin in the nucleoplasm and regulating the transcriptional state of genes. Moreover, the nup-mediated regulation seems to predominantly target genes involved in developmental regulation and the cell cycle.
- 69.Kalverda B, Pickersgill H, Shloma VV, Fornerod M. Nucleoporins directly stimulate expression of developmental and cell-cycle genes inside the nucleoplasm. Cell. 2010;140:360–371. doi: 10.1016/j.cell.2010.01.011. • See comment in Ref. [68•].
- 70.Kohler A, Hurt E. Gene regulation by nucleoporins and links to cancer. Mol Cell. 2010;38:6–15. doi: 10.1016/j.molcel.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 71.Strambio-De-Castillia C, Niepel M, Rout MP. The nuclear pore complex: bridging nuclear transport and gene regulation. Nat Rev Mol Cell Biol. 2010;11:490–501. doi: 10.1038/nrm2928. [DOI] [PubMed] [Google Scholar]
- 72.Jovanovic-Talisman T, Tetenbaum-Novatt J, McKenney AS, Zilman A, Peters R, Rout MP, Chait BT. Artificial nanopores that mimic the transport selectivity of the nuclear pore complex. Nature. 2009;457:1023–1027. doi: 10.1038/nature07600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kowalczyk SW, Kapinos L, Blosser TR, Magalhaes T, van Nies P, Lim RY, Dekker C. Single-molecule transport across an individual biomimetic nuclear pore complex. Nat Nanotechnol. 2011;6:433–438. doi: 10.1038/nnano.2011.88. [DOI] [PubMed] [Google Scholar]
- 74.Chahine MN, Pierce GN. Therapeutic targeting of nuclear protein import in pathological cell conditions. Pharmacol Rev. 2009;61:358–372. doi: 10.1124/pr.108.000620. [DOI] [PubMed] [Google Scholar]
- 75.Funasaka T, Wong RW. The role of nuclear pore complex in tumor microenvironment and metastasis. Cancer Metastasis Rev. 2011;30:239–251. doi: 10.1007/s10555-011-9287-y. [DOI] [PubMed] [Google Scholar]
- 76.Woodward CL, Chow SA. The nuclear pore complex: a new dynamic in HIV-1 replication. Nucleus. 2010;1:18–22. doi: 10.4161/nucl.1.1.10571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu S, Powers MA. Nuclear pore proteins and cancer. Semin Cell Dev Biol. 2009;20:620–630. doi: 10.1016/j.semcdb.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]