Abstract
Reactive oxygen species (ROS) have been implicated in the pathogenesis of pulmonary hypertension. Since iron is an important regulator of ROS biology, the present study examined the effect of iron chelation on the development of pulmonary vascular remodeling. The administration of an iron chelator, deferoxamine, to rats prevented chronic hypoxia-induced pulmonary hypertension and pulmonary vascular remodeling. Various iron chelators inhibited growth of cultured pulmonary artery smooth muscle cells. Protein carbonylation, an important iron-dependent biological event, was promoted in association with pulmonary vascular remodeling and cell growth. A proteomic approach identified that Rho GDP-dissociation inhibitor (a negative regulator of RhoA) is carbonylated. In human plasma, the protein carbonyl content was significantly higher in patients with idiopathic pulmonary arterial hypertension than in healthy controls. These results suggest that iron plays an important role in the ROS-dependent mechanism underlying the development of pulmonary hypertension.
Keywords: carbonylation, iron, pulmonary hypertension, reactive oxygen species
Introduction
Pulmonary hypertension is a devastating disease that currently has no therapeutic strategies for prevention or cure.1 The disease is characterized by increased pulmonary vascular resistance, which translates into pressure overload of the right ventricle, with progressive hypertrophy and dilation, followed by right heart failure and death. While different types of pulmonary hypertension have different pathologic features, structural remodeling of the pulmonary arteries, in part, due to the growth of pulmonary artery smooth muscle are common characteristics.1
Accumulating evidence suggests that reactive oxygen species (ROS) are involved in cell signaling mechanisms,2 including those which trigger the growth of pulmonary artery smooth muscle cells (SMCs).3–6 Patients with pulmonary arterial hypertension (PAH) are under oxidative stress as indicated by increased lipid peroxidation,7 increased DNA oxidation,8 and decreased levels of tocopherols and carotenoids.9
Sequential reduction of molecular oxygen produces ROS, which include superoxide anion radical (·O2−), hydrogen peroxide (H2O2), and hydroxyl radicals (HO·). ·O2− is formed through various pathways, some of which includes heme-containing NAD(P)H oxidase. Notably, NAD(P)H oxidase has been shown to participate in the development of hypoxia-induced pulmonary hypertension.10–13 H2O2 is reduced to HO· via reduced metal ions such as iron in a Fenton reaction. Iron ions are normally present in cytosol in their oxidized form, ferric ion (Fe3+). During a ·O2−-driven Fenton reaction, Fe3+ is reduced to ferrous ions (Fe2+) with subsequent HO· formation.14 HO· is a highly reactive oxidant, which can cause lipid peroxidation, DNA oxidation, and protein oxidation such as carbonylation.14 These multiple functions of iron in formation of ROS suggest that it may play a role in the development of conditions in which oxidative stress is present, such as PAH. Thus, inhibiting iron-catalyzed oxidation may have potential therapeutic effects.
In contrast with the hypothesis that iron may promote pulmonary hypertension through increased oxidative stress, studies using iron chelators mimicked hypoxia,15,16 and promoted pulmonary hypertension. In addition, studies on healthy volunteers have shown that iron chelators can increase hypoxia-induced pulmonary vasoconstriction.15,17,18 More recently, iron deficiency was reported to be common in patients with PAH,19–22 although a cause and effect was not established.
To clarify the role of iron in pulmonary hypertension, the present study tested the hypothesis that iron chelators inhibit pulmonary vascular SMC growth and pulmonary vascular remodeling of experimental pulmonary hypertension. Furthermore, we hypothesized that protein carbonylation, which is an iron-catalyzed protein oxidation process, would occur in response to the stimulation of cultured pulmonary artery SMCs with mediators of pulmonary hypertension, as well as in response to chronic hypoxia in intact rats. We also sought evidence of increased carbonylation in the plasma of patients with PAH.
Materials and Method
Animal model of pulmonary hypertension
Male Sprague Dawley rats (275 – 300 g) were subjected to chronic sustained hypoxia in a chamber regulated by an OxyCycler Oxygen Profile Controller (BioSpherix, Redfield, NY) that was set to maintain 10% O2 for 24 h a day. Normoxic controls were subjected to ambient 21% O2 in another chamber. Animals were fed normal rat chow. Some animals were injected daily with saline or 20 mg/kg body weight of deferoxamine mesylate (Sigma-Aldrich, St. Louis, MO) via intraperitoneal (i.p.) injection during 2-week normoxic or hypoxic treatment. The injection began the day before the initiation of hypoxic treatment. Georgetown University Animal Care and Use Committee approved all animal experiments, and the investigation conforms to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
At the end of the normoxic or hypoxic treatment, rats were anesthetized with intraperitoneal injection of xylazine (10 mg/kg) and ketamine (100 mg/kg). The rats were intubated and mechanically ventilated with a volume-controlled Inspira Advanced Safety Ventilator (Harvard Apparatus, Holliston, MA). Rats were maintained on a heat pad; their temperature was kept at 37°C using a TR-200 Temperature Controller connected to a rectal probe (Fine Scientific Tools, North Vancouver, Canada). After a thoracotomy through the third left intercostal space, a Millar catheter (1.4 F) was inserted to the right ventricle. Right ventricular pressure and right ventricular force (dP/dtmax) were recorded using PowerLab with Chart 5 software (ADInstruments, Colorado Springs, CO). Hearts and lungs were surgically removed. The right ventricle (RV), septum (S), and left ventricle (LV) were weighed, and the Fulton Index value RV/(LV+S) was calculated as an indication of right ventricular hypertrophy. For histological analysis, lungs were incubated in 10% neutral buffered formalin solution at 4°C for 24 h and were then embedded in paraffin. Embedded tissues were cut into 8-μm-thick slices and mounted on glass slides. Tissue sections were stained with hematoxylin and eosin (H & E) for microscopic evaluation at 400x magnification. The values of wall thickness and radius of pulmonary arteries were determined using the IP Lab Software (Scanalytics, Fairfax, VA). The values for % wall thickness, (wall thickness)/(radius), were calculated.
Cell culture
Human pulmonary artery SMCs were purchased from Cell Applications (San Diego, CA) and used in accordance with the manufacture’s instructions. Bovine pulmonary artery SMCs were isolated and maintained in an RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin, 0.5% fungisone, and 2.5 μg/mL plasmocin at 5% CO2 and 37°C as previously described (Wong et al., 2008). Cells were starved overnight in 0.01% FBS-containing medium before treatment with 30 nmol/L ET-1 (Sigma), 1 μmol/L serotonin (5-HT; Sigma) or 10 ng/mL platelet-derived growth factor-BB (PDGF; BioSource International, Inc., Camarillo, CA).
To determine cell growth, a TACS XTT cell proliferation assay (Trevigen, Gaithersburg, MD) was performed in accordance with the manufacturer’s instructions. Cells were grown to 50% confluence and then growth-arrested before being treated with inhibitors and mitogens. Cells were washed with PBS, and XTT working solution was added for 4 h incubation at 37°C. Absorbance values were measured at 450 nm using a SpectraMax 340 Absorbance Microplate Reader (Molecular Devices, Sunnyvale, CA).
Patients and control subjects
Patients attending the outpatient Pulmonary Hypertension Clinic at the Rhode Island Hospital (Providence, RI) were recruited for prospective studies.9 Volunteers were included in the study if they had a mean pulmonary arterial pressure of >25 mm Hg, a pulmonary arterial wedge pressure of <15 mmHg, and if they had not received treatment for IPAH, such as calcium-channel blockers, prostacyclins, or endothelin-receptor antagonists. Patients were excluded if they had other forms of pulmonary hypertension, such as pulmonary hypertension related to connective tissue disease, porto-pulmonary hypertension, HIV-related pulmonary hypertension, or pulmonary hypertension secondary to interstitial lung disease, chronic obstructive lung disease, thromboembolic disease, or left heart failure. Patients were also excluded if they consumed ethanol daily, had a body mass index (BMI) <20 or >30 kg/m2, or if they had a history of liver disease or abnormal liver function tests. Control subjects were matched for age and gender and were healthy, receiving no medication and having no chronic, underlying medical conditions. The Institutional Review Board at Rhode Island Hospital and Georgetown University approved the protocols, and all participants gave written, informed consent. Samples were stored at −80 to −70°C until analyses.
Gel electrophoresis and Western blotting
Equal amounts of protein were electrophoresed through a reducing SDS polyacrylamide gel and electroblotted onto a membrane. The membrane was blocked and incubated with antibodies for ferritin heavy chain, transferrin, RhoGDIα, MYPT1 (Santa Cruz Biotechnology, Santa Cruz, CA), and phospho-MYPT1 (Cell Signaling Technology, Danvers, MA). Levels of proteins were detected using horseradish peroxidase (HRP)-linked secondary antibodies and an Enhanced Chemiluminescence (ECL) System (Amersham Life Science, Arlington Heights, IL).
To detect carbonylated proteins, equal amounts of protein were denatured with SDS and derivatized with 2,4-dinitrophenylhydrazine (DNPH) to form the 2,4-dinitrophenyl (DNP) hydrazone derivative using the Oxyblot Protein Oxidation Detection Kit (Millipore, Billerica, MA) and were electrophoresed through a reducing 12% SDS polyacrylamide gel and electroblotted onto a nitrocellulose membrane.6 The membrane was blocked with 1% BSA and incubated with the rabbit polyclonal IgG for DNP (Oxyblot Kit), followed by incubation with HRP-linked secondary antibodies and ECL. Carbonyl contents were determined using densitometry analysis of band areas.
For 2-dimesional gel electrophoresis, proteins were denatured with 6% SDS and derivatized with DNPH, precipitated with 15% trichloroacetic acid, and washed three times with ethanol:ethyl acetate solution. Protein pellets were dissolved in a rehydration buffer and loaded onto IPG strips (pH 3 – 10 or 5 – 8) for isoelectric focusing of proteins for the first separation using a PROTEAN IEF cell (Bio-Rad Laboratories, Hercules, CA). The strip was then electrophoresed through a reducing 10.4–14% SDS polyacrylamide gel and electroblotted onto a nitrocellulose membrane. The membrane was blocked with 1% BSA and incubated with rabbit polyclonal IgG for DNP in order to detect carbonylated proteins with the HRP-linked secondary antibody and ECL.6
Mass spectrometry
Mass spectrometric analyses were performed using excised gel spots from Coomassie Blue-stained two-dimensional gels as previously described.6 Excised gel pieces were transferred to a 96-well ZipPlate (Millipore) and destained with 50% acetonitrile in 25 mmol/L ammonium bicarbonate, dehydrated with acetonitrile for 15 min, vacuum dried, and then rehydrated with ammonium bicarbonate (25 mmol/L) supplemented with trypsin (5 ng/μL, Promega, Madison, WI, USA) at 30°C for 16 h. Tryptic peptides were then extracted in 0.2% TFA and captured by C18 resin at the bottom of each well. Peptides were then eluted in 0.1% TFA/50% acetonitrile, which contains 2.5 mg/mL CHCA (Acros Organics, Morris Plains, NJ, USA). Mass spectra were recorded with a matrix-assisted laser desorption/ionization time-of-flight, time-of-flight (MALDI-TOF-TOF) spectrometer (4800 Proteomics Analyzer, Framingham, MA, USA) set in reflector-positive mode and onto the plate of which the samples were spotted. The samples were ionized with a fixed laser intensity of 3,500 J, and 1,000 laser shots were collected per spectrum. The detector voltage was 2.1 kvolts, the bin size was set at 0.5 nsec, and the signal/noise threshold was set at 10. The spectra were collected with a specified mass range of 799 – 4,000 daltons, with a focus mass of 2,000 daltons. Peptide masses were compared with the theoretical masses derived from the sequences contained in SWISS-PROT/NCBI databases using MASCOT.
Statistical analysis
Comparisons between 2 groups were analyzed by a two-tailed Student’s t test, and comparisons between 3 or more groups were analyzed by ANOVA with a Student-Newman-Keuls post-hoc test. p < 0.05 was considered to be significant.
Results
Effects of iron chelation on pulmonary vascular remodeling and SMC growth
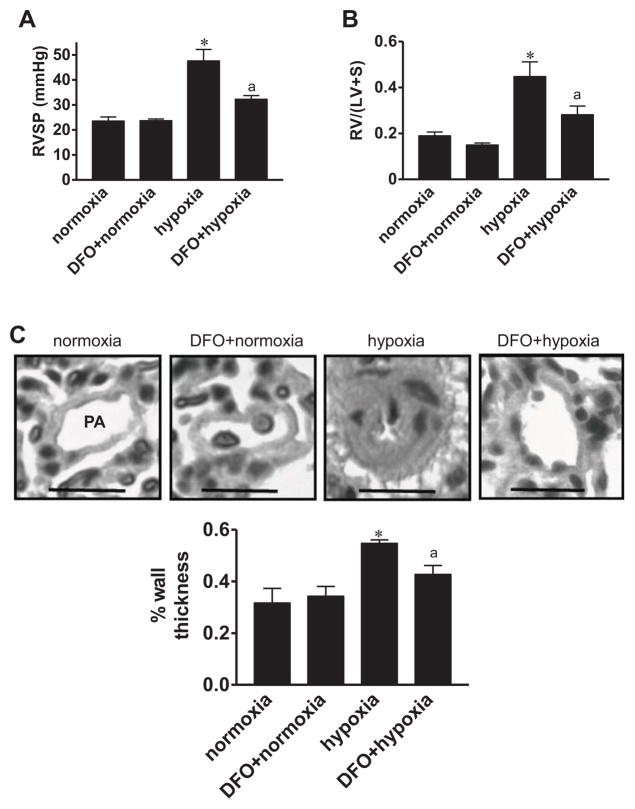
To study the role of iron in pulmonary hypertension and pulmonary vascular remodeling, rats were subjected to chronic hypoxia at 10% O2 and were intraperitoneally injected with deferoxamine (20 mg/kg body weight) once a day for 2 wks during exposure to chronic hypoxia treatment. Deferoxamine attenuated the elevation in right ventricular pressure (Fig. 1A), right ventricular hypertrophy as indicated by the Fulton Index (Fig. 1B), and pulmonary vascular remodeling of small (Fig. 1C) and large (Fig. S1) pulmonary arteries. The right ventricular contractility as measured by dP/dtmax was unaltered (Fig. S2). Deferoxamine did not influence pulmonary arterial ferritin levels (Fig. S3). In separate experiments, rats were first subjected to chronic hypoxia for 2 weeks to develop pulmonary vascular remodeling, then given deferoxamine, and placed back in the hypoxia environment. Within 3 days of deferoxamine administration, pulmonary vascular thickness was significantly reduced (Fig. S4).
Fig. 1. Effects of deferoxamine (DFO) on chronic hypoxia-induced pulmonary hypertension in intact rats.
Rats were intraperitoneally injected daily with saline (vehicle control) or 20 mg/kg body weight of DFO during 2-week exposure to normoxia or hypoxia (10% O2). (A) Right ventricular systolic pressure (RVSP) was measured using a Millar catheter (n = 3). (B) RV/(LV+S) values (n = 3 – 6). (C) H & E staining showing small pulmonary arteries (PA) and arterioles with the diameter ranging 53–113 μm. Scale bars, 50 μm. The bar graph represents means ± SEM of % wall thickness (n = 3). *, P<0.05 vs. normoxia; a, P<0.05 vs. hypoxia.
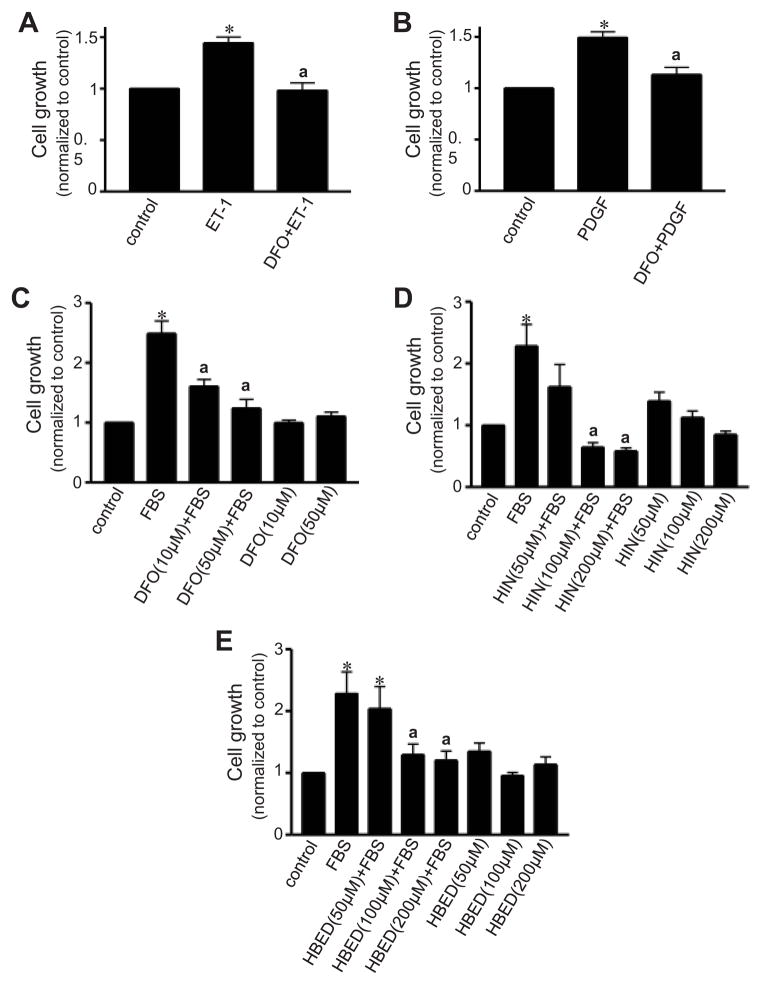
To test the hypothesis that iron chelation may influence the growth of pulmonary artery SMCs, cultured pulmonary artery SMCs were pre-treated with various iron chelators before being stimulated with cell-growth promoting agents and metabolically active living cells were monitored. Deferoxamine inhibited cell growth induced by endothelin-1 (ET-1; Fig. 2A), platelet-derived growth factor (PDGF; Fig. 2B), and fetal bovine serum (FBS; Fig. 2C). Cell growth was also inhibited by other iron chelators, indicating a possible class effect (Figs. 2D & 2E).
Fig. 2. Effects of metal chelators on pulmonary artery SMC growth.
Growth-arrested bovine pulmonary artery SMCs were pre-treated for 30 min with (A, B, and C) deferoxamine (DFO), (D) hinokitiol (HIN), and (E) HBED. Cells were then treated with (A) 30 nM ET-1 for 6 days, (B) 10 ng/ml PDGF for 6 days, or (C, D & E) 10% FBS for 3 days. Cell growth was monitored by XTT assay. Bar graphs represent means ± SEM (n = 7 – 24). *, P<0.05 vs. untreated; a, P<0.05 vs. ET-1, PDGF or FBS.
Occurrence of metal-catalyzed oxidation in pulmonary hypertension
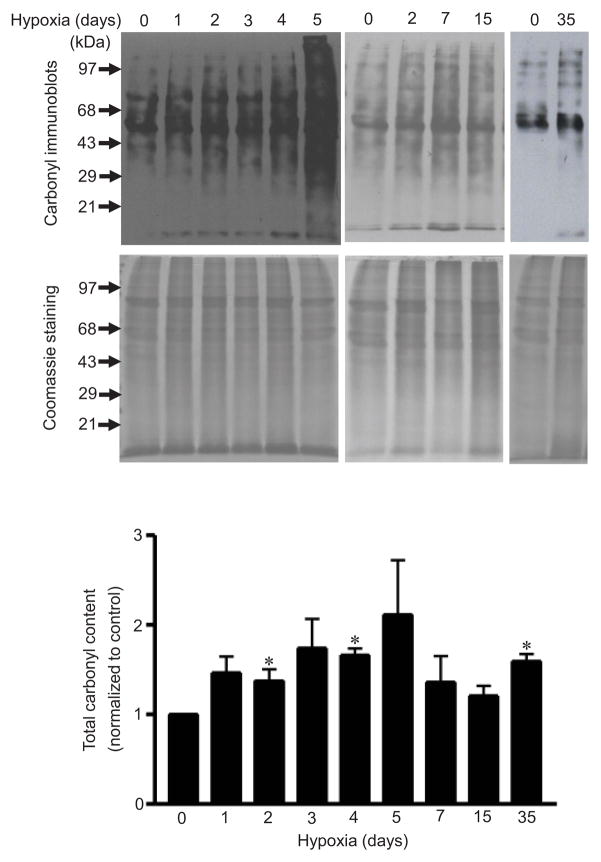
One iron-dependent process involves promoting the formation of protein carbonyls via a metal-catalyzed Fenton reaction.23–25 We examined the occurrence of protein carbonylation in an in vivo model of pulmonary hypertension in rats. Rats were subjected to chronic hypoxia for various durations. Isolated pulmonary arterial tissues were homogenized and derivatized with 2,4-dinitrophenylhydrazine (DNPH), and carbonyl content was monitored by immunoblotting. As shown in Fig. 3, chronic hypoxia increased total protein carbonyl content as early as 2 days after the onset of hypoxia treatment. Elevated total protein carbonyl content was also observed after 35 days of hypoxia, at which point pulmonary vascular remodeling was present.
Fig. 3. Effects of chronic hypoxia on protein carbonylation in an in vivo model of pulmonary hypertension.
Rats were subjected to chronic hypoxia (10% O2) for indicated durations. After treatments, pulmonary arteries were isolated and homogenized. Proteins were derivatized with DNPH and subjected to Western blotting, with rabbit polyclonal IgG for DNP used to monitor carbonylated proteins. Total protein levels were monitored by Coomassie Blue staining. The bar graph represents means ± SEM (n = 3 – 4). *, P<0.05 vs. normoxia (0 day hypoxia).
Immunoblotting of one-dimensional gel electrophoresis, which showed protein carbonylation, was further analyzed for each band by designating carbonylated protein band (CPB) numbers6 as shown in Fig. S5A. Results of the analysis of these bands are shown in Fig. S5B. A large number of bands (CPBs 2, 4, 5, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, and 17) exhibited increased protein carbonyl content in response to 35 days of chronic hypoxia. Some bands (CPBs 7, 8, 11, 14, 15, 16, and 17) also exhibited significant promotion of protein carbonylation at earlier points in time. Promotion of protein carbonylation was noted as early as 2 days after the initiation of chronic hypoxia for CPBs 15, 16, and 17. No increases in carbonyl content at any of these examined points in time were noted for CPBs 1, 3, and 6.
DNPH can react with the carbonyl groups of aldehydes and ketones, which include primary protein carbonyls as well as secondary protein carbonyls. Primary protein carbonyls are products of the direct oxidation of protein amino acid residues (i.e., proline, arginine, lysine and threonine), while secondary protein carbonyls are derived from the addition of lipid peroxidation products, such as 4-hydroxylnonenal (4-HNE) and malondialdehyde (MDA).26 Immunoblotting with antibodies against 4-HNE and MDA demonstrated that secondary protein carbonylation did not occur in response to chronic hypoxia (Fig. S6).
We previously reported that ET-1 promotes metal-catalyzed protein carbonylation in cultured bovine pulmonary artery SMCs.6 Similarly, the treatment of these cells with PDGF (Fig. S7A) or serotonin (5-HT; Fig. S7B) increased the levels of overall protein carbonylation. We also found that ET-1 promoted protein carbonylation in cultured human pulmonary artery SMCs (Fig. S7C). This is concordance with prior reports showing that ET-1, PDGF, and 5-HT promote SMC growth by producing ROS.3,5,27
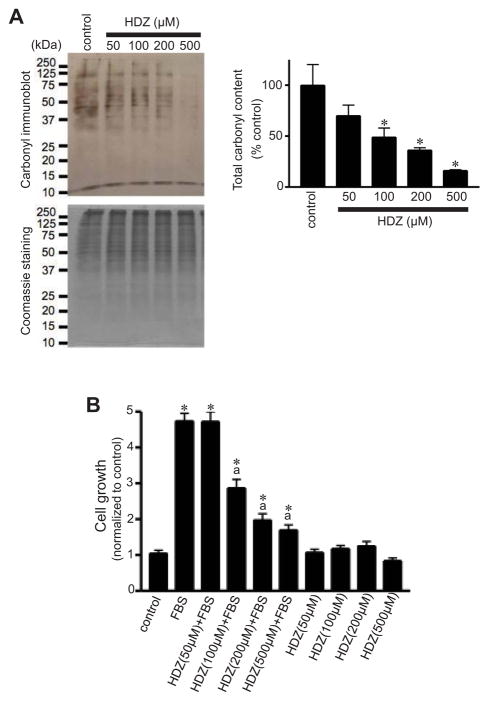
Hydralazine has been described as a carbonyl scavenger.28 Treatment of cell lysates from FBS-treated pulmonary artery SMCs with hydralazine before the addition of DNPH reduced the interactions between protein carbonyl groups and DNPH in a dose-dependent fashion (Fig. 4A). In addition, treatment of cultured pulmonary artery SMCs with hydralazine inhibited FBS-induced cell growth (Fig. 4B).
Fig. 4. Effects of hydralazine (HDZ) on pulmonary artery SMC growth.
(A) Cell lysates from FBS-stimulated pulmonary artery SMCs (control) were treated in vitro with HDZ before derivatization with DNPH and subsequent immunoblotting to demonstrate that HDZ interacts with protein carbonyl groups and competes with DNPH. The bar graph represents means ± SEM (n = 3 – 4). *, P<0.05 vs. untreated control. (B) Growth-arrested bovine pulmonary artery SMCs were pre-treated for 30 min with HDZ and then treated with 10% FBS for 3 days. Cell growth was monitored by XTT assay. The bar graph represents means ± SEM (n = 8).*, P<0.05 vs. untreated control; a, P<0.05 vs. FBS.
Identification of a carbonylated protein as Rho guanine nucleotide dissociation inhibitor-α (RhoGDI-α)
A proteomic approach was used to identify proteins that are carbonylated in response to mediators of SMC growth in pulmonary hypertension. Lysates from pulmonary artery SMCs treated with 5-HT were derivatized with DNPH and subjected to 2-dimensional gel electrophoresis and immunoblotting with DNPH. We performed mass spectrometric analysis on three carbonylated spots and identified these proteins as RhoGDI-α, peroxiredoxin 6, and transgelin (Fig. S8).
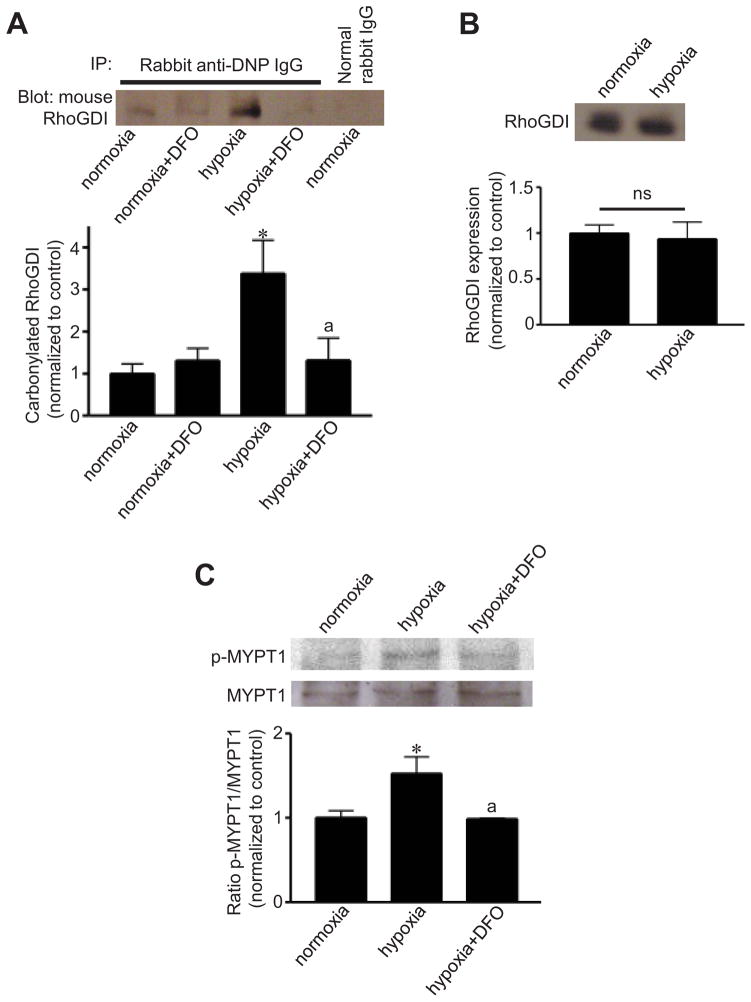
RhoGDI-α is a negative regulator of RhoA, which stimulates smooth muscle contraction and contribute to pulmonary artery SMC growth and pulmonary hypertension.29,30 In our in vivo model of pulmonary hypertension, RhoGDI was carbonylated in the pulmonary artery in response to chronic hypoxia before the onset of pulmonary vascular remodeling, and deferoxamine inhibited RhoGDI carbonylation in response to hypoxia (Fig. 5A). Protein expression levels of RhoGDI did not change in response to hypoxia (Fig. 5B). Deferoxamine also inhibited Rho kinase activation by hypoxia, as expressed by the phosphorylation of myosin phosphatase target subunit 1 (MYPT1) (Fig. 5C).
Fig. 5. Chronic hypoxia promotes RhoGDI-α carbonylation and activates RhoA signaling in an iron-dependent fashion in the pulmonary arteries of intact rats.
(A) Rats were intraperitoneally injected with saline or 20 mg/kg body weight of deferoxamine (DFO) and were then subjected to chronic hypoxia (10% O2) for 1 day. Isolated pulmonary arteries were homogenized. Proteins were derivatized with DNPH and subjected to immunoprecipitation with anti-DNP IgG and to Western blotting with anti-RhoGDI-α IgG in order to monitor carbonylated RhoGDI-α. (B) Western blotting to monitor total RhoGDI-α expression. (C) Rats were injected with saline or DFO and were then subjected to chronic hypoxia for 4 days. Western blotting was performed on pulmonary arterial homogenates to show phosphorylation of MYPT1 at Serine 507 (p-MYPT1) and total MYPT1 expression. Bar graphs represent means ± SEM (n = 3 – 4). *, P<0.05 vs. normoxia; a, P<0.05 vs. hypoxia. ns: not significantly different from each other.
Protein oxidation in patients with human pulmonary arterial hypertension
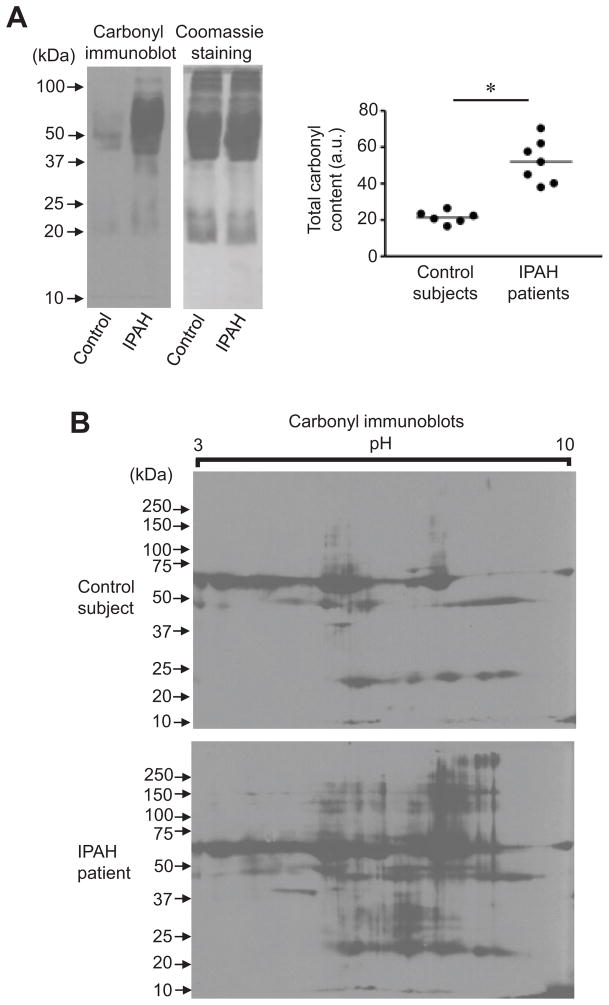
To examine if protein carbonylation occurs in PAH, plasma samples from patients with idiopathic PAH (IPAH) and those from age- and gender-matched control subjects were derivatized with DNPH and subjected to immunoblotting. Patient samples were from seven IPAH patients diagnosed according to the current definition31 with a mean age of 52.6 ± 5.8 (57% females) and a mean pulmonary arterial pressure of 55.7 ± 5.3 mmHg. Healthy control subjects consisted of six individuals with a mean age of 48.0 ± 2.9 (50% females). As shown in Fig. 6A, IPAH patients exhibited significantly higher carbonyl content compared to control subjects. Coomassie Blue staining showed equal loading of proteins. These results using one-dimensional gel electrophoresis coupled with immunoblotting were confirmed using two-dimensional gel electrophoresis coupled with immunoblotting, which shows increased protein carbonyl content in IPAH samples compared to controls (Fig. 6B).
Fig. 6. Total protein carbonyl contents in IPAH patients and control subjects.
Plasma samples from IPAH patients and age- and gender-matched control subjects were derivatized with DNPH and subjected to (A) 1-dimensional SDS-PAGE or (B) 2-dimensional gel electrophoresis consisting of isoelectric focusing and SDS-PAGE. Samples were then immunoblotted with DNP antibody. The graph represents means ± SEM (n = 6 – 7) of total carbonyl content in arbitrary units (a.u.). The symbol (*) denotes that the two groups are significantly different from each other at P < 0.05. No signals were detected without DNPH derivatization (data not shown).
To further identify selective carbonylated proteins in plasma of IPAH patients, we took a proteomic approach. Through the analysis of two-dimensional gels, we identified nine spots (denoted as CPS 1 – 9) that exhibited a consistent and statistically significant increase in carbonyl content in IPAH samples compared to controls in the pH 5 – 8 regions with molecular weights between 10 and 250 kDa (Fig. S9A). While the carbonyl content of these spots was significantly different (Fig. S9B), expression levels of these proteins did not significantly differ between IPAH patients and controls (Fig. S9C). The ratio of carbonyl content to protein expression showed a significant difference for all the spots (Fig. S9D). Mass spectrometry identified that these nine spots consisted of four proteins (CPS 1–3, fibrinogen beta chain; CPS 4–5, Ig lambda chain C region; CPS 6–7, haptoglobin; and CPS 8–9, fibrinogen gamma chain). Analysis of carbonylated amino acids by mass spectrometry indicated that total carbonyl content of the fibrinogen beta chain is ~2.5 fold higher in IPAH compared to control subjects, consistent with the results of total carbonyl content as monitored by immunoblotting and as shown in Fig. 6A. Neither plasma transferrin nor ferritin levels were significantly different between control subjects and IPAH patients, suggesting that these patients are not iron-deficient, although ferritin levels could be misleading in inflammatory states (Fig. S10).
Discussion
Experimental results from the present study support the concept that iron plays a role in the development of pulmonary vascular remodeling. The administration of the iron chelator deferoxamine32 in vivo attenuated chronic hypoxia-induced pulmonary hypertension and vascular remodeling in rats. Cell culture experiments showed that iron chelators, including deferoxamine, hinokitiol, and N,N′-bis (2-hydroxybenzyl) ethylenediamine-N,N′-diacetic acid (HBED), inhibited pulmonary artery SMC growth. On the other hand, previously published studies have shown that iron inhibits acute hypoxic pulmonary vasoconstriction and iron chelation promotes acute pulmonary vasoconstriction,15,17,18 despite the increased production of ROS with acute hypoxia. The present study and these previous studies together suggest that iron has paradoxical effects by inhibiting acute pulmonary vasoconstriction, but promoting chronic pulmonary vascular remodeling.
ROS have been shown to be involved in the mechanism of pulmonary artery SMC growth,3–5 and iron is essential for the formation of ROS.14 Therefore, the possible mechanism of action for iron chelators is to inhibit the ROS-dependent signaling pathway for SMC growth. Iron chelation can inhibit the activity of the heme-containing NAD(P)H oxidase that produces ROS, or it can inhibit the Fenton reaction that produces HO· from H2O2. HO· can subsequently promote protein oxidation, such as carbonylation, as well as lipid peroxidation. The observation that iron is not increased in pulmonary hypertension supports the notion that elevation of ROS, rather than iron per se, regulates pulmonary vascular remodeling.
The present study demonstrated that mediators of pulmonary hypertension promote protein carbonylation, both in cultured cells and in intact animals. Protein carbonylation is defined as the formation of aldehyde or ketone groups on amino acid side chains. Lysine, arginine, proline, and threonine residues are susceptible to direct oxidation to form carbonyls.24,25,33 Oxidation of arginine and proline results in the formation of glutamic semialdehyde, oxidation of lysine forms aminoadipic semialdehyde, and that of threonine forms 2-amino-3-ketobutyric acid, introducing carbonyl groups into the protein structure.23,34–36 DNPH can react with the carbonyl groups of ketone and aldehyde to form a hydrazone derivative. This hydrazone derivative can be detected using immunological techniques.25,37–39 DNPH can also react with lipid-derived aldehydes, such as 4-HNE and MDA, which are the products of lipid peroxidation and form an adduct with amino acid side chains. In the present study, chronic hypoxia did not increase 4-HNE or MDA adduct formation. A molecule with a carbonyl scavenging activity, hydralazine28 inhibited pulmonary artery SMC growth. Although there was considerable interest in hydralazine as a pulmonary vasodilator during the 1980s40,41 with conflicting findings,42,43 our results suggest that the therapeutic potential of hydralazine or other carbonyl scavenging agents for pulmonary hypertension may deserve another look.
A proteomic approach identified that carbonylated RhoGDI-α protein is formed in response to various mediators of pulmonary hypertension, both in cultured cells and in intact animals. Since RhoGDI-α is a negative regulator of RhoA signaling, the oxidation and possible inhibition of this protein result in the activation of RhoA. The RhoA-Rho kinase pathway has been shown to mediate pulmonary artery SMC growth as well as vasoconstriction, contributing to the development of pulmonary hypertension.29,30,44,45 Consistently, the administration of deferoxamine inhibited chronic hypoxia-mediated Rho-kinase activation, supporting the hypothesis that iron-catalyzed protein oxidation inhibits RhoGDI-α, which permits activation of RhoA signaling. Jernigan et al.46 reported that ROS activate the RhoA/Rho-kinase pathway in pulmonary vascular smooth muscle following chronic hypoxia. Carbonylation of RhoGDI-α may be the underlying mechanism for this cell growth process.
The present study revealed higher total carbonyl contents in the plasma of IPAH patients compared to control subjects. This is in accordance with previous studies, which have shown increased oxidative stress in IPAH patients, such as increased levels of lipid peroxidation products in plasma,7 increased DNA oxidation,8 and decreased levels of antioxidants, α-tocopherol and β-carotene.9 These results suggest that patients with PAH are under a higher oxidation state, therefore therapeutic strategies to inhibit ROS actions may be beneficial. However, it is not known whether the increased oxidation state has a causal relation to the development of this disease in humans. In experimental animals, the present data provide a support for the link between iron-catalyzed oxidation and the development of pulmonary vascular remodeling.
Recently, however, several studies suggested that iron deficiency is common in patients with PAH19–22, and iron supplementation has been suggested as a therapeutic strategy.22 Ruiter et al. (2011)20 report that 43% of IPAH patients they studied were iron deficient. These findings were from patients who have already developed pulmonary hypertension, while our data from experimental animals indicate that iron mediates the development of this disease. It is possible that iron deficiency serves as a negative feedback mechanism to protect against iron-dependent pulmonary vascular remodeling. In this case, it should be cautioned that iron supplementation may further increase oxidative stress in IPAH patients and promote cell signaling for pulmonary vascular remodeling.
In summary, the present study provides evidence that iron-dependent biological oxidation plays a role in the development of pulmonary vascular remodeling and pulmonary hypertension. These results highlight the need for more clarification of the recently developed concept that iron supplementation may present a useful strategy for treating PAH.
Supplementary Material
Highlights.
Iron chelation prevents the development of pulmonary vascular remodeling in rats
Iron chelation inhibits the growth of pulmonary artery smooth muscle cells.
The mediators of pulmonary hypertension promote iron-dependent protein oxidation.
A regulator of RhoA is one protein that is oxidized
Patients with pulmonary arterial hypertension have elevated protein oxidation.
Acknowledgments
This work was supported in part by National Institutes of Health (R01HL72844 and R01HL97514) to YJS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Runo JR, Loyd JE. Primary pulmonary hypertension. Lancet. 2003;361:1533–1544. doi: 10.1016/S0140-6736(03)13167-4. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki YJ, Forman HJ, Sevanian A. Oxidants as stimulators of signal transduction. Free Radic Biol Med. 1997;22:269–285. doi: 10.1016/s0891-5849(96)00275-4. [DOI] [PubMed] [Google Scholar]
- 3.Lee SL, Wang WW, Fanburg BL. Superoxide as an intermediate signal for serotonin-induced mitogenesis. Free Radic Biol Med. 1998;24:855–858. doi: 10.1016/s0891-5849(97)00359-6. [DOI] [PubMed] [Google Scholar]
- 4.Lee SL, Simon AR, Wang WW, Fanburg BL. H2O2 signals 5-HT-induced ERK MAP kinase activation and mitogenesis of smooth muscle cells. Am J Physiol. 2001;281:L646–L652. doi: 10.1152/ajplung.2001.281.3.L646. [DOI] [PubMed] [Google Scholar]
- 5.Wedgwood S, Dettman RW, Black SM. ET-1 stimulates pulmonary arterial smooth muscle cell proliferation via induction of reactive oxygen species. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1058–L1067. doi: 10.1152/ajplung.2001.281.5.L1058. [DOI] [PubMed] [Google Scholar]
- 6.Wong CM, Cheema AK, Zhang L, Suzuki YJ. Protein carbonylation as a novel mechanism in redox signaling. Circ Res. 2008;102:310–318. doi: 10.1161/CIRCRESAHA.107.159814. [DOI] [PubMed] [Google Scholar]
- 7.Irodova NL, Lankin VZ, Konovalova GK, Kochetov AG, Chazova IE. Oxidative stress in patients with primary pulmonary hypertension. Bull Exp Biol Med. 2002;133:580–582. doi: 10.1023/a:1020238026534. [DOI] [PubMed] [Google Scholar]
- 8.Bowers R, Cool C, Murphy RC, Tuder RM, Hopken MW, Flores SC, Voelkel NF. Oxidative stress in severe pulmonary hypertension. Am J Respir Crit Care Med. 2004;169:764–769. doi: 10.1164/rccm.200301-147OC. [DOI] [PubMed] [Google Scholar]
- 9.Preston IR, Tang G, Tilan JU, Hill NS, Suzuki YJ. Retinoids and pulmonary hypertension. Circulation. 2005;111:782–790. doi: 10.1161/01.CIR.0000155254.86840.47. [DOI] [PubMed] [Google Scholar]
- 10.Liu JQ, Zelko IN, Erbynn EM, Sham JS, Folz RJ. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91phox) Am J Physiol Lung Cell Mol Physiol. 2006;290:L2–L10. doi: 10.1152/ajplung.00135.2005. [DOI] [PubMed] [Google Scholar]
- 11.Grobe AC, Wells SM, Benavidez E, Oishi P, Azakie A, Fineman JR, Black SM. Increased oxidative stress in lambs with increased pulmonary blood flow and pulmonary hypertension: role of NADPH oxidase and endothelial NO synthase. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1069–L1077. doi: 10.1152/ajplung.00408.2005. [DOI] [PubMed] [Google Scholar]
- 12.Fike CD, Slaughter JC, Kaplowitz MR, Zhang Y, Aschner JL. Reactive oxygen species from NADPH oxidase contribute to altered pulmonary vascular responses in piglets with chronic hypoxia-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2008;295:L881–L888. doi: 10.1152/ajplung.00047.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dennis KE, Aschner JL, Milatovic D, Schmidt JW, Aschner M, Kaplowitz MR, Zhang Y, Fike CD. NADPH oxidases and reactive oxygen species at different stages of chronic hypoxia-induced pulmonary hypertension in newborn piglets. Am J Physiol Lung Cell Mol Physiol. 2009;297:L596–L607. doi: 10.1152/ajplung.90568.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freeman BA, Crapo JD. Biology of disease: free radicals and tissue injury. Lab Invest. 1982;47:412–426. [PubMed] [Google Scholar]
- 15.Balanos GM, Dorrington KL, Robbins PA. Desferrioxamine elevates pulmonary vascular resistance in humans: potential for involvement of HIF-1. J Appl Physiol. 2002;92:2501–2507. doi: 10.1152/japplphysiol.00965.2001. [DOI] [PubMed] [Google Scholar]
- 16.Ren X, Dorrington KL, Maxwell PH, Robbins PA. Effects of desferrioxamine on serum erythropoietin and ventilatory sensitivity to hypoxia in humans. J Appl Physiol. 2000;89:680–686. doi: 10.1152/jappl.2000.89.2.680. [DOI] [PubMed] [Google Scholar]
- 17.Smith TG, Balanos GM, Croft QP, Talbot NP, Dorrington KL, Ratcliffe PJ, Robbins PA. The increase in pulmonary arterial pressure caused by hypoxia depends on iron status. J Physiol. 2008;586:5999–6005. doi: 10.1113/jphysiol.2008.160960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith TG, Talbot NP, Privat C, Rivera-Ch M, Nickol AH, Ratcliffe PJ, Dorrington KL, León-Velarde F, Robbins PA. Effects of iron supplementation and depletion on hypoxic pulmonary hypertension: two randomized controlled trials. JAMA. 2009;302:1444–1450. doi: 10.1001/jama.2009.1404. [DOI] [PubMed] [Google Scholar]
- 19.Soon E, Treacy CM, Toshner MR, MacKenzie-Ross R, Manglam V, Busbridge M, Sinclair-McGarvie M, Arnold J, Sheares KK, Morrell NW, Pepke-Zaba J. Unexplained iron deficiency in idiopathic and heritable pulmonary arterial hypertension. Thorax. 2011;66:326–332. doi: 10.1136/thx.2010.147272. [DOI] [PubMed] [Google Scholar]
- 20.Ruiter G, Lankhorst S, Boonstra A, Postmus PE, Zweegman S, Westerhof N, van der Laarse WJ, Vonk-Noordegraaf A. Iron deficiency is common in idiopathic pulmonary arterial hypertension. Eur Respir J. 2011;37:1386–1391. doi: 10.1183/09031936.00100510. [DOI] [PubMed] [Google Scholar]
- 21.Rhodes CJ, Howard LS, Busbridge M, Ashby D, Kondili E, Gibbs JS, Wharton J, Wilkins MR. Iron deficiency and raised hepcidin in idiopathic pulmonary arterial hypertension: clinical prevalence, outcomes, and mechanistic insights. J Am Coll Cardiol. 2011;58:300–309. doi: 10.1016/j.jacc.2011.02.057. [DOI] [PubMed] [Google Scholar]
- 22.Rhodes CJ, Wharton J, Howard L, Gibbs JS, Vonk-Noordegraaf A, Wilkins MR. Iron deficiency in pulmonary arterial hypertension: a potential therapeutic target. Eur Respir J. 2011;38:1453–1460. doi: 10.1183/09031936.00037711. [DOI] [PubMed] [Google Scholar]
- 23.Stadtman ER. Oxidation of free amino acids and amino acid residues in proteins by radiolysis and by metal-catalyzed reactions. Annu Rev Biochem. 1993;62:797–821. doi: 10.1146/annurev.bi.62.070193.004053. [DOI] [PubMed] [Google Scholar]
- 24.Stadtman ER, Berlett BS. Fenton chemistry. Amino acid oxidation. J Biol Chem. 1991;266:17201–17211. [PubMed] [Google Scholar]
- 25.Levine RL. Carbonyl modified proteins in cellular regulation, aging, and disease. Free Radic Biol Med. 2002;32:790–796. doi: 10.1016/s0891-5849(02)00765-7. [DOI] [PubMed] [Google Scholar]
- 26.Wong CM, Marcocci L, Liu L, Suzuki YJ. Cell signaling by protein carbonylation and decarbonylation. Antioxid Redox Signal. 2010;12:393–404. doi: 10.1089/ars.2009.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 28.Galvani S, Coatrieux C, Elbaz M, Grazide MH, Thiers JC, Parini A, Uchida K, Kamar N, Rostaing L, Baltas M, Salvayre R, Nègre-Salvayre A. Carbonyl scavenger and antiatherogenic effects of hydrazine derivatives. Free Radic Biol Med. 2008;45:1457–1467. doi: 10.1016/j.freeradbiomed.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 29.McMurtry IF, Bauer NR, Fagan KA, Nagaoka T, Gebb SA, Oka M. Hypoxia and Rho/Rho-kinase signaling. Lung development versus hypoxic pulmonary hypertension. Adv Exp Med Biol. 2003;543:127–137. [PubMed] [Google Scholar]
- 30.Liu Y, Suzuki YJ, Day RM, Fanburg BL. Rho kinase-induced nuclear translocation of ERK1/ERK2 in smooth muscle cell mitogenesis caused by serotonin. Circ Res. 2004;595:579–586. doi: 10.1161/01.RES.0000141428.53262.a4. [DOI] [PubMed] [Google Scholar]
- 31.Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery J-L, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, Gomez-Sanchez MA, Jondeau G, Klepetko W, Opitz C, Peacock A, Rubin L, Zellweger M, Simonneau G. Guidelines for the diagnosis and treatment of pulmonary hypertension. European Heart J. 2009;30:2493–2537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 32.Stark PE, Farber JL. Ferric iron and superoxide ions are required for the killing of cultured hepatocytes by hydrogen peroxide. J Biol Chem. 1985;260:10099–10104. [PubMed] [Google Scholar]
- 33.Stadtman ER, Levine RL. Protein oxidation. Ann N Y Acad Sci. 2000;899:191–208. doi: 10.1111/j.1749-6632.2000.tb06187.x. [DOI] [PubMed] [Google Scholar]
- 34.Amici A, Levine RL, Tsai L, Stadtman ER. Conversion of amino acid residues in proteins and amino acid homopolymers to carbonyl derivatives by metal-catalyzed oxidation reactions. J Biol Chem. 1989;264:3341–3346. [PubMed] [Google Scholar]
- 35.Berlett BS, Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. J Biol Chem. 1997;272:20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- 36.Requena JR, Chao CC, Levine RL, Stadtman ER. Glutamic and aminoadipic semialdehydes are the main carbonyl products of metal-catalyzed oxidation of proteins. Proc Natl Acad Sci USA. 2001;98:69–74. doi: 10.1073/pnas.011526698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levine RL, Williams JA, Stadtman ER, Shacter E. Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol. 1994;233:346–357. doi: 10.1016/s0076-6879(94)33040-9. [DOI] [PubMed] [Google Scholar]
- 38.Levine RL, Wehr N, Williams JA, Stadtman ER, Shacter E. Determination of carbonyl groups in oxidized proteins. Methods Mol Biol. 2000;99:15–24. doi: 10.1385/1-59259-054-3:15. [DOI] [PubMed] [Google Scholar]
- 39.Shacter E, Williams JA, Lim M, Levine RL. Differential susceptibility of plasma proteins to oxidative modification: examination by western blot immunoassay. Free Radic Biol Med. 1994;17:429–437. doi: 10.1016/0891-5849(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 40.Rubin LJ, Peter RH. Oral hydralazine therapy for primary pulmonary hypertension. N Engl J Med. 1980;302:69–73. doi: 10.1056/NEJM198001103020201. [DOI] [PubMed] [Google Scholar]
- 41.Lupi-Herrera E, Sandoval J, Seoane M, Bialostozky D. The role of hydralazine therapy for pulmonary arterial hypertension of unknown cause. Circulation. 1982;65:645–650. doi: 10.1161/01.cir.65.4.645. [DOI] [PubMed] [Google Scholar]
- 42.McGoon MD, Seward JB, Vlietstra RE, Choo MH, Moyer TP, Reeder GS. Haemodynamic response to intravenous hydralazine in patients with pulmonary hypertension. Br Heart J. 1983;50:579–585. doi: 10.1136/hrt.50.6.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rich S, Ganz R, Levy PS. Comparative actions of hydralazine, nifedipine and amrinone in primary pulmonary hypertension. Am J Cardiol. 1983;52:1104–1107. doi: 10.1016/0002-9149(83)90541-6. [DOI] [PubMed] [Google Scholar]
- 44.Nagaoka T, Morio Y, Casanova N, Bauer N, Gebb S, McMurtry I, Oka M. Rho/Rho kinase signaling mediates increased basal pulmonary vascular tone in chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol. 2004;287:L665–L672. doi: 10.1152/ajplung.00050.2003. [DOI] [PubMed] [Google Scholar]
- 45.Abe K, Shimokawa H, Morikawa K, Uwatoku T, Oi K, Matsumoto Y, Hattori T, Nakashima Y, Kaibuchi K, Sueishi K, Takeshit A. Long-term treatment with a Rho-kinase inhibitor improves monocrotaline-induced fatal pulmonary hypertension in rats. Circ Res. 2004;94:385–393. doi: 10.1161/01.RES.0000111804.34509.94. [DOI] [PubMed] [Google Scholar]
- 46.Jernigan NL, Walker BR, Resta TC. Reactive oxygen species mediate RhoA/Rho kinase-induced Ca2+ sensitization in pulmonary vascular smooth muscle following chronic hypoxia. Am J Physiol Lung Cell Mol Physiol. 2008;295:L515–L529. doi: 10.1152/ajplung.00355.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.