Abstract
An efficient approach to a wide range of isoindolinones, including 3-monosubstituted and 3,3-disubstituted isoindolinones, from the annulation of N-benzoylsulfonamides with olefins and diazoacetate has been developed. The transformation is broadly compatible with both terminal and internal olefins. Moreover, diazoacetate is for the first time incorporated into an amide-directed C-H functionalization reaction. Specifically, the rhodium complex [{RhCl2Cp*}2] enables the in situ dimerization of diazoacetate in addition to its role in catalyzing C-H functionalization/cross-coupling.
Keywords: N-benzoylsulfonamide, C-H activation, diazoacetate, isoindolinone, rhodium catalysis
1. Introduction
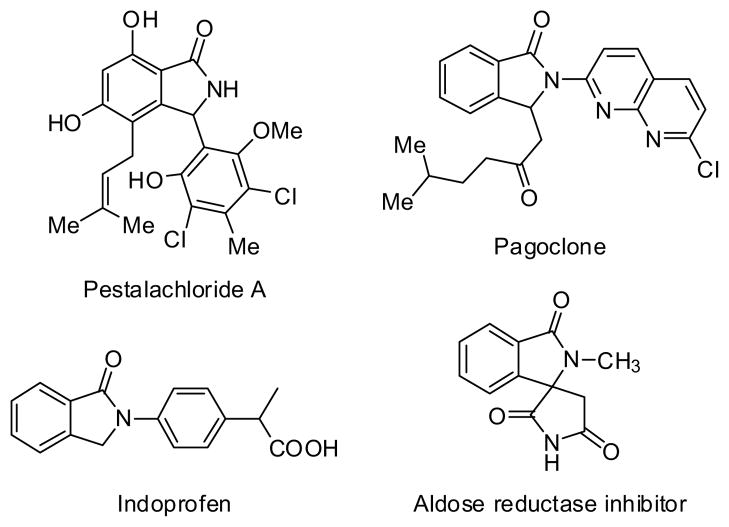
Transition-metal catalyzed direct transformations of C-H bonds has becomes a promising strategy for the construction of complex structures due to its undeniable synthetic efficiency and atom economy.1 Isoindolinone represents a significant subunit of nitrogen-containing heterocycles well represented amongst natural products and biologically active compounds (Figure 1).2 Apart from a variety of documented conventional methods,3 isoindolinone can be readily prepared by the means of C-H activation. Our group,4 the Lloyd-Jones/Booker-Milburn groups,5 and the Wang group6 recently revealed palladium-catalyzed sequences of C-H olefination/annulation to generate 3-monosubstituted isoindolinones; the Li group,7 the Glorius group8 and the Ackermann group9 also disclosed a similar process but accomplished by rhodium or ruthenium catalysis. Regarding the broadly structural diversity of isoindolinones, it is clear that it will continue to remain an area of active investigations.
Fig. 1.
Representative structures containing isoindolinone
Amongst many efficient catalytic systems for C-H activation, the utility of the rhodium complex [{RhCl2Cp*}2] has been cogently demonstrated in numerous concise syntheses of oxygen- and nitrogen-containing heterocycles.10,11 We recently developed an efficient rhodium-catalyzed approach to a wide range of 3,3-disubstituted isoindolinones from the annulation of N-benzoylsulfonamides with internal olefins by means of C-H olefination.12 Two intriguing facts were referred in the preliminary study: the internal olefins were the first time systematically investigated in the C-H olefination and a new N-substituted quaternary centre was constructed during the reaction.
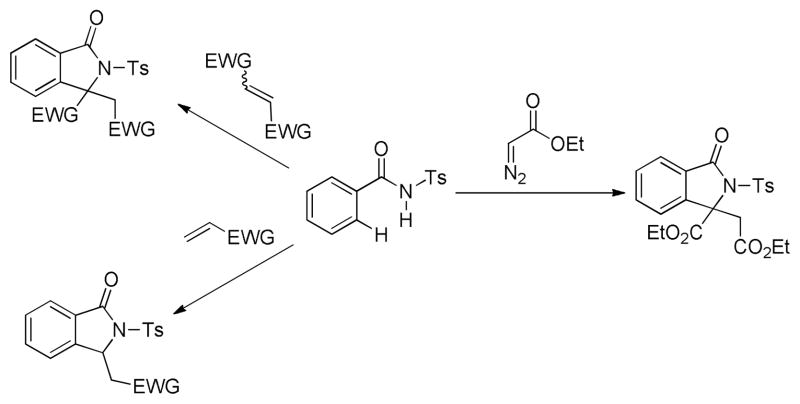
Based on that, herein we wish to report a full article about rhodium-catalyzed C-H activation of N-benzoylsulfonamides, that N-benzoylsulfonamides are annulated with a variety of olefins including terminal and internal olefins to generate both 3-monosubstituted and 3,3-disubstituted isoindolinones (Scheme 1). Moreover, the one-pot synthesis of 3,3-disubstituted isoindolinones via the coupling with diazoacetate is also described. It is noteworthy that, besides the known effect of facilitating C-H bond cleavage, a novel function of the rhodium complex [{RhCl2Cp*}2], i.e., in situ dimerization of methyl diazoacetate, is demonstrated in the transformation.
Scheme 1.
Rhodium-catalyzed synthesis of isoindolinones
2. Results and discussion
2.1 Annulation with olefins
For the initial survey of reaction conditions and optimization, tert-butyl acrylate was selected as model substrate. Subsequent screening experiments established some reaction parameters: (a) toluene affords a better yield than other common solvents; (b) the reaction at 130 °C ensures the full conversions within 24 h.
With the optimized conditions in hand, we applied the method to a variety of substituted N-benzoylsulfonamides (Table 1). The tandem process readily provided 3-monosubstituted isoindolinones regardless of the electronic properties of the substituents. The electron-rich methyl (1b) and methoxy groups (1c) as well as electron-deficient fluoro (1d) and trifluoromethyl groups (1e) all furnished their respective products in high chemical yields (entries 2–5). Even the ortho-substituted substrate 1f could afford the corresponding adduct 3f in good yield with prolonged reaction time (entry 6).
Table 1.
Substrate scope of reaction with tert-butyl acrylate.a
Reaction conditions: 1 (0.10 mmol), 2a (0.12 mmol), [RhCl2Cp*]2 (0.002 mmol) and Cu(OAc)2·H2O (0.20 mmol) in toluene, 130 °C for 24 h.
Isolated yield.
48 h.
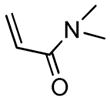
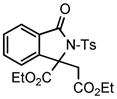
We next investigated the range of suitable alkenes (Table 2). When subjected to the standard reaction conditions, the terminal olefins (conjugated ketone 2b and amide 2c) smoothly evolved the corresponding products (entries 1–2). Subsequently, we extended the methodology into the annulation with internal olefins that would result in the formation of 3,3-disubstituted isoindolinones. The olefin configuration was observed to be unimportant to the reaction, since either fumarate 2d or maleate 2e led to the similar results (entries 3–4). E-1,2-Diketone conjugated olefin 2f was also a suitable substrate and gave rise to the corresponding adduct 3j in useful yield (entry 5). The transformation displayed excellent electronic discrimination with respect to the unsymmetric olefin 2g so that the regioisomeric product 3k was exclusively generated (entry 6). Cyclic olefins were also compatible with the reaction conditions (entry 7). Coupling with maleimide 2h offered a facile access to the spiroisoindolinone 3l.
Table 2.
Substrate scope of various alkenes.a
| |||
|---|---|---|---|
| Entry | Alkene | Product | Yield (%)b |
| 1 |
 2b |
 3g |
78 |
| 2 |
 2c |
 3h |
82 |
| 3 |
2d |
 3i |
82 |
| 4 |
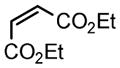 2e |
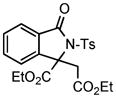 3i |
80 |
| 5 |
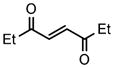 2f |
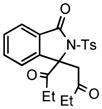 3j |
51 |
| 6 |
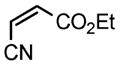 2g |
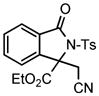 3k |
66 |
| 7c |
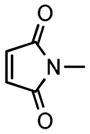 2h |
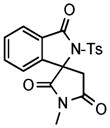 3l |
73 |
Reaction conditions: 1a (0.10 mmol), 2 (0.12 mmol), [RhCl2Cp*]2 (0.004 mmol) and Cu(OAc)2·H2O (0.20 mmol) in toluene, 130 °C for 24 h.
Isolated yield.
48 h.
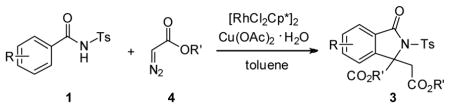
2.2 Annulation with diazoacetate
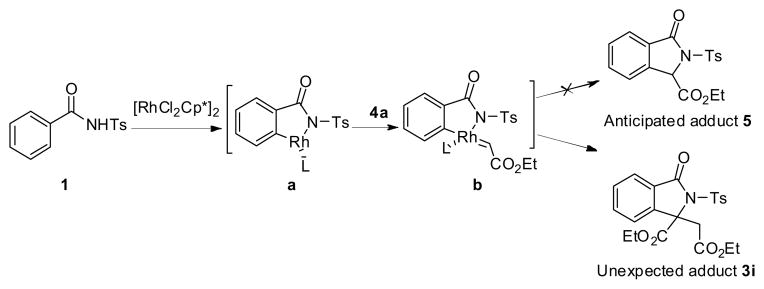
As part of our contribution to the construction of isoindolinones from N-benzoylsulfonamides,4,12,13 we envisioned that if the rhodacycle a formed at the stage of C-H activation encounters diazoacetate 4a, the rhodium-carbene species b might be in situ generated followed by carbene insertion, affording 3-carboxy isoindolinone adduct 5, a new type of isoindolinone which could not be produced via benzamide-directed C-H olefination (Scheme 2).
Scheme 2.
Proposed reaction pathway
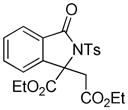
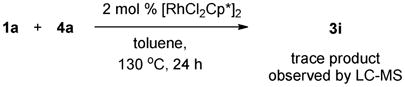
Under the previous reaction conditions, the proposed product 5 was not detected. But, an unexpected adduct 3i was alternatively isolated in good yield. This finding intrigued us since it involved the first incorporation of diazoacetate into the annulation reaction of an amide and offered another efficient approach for the synthesis of 3,3-disubstituted isoindolinones.
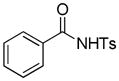
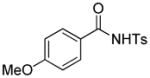
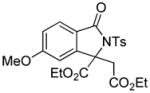
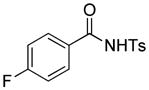
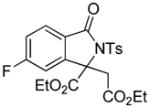
Encouraged by the above results, we commenced to determine the substrate scope (Table 3). The satisfactory results were obtained with a wide range of N-benzoylsulfonamides in spite of their electronic or steric properties. The electron-donating 4-methyl and 4-methoxy groups furnished their respective adducts in high yields (entries 3–4). Weak or even strong electron-deficient substitution, such as 4-fluoro and 4-trifluoromethyl groups, consistently resulted in good outcomes (entries 5–6). The chemoselective transformation in the presence of aryl bromide is noteworthy, as the bromide could incur subsequently competitive cross coupling reactions (entry 7). Moreover, meta-substituted substrates delivered not only good chemical yields but excellent regioselectivities, so that para-positional products were predominantly obtained (entries 8–9). Even the ortho-fluoro group was tolerated and the reaction readily proceeded without compromising the chemical yield (entry 10). However, significantly increasing the steric hindrance on either substrate hampered the reaction. For example, conversions were sluggish when ethyl diazoacetate 2a was replaced with tert-butyl diazoacetate 2b (entry 2), or possessed a crowded spatial environment around the reaction site (entry 11).
Table 3.
Substrate scope of reaction with diazoacetate.a
| ||||
|---|---|---|---|---|
| Entry | Amide | Diazoacetate | Product | Yield (%)b |
| 1 |
 1a |
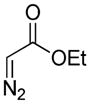 4a |
 3i |
80 |
| 2 | 1a |
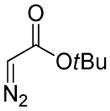 4b |
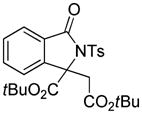 3m |
< 10 |
| 3 |
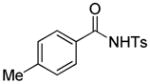 1b |
4a |
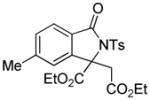 3n |
81 |
| 4 |
 1c |
4a |
 3o |
75 |
| 5 |
 1d |
4a |
 3p |
82 |
| 6 |
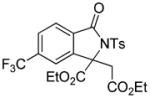
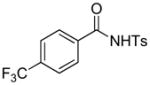 1e |
4a |
 3q |
74 |
| 7 |
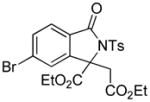
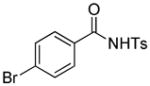 1g |
4a |
 3r |
66 |
| 8c,d |
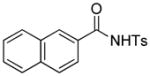 1h |
4a |
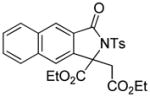 3s |
76 |
| 9c,e |
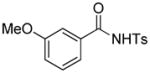 1i |
4a |
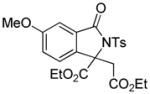 3t |
70 |
| 10c |
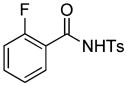 1f |
4a |
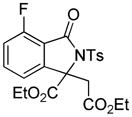 3u |
78 |
| 11c |
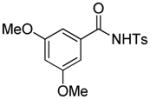 1j |
4a |
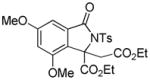 3v |
45 |
Reaction conditions: 1 (0.10 mmol), 4 (0.30 mmol), [RhCl2Cp*]2 (0.002 mmol) and Cu(OAc)2·H2O (0.20 mmol) in toluene, 130 °C for 24 h.
Isolated yield.
48 h.
Regioisomeric ratio: β/α > 19:1.
Regioisomeric ratio: para/ortho = 15:1.
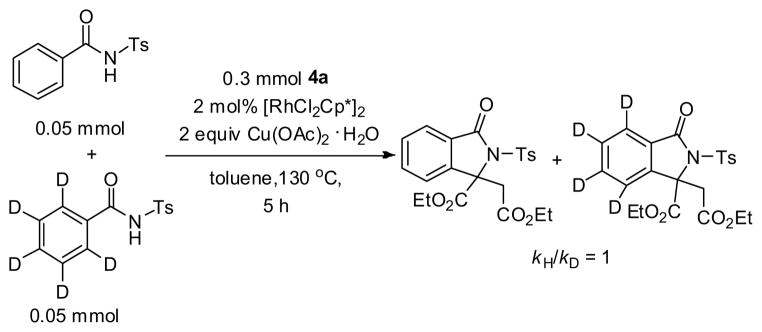
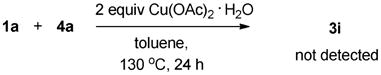
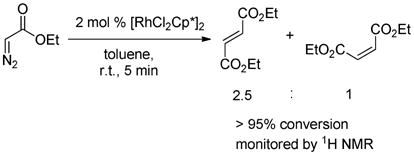
Some control experiments were carried out to elucidate the reaction pathways. The evidence in eqs 1 and 2 suggested that the rhodium complex rather than copper acetate is essential to the transformation. The experiment that exposure of diazoacetate to the complex [{RhCl2Cp*}2] rapidly generated the dimerization product (a 2.5:1 mixture of fumarate and maleate) is noteworthy (eq 3), as the decomposition of diazo compounds is prompted generally by Rh(II),14 rarely by Rh(III) species.15,16 Based on these observations, we speculate that at the beginning of the overall transformations, the diazoacetate is quickly converted into the corresponding internal olefin, which is the reactive species in the subsequent C-H olefination and annulation (Table 2, entries 3–4).
 |
(1) |
 |
(2) |
 |
(3) |
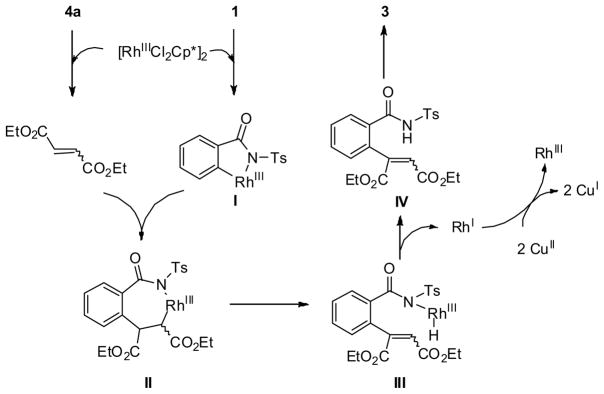
The KIE value (kH/kD = 1) might suggest that the C-H cleavage is fast, thus not involved as the rate-limiting step (Scheme 3). The postulated mechanism is depicted in Figure 2. Two roles of the rhodium complex [{RhCl2Cp*}2] were involved in the transformation. At first, the diazoacetate 4a is rapidly converted into the corresponding fumarate/maleate under the rhodium catalysis. Rapid C-H functionalization of 1 generates five-membered rhodacycle (I), which subsequently undergoes the insertion of fumarate/maleate and β-H elimination to generate Rh-H complex (III). Then, the following reductive elimination and Michael addition are proposed to give rise to the annulated isoindolinone product 3. Meanwhile, the rhodium catalyst is regenerated by copper oxidation.
Scheme 3.
KIE experiment
Fig. 2.
Plausible mechanism
3. Conclusion
We have described the rhodium catalyzed synthesis of isoindolinones from the annulation of N-benzoylsulfonamides with a variety of olefins. The transformation is broadly compatible with both terminally and internally electron-deficient olefins, efficiently producing 3-monosubstituted and 3,3-disubstituted isoindolinones.
Moreover, diazoacetate is for the first time incorporated into an amide-directed C-H functionalization reaction. The tandem process that dimerization of diazoacetate followed by subsequent C-H olefination offers another interesting approach to 3,3-disubstituted isoindolinones. In addition to facilitating C-H bond cleavage, the rhodium complex [{RhCl2Cp*}2] unexpectedly dimerized diazoacetate in situ to a mixture of fumarate/maleate that then participated in the annulation.
4. Experimental section
General Methods
All reactions were maintained under an argon atmosphere unless otherwise stated. Anhydrous solvents (THF, DME) were freshly distilled from sodium benzophenone ketyl or from CaH2 (CH2Cl2, toluene) under argon. Commercially available reagents were used without further purification. Flash chromatography (FC) was performed using E. Merck silica gel 60 (240–400 mesh). Thin layer chromatography (TLC) was performed using pre-coated plates purchased from E. Merck (silica gel 60 PF254, 0.25 mm). NMR spectra were recorded in CDCl3, unless otherwise stated, on spectrometers at operating frequencies of 400/500 MHz (1H) or 100/125 MHz (13C) as indicated in the individual spectrum.
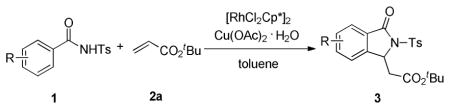
Typical Procedure for Annulation of N-Benzoylsulfonamide with Olefins
N-Benzoylsulfonamide 1a (27.5 mg, 0.1 mmol), [RhCl2Cp*]2 (1.2 mg, 0.002 mmol) and Cu(OAc)2 ·H2O (40.0 mg, 0.20 mmol) were loaded in a dry vial which was subjected to evacuation/flushing with dry argon three times. Anhydrous toluene (1.0 mL) solution of tert-butyl acrylate 2a (17.4 uL, 0.12 mmol) was syringed into the mixture which was then stirred at 130 °C for 24 h or until the starting material had been consumed as determined by TLC. Upon cooling to room temperature, all volatiles were evaporated and the residue was purified by preparative TLC (ethyl acetate/hexane 1:2) to give isoindolinone 3a in 88% yield.
Typical Procedure for Annulation of N-Benzoylsulfonamide with Ethyl Diazoacetate
N-Benzoylsulfonamide 1a (27.5 mg, 0.1 mmol), [RhCl2Cp*]2 (1.2 mg, 0.002 mmol) and Cu(OAc)2·H2O (40.0 mg, 0.20 mmol) were loaded in a dry vial which was subjected to evacuation/flushing with dry argon three times. An anhydrous toluene (1.0 mL) solution of ethyl diazoacetate 4a (30 uL, 0.30 mmol) was syringed into the mixture which was then stirred at 130 °C for 24 h or until the starting material had been consumed as determined by TLC. Upon cooling to room temperature, all volatiles were evaporated and the residue was purified by preparative TLC (ethyl acetate/hexane = 1:2) to give isoindolinone 3i in 80% yield.
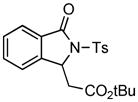
4.1 3-tert-Butoxycarbonylmethyl-2-tosylisoindolin-1-one (3a)
1H NMR (500 MHz, CDCl3) δ 1.24 (s, 9H), 2.42 (s, 3H), 3.01 (dd, J = 7.5, 16.5 Hz, 1H), 3.41 (dd, J = 3.5, 16.5 Hz, 1H), 5.55 (dd, J = 3.0, 7.5 Hz, 1H), 7.33 (d, J = 8.5 Hz, 2H), 7.47 (dd, J = 7.5, 8.0 Hz, 1H), 7.50 (d, J = 8.0 Hz, 1H), 7.63 (dd, J = 7.5, 8.0 Hz, 1H), 7.79 (d, J = 7.5 Hz, 1H), 8.05 (d, J = 8.5 Hz, 2H); 13C NMR (125 MHz, CDCl3) δ 21.9, 28.0, 40.2, 58.8, 82.0, 123.2, 125.1, 128.7, 129.3, 129.8, 129.9, 134.4, 136.0, 145.4, 145.6, 166.8, 169.0. FT-IR (CH2Cl2) 2360, 1727, 1597, 1366, 1292, 1170, 1090, 668 cm−1. HRMS calcd for C21H24NO5S [M+H]+ 402.1370, found 402.1358.
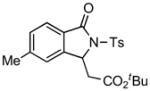
4.2 3-tert-Butoxycarbonylmethyl-5-methyl-2-tosylisoindolin-1-one (3b)
1H NMR (500 MHz, CDCl3) δ 1.27 (s, 9H), 2.41 (s, 3H), 2.44 (s, 3H), 2.96 (dd, J = 7.5, 16.0 Hz, 1H), 3.40 (dd, J = 3.5, 16.5 Hz, 1H), 5.50 (dd, J = 3.5, 7.5 Hz, 1H), 7.27 (d, J = 7.5 Hz, 1H), 7.29 (s, 1H), 7.33 (d, J = 8.0 Hz, 2H), 7.66 (d, J = 8.0 Hz, 1H), 8.04 (d, J = 7.5 Hz, 2H); 13C NMR (125 MHz, CDCl3) δ 21.9, 22.4, 28.0, 40.5, 58.6, 81.9, 123.6, 124.9, 127.1, 128.6, 129.8, 130.4, 136.1, 145.3, 145.7, 146.0, 166.7, 169.1. FT-IR (CH2Cl2) 2979, 1731, 1618, 1366, 1337, 1284, 1171, 1091, 815, 691, 659 cm−1. HRMS calcd for C22H26NO5S [M+H]+ 416.1526, found 416.1511.
4.3 3-tert-Butoxycarbonylmethyl-5-methoxy-2-tosylisoindolin-1-one (3c)
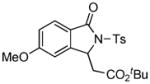
1H NMR (500 MHz, CDCl3) δ 1.33 (s, 9H), 2.41 (s, 3H), 2.89 (dd, J = 8.0, 16.5 Hz, 1H), 3.46 (dd, J = 2.0, 16.5 Hz, 1H), 3.84 (s, 3H), 5.63 (dd, J = 2.0, 7.5 Hz, 1H), 6.96 (s, 1H), 6.97 (d, J = 9.0 Hz, 1H), 7.32 (d, J = 8.0 Hz, 2H), 7.68 (d, J = 9.0 Hz, 1H), 8.03 (d, J = 8.0 Hz, 2H); 13C NMR (125 MHz, CDCl3) δ 21.9, 28.1, 40.7, 56.0, 58.4, 82.0, 107.3, 116.8, 121.9, 126.8, 128.6, 129.8, 136.2, 145.3, 148.4, 165.0, 166.4, 169.4. FT-IR (CH2Cl2) 2978, 1728, 1608, 1493, 1366, 1260, 1170, 1090, 693, 659 cm−1. HRMS calcd for C22H26NO6S [M+H]+ 432.1475, found 432.1472.
4.4 3-tert-Butoxycarbonylmethyl-5-fluoro-2-tosylisoindolin-1-one (3d)
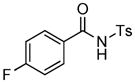
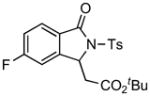
1H NMR (500 MHz, CDCl3) δ 1.31 (s, 9H), 2.42 (s, 3H), 2.93 (dd, J = 8.0, 16.5 Hz, 1H), 3.44 (dd, J = 3.5, 16.5 Hz, 1H), 5.52 (dd, J = 3.0, 8.0 Hz, 1H), 7.15–7.19 (m, 1H), 7.21–7.23 (m, 1H), 7.34 (d, J = 8.0 Hz, 2H), 7.76–7.80 (m, 1H), 8.04 (d, J = 8.5 Hz, 2H); 13C NMR (125 MHz, CDCl3) δ 21.9, 28.1, 40.2, 58.4, 82.3, 110.9 (d, JC-F = 29.2 Hz), 117.5 (d, JC-F = 24.2 Hz), 125.7 (d, JC-F = 11.5 Hz), 127.5 (q, JC-F = 5.0 Hz), 128.6, 129.9, 135.8, 145.6, 148.3 (d, JC-F = 10.6 Hz), 165.6 (d, JC-F = 267 Hz), 167.7, 168.9. FT-IR (CH2Cl2) 2981, 2360, 2342, 1733, 1624, 1603, 1485, 1358, 1245, 1173, 1105, 860, 813, 690, 668 cm−1. HRMS calcd for C21H23FNO5S [M+H]+ 420.1275, found 420.1275.
4.5 3-tert-Butoxycarbonylmethyl-5-trifluoromethyl-2-tosylisoindolin-1-one (3e)
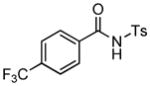
1H NMR (500 MHz, CDCl3) δ 1.30 (s, 9H), 2.43 (s, 3H), 2.92 (dd, J = 8.0, 16.5 Hz, 1H), 3.51 (dd, J = 3.5, 16.5 Hz, 1H), 5.62 (dd, J = 3.5, 8.0 Hz, 1H), 7.35 (d, J = 8.5 Hz, 2H), 7.74 (d, J = 8.0 Hz, 1H), 7.82 (s, 1H), 7.91 (d, J = 8.0 Hz, 1H ), 8.05 (d, J = 8.0 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 21.9, 28.0, 40.1, 58.8, 82.4, 120.9, 122.1, 125.8, 126.5, 128.7, 130.0, 132.9, 135.9, 136.0 (q, JC-F = 32.3 Hz), 145.8, 146.0, 165.3, 168.8. FT-IR (CH2Cl2) 2980, 1729, 1598, 1440, 1367, 1312, 1171, 1131, 1092, 1061, 841, 696, 672 cm−1. HRMS calcd for C22H23F3NO5S [M++H] 470.1244, found 470.1230.
4.6 3-tert-Butoxycarbonylmethyl-7-fluoro-2-tosylisoindolin-1-one (3f)
1H NMR (500 MHz, CDCl3) δ 1.25 (s, 9H), 2.43 (s, 3H), 3.05 (dd, J = 7.0, 16.5 Hz, 1H), 3.36 (dd, J = 3.5, 16.5 Hz, 1H), 5.55 (dd, J = 3.0, 7.0 Hz, 1H), 7.10 (dd, J = 8.5, 8.5 Hz, 1H), 7.27 (d, J = 7.5 Hz, 1H), 7.34 (d, J = 8.0 Hz, 2H), 7.58–7.63 (m, 1H), 8.05 (d, J = 8.5 Hz, 2H); 13C NMR (125 MHz, CDCl3) δ 21.9, 28.0, 40.1, 58.3, 82.1, 116.4, 116.5, 117.5, 117.6, 119.08, 119.12, 128.8, 129.9, 135.7, 136.4, 136.5, 145.6, 147.9, 158.6, 160.7, 163.4, 168.7; FT-IR (CH2Cl2) 2979, 2360, 2341, 1731, 1626, 1599, 1482, 1366, 1313, 1292, 1252, 1207, 1172, 1102, 1036, 979, 684, 668 cm−1. HRMS calcd for C21H23FNO5S [M+H]+ 420.1275, found 420.1259.
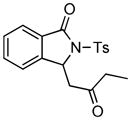
4.7 3-Ethylcarbonylmethyl-2-tosylisoindolin-1-one (3g)
1H NMR (500 MHz, CDCl3) δ 1.12 (t, J = 7.0 Hz, 3H), 2.41 (s, 3H), 2.41–2.46 (m, 1H), 2.56–2.62 (m, 1H), 2.90 (dd, J = 9.0, 18.0 Hz, 1H), 3.81 (dd, J = 3.0, 18.0 Hz, 1H), 5.69 (dd, J = 3.0, 9.0 Hz, 1H), 7.33 (d, J = 8.0 Hz, 2H), 7.42–7.46 (m, 2H), 7.59 (dd, J = 7.5, 7.5 Hz, 1H), 7.76 (d, J = 7.5 Hz, 1H), 7.99 (d, J = 8.5 Hz, 2H); 13C NMR (125 MHz, CDCl3) δ 7.83, 21.9, 36.8, 48.0, 58.2, 123.7, 125.2, 128.5, 129.1, 129.3, 130.0, 134.6, 135.7, 145.6, 146.6, 166.7, 208.5. FT-IR (CH2Cl2) 2938, 1729, 1597, 1358, 1290, 1169, 1092, 694, 664 cm−1. HRMS calcd For C19H20NO4S [M+H]+ 358.1108, found 358.1093.
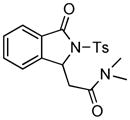
4.8 3-Dimethylaminocarbonylmethyl-2-tosylisoindolin-1-one (3h)
1H NMR (500 MHz, CDCl3) δ 2.40 (s, 3H), 2.69 (dd, J = 10.0, 16.5 Hz, 1H), 2.99 (s, 3H), 3.04 (s, 3H), 3.78 (dd, J = 2.5, 16.5 Hz, 1H), 5.77 (dd, J = 2.5, 10.0 Hz, 1H), 7.32 (d, J = 8.5 Hz, 2H), 7.43 (dd, J = 7.5, 7.5 Hz, 1H), 7.58 (dd, J = 7.5, 8.0 Hz, 1H), 7.70 (d, J = 8.0 Hz, 1H), 7.75 (d, J = 7.5 Hz, 1H), 8.00 (d, J = 8.0 Hz, 2H); 13C NMR (125 MHz, CDCl3) δ 21.9, 35.7, 37.4, 39.9, 59.8, 124.7, 125.0, 128.5, 129.0, 129.2, 129.9, 134.6, 135.7, 145.5, 146.9, 166.9, 169.5; FT-IR (CH2Cl2) 2360, 1732, 1645, 1402, 1358, 1290, 1169, 1090, 695, 663 cm−1. HRMS calcd for C19H21N2O4S [M+H]+ 373.1217, found 373.1217.
4.9 3-Ethoxycarbonyl-3-ethoxycarbonylmethyl-2-tosylisoindolin-1-one (3i)
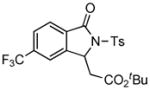
1H NMR (500 MHz, CDCl3) δ 0.79 (t, J = 7.0 Hz, 3H), 1.25 (t, J = 7.0 Hz, 3H), 2.42 (s, 3H), 3.52–3.64 (m, 2H), 3.70 (d, J = 17.5 Hz, 1H), 3.94 (d, J = 17.5 Hz, 1H), 4.16–4.24 (m, 1H), 4.28–4.36 (m, 1H), 7.33 (d, J = 8.5 Hz, 2H), 7.44 (d, J = 7.5 Hz, 1H), 7.51 (dd, J = 7.5, 7.5 Hz, 1H), 7.63 (dd, J = 7.5, 7.5 Hz, 1H), 7.82 (d, J = 7.5 Hz, 1H), 8.10 (d, J = 8.5 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 13.7, 14.0, 21.9, 38.5, 60.8, 63.4, 70.5, 121.3, 125.1, 129.2, 129.4, 129.9, 130.1, 134.4, 136.1, 143.7, 145.4, 166.6, 167.9, 168.6. FT-IR (CH2Cl2) 2982, 1738, 1468, 1366, 1248, 1169, 1123, 1089, 1028, 693, 664 cm−1. HRMS calcd for C22H24NO7S [M+H]+ 446.1268, found 446.1252.
4.10 3-Ethylcarbonyl-3-ethylcarbonylmethyl-2-tosylisoindolin-1-one (3j)
1H NMR (400 MHz) δ 0.57 (t, J = 7.2 Hz, 3H), 0.95 (t, J = 7.2 Hz, 3H), 2.06–2.16 (m, 3H), 2.41 (s, 3H), 2.80–2.92 (m, 1H), 3.70 (d, J = 19.2 Hz, 1H), 3.78 (d, J = 19.2 Hz, 1H), 7.14 (d, J = 7.6 Hz, 1H), 7.30 (d, J = 8.4 Hz, 2H), 7.51 (dd, J = 7.2, 7.6 Hz, 1H), 7.58 (dd, J = 7.2, 7.6 Hz, 1H), 7.93 (d, J = 7.6 Hz, 1H), 8.02 (d, J = 8.4 Hz, 2H); 13C NMR (100 MHz) δ 6.9, 8.5, 21.9, 28.6, 36.3, 42.5, 75.4, 121.1, 125.6, 128.3, 129.8, 130.0, 130.6, 134.6, 135.8, 143.1, 145.7, 167.0, 205.3, 205.6. FT-IR (CH2Cl2) 1745, 1716, 1357, 1169, 1123, 1087, 1057, 822, 702, 658 cm−1. HRMS calcd for C22H24NO5S [M+H]+ 414.1370, found 414.1368.
4.11 3-Ethoxycarbonyl-3-cyanomethyl-2-tosylisoindolin-1-one (3k)
1H NMR (400 MHz) δ 1.25 (t, J = 7.2 Hz, 3H), 2.43 (s, 3H), 3.78 (d, J = 17.6 Hz, 1H), 3.90 (d, J = 17.6 Hz, 1H), 4.16–4.24 (m, 1H), 4.32–4.40 (m, 1H), 7.37 (d, J = 8.0 Hz, 2H), 7.52 (d, J = 7.6 Hz, 1H), 7.60 (dd, J = 7.6, 7.6 Hz, 1H), 7.73 (dd, J = 7.6, 7.6 Hz, 1H), 7.87 (d, J = 7.6 Hz, 1H), 8.09 (d, J = 8.4 Hz, 2H); 13C NMR (100 MHz) δ 14.0, 22.0, 26.2, 64.1, 69.5, 114.7, 121.5, 126.0, 129.1, 129.2, 129.8, 131.3, 135.2, 135.3, 141.9, 146.2, 165.5, 167.3. FT-IR (CH2Cl2) 2987, 1743, 1598, 1468, 1365, 1294, 1267, 1169, 1128, 1088, 815, 748, 698, 666 cm−1. HRMS calcd for C20H19N2O5S [M+H]+ 399.1009, found 399.1009.
4.12 3,3-spiro[3′-N-Methyl-2′,4′-dicarbonylpyrrolidine]-2-tosylisoindolin-1-one (3l)
1H NMR (400 MHz) δ 2.44 (s, 3H), 3.21 (d, J = 14.4 Hz, 1H), 3.27 (s, 3H), 3.89 (d, J = 14.4 Hz, 1H), 7.27 (d, J = 7.6 Hz, 1H), 7.36 (d, J = 8.4 Hz, 2H), 7.55 (dd, J = 7.6, 7.6 Hz, 1H), 7.68 (dd, J = 7.6, 7.6 Hz, 1H), 7.81 (d, J = 7.6 Hz, 1H), 8.05 (d, J = 8.4 Hz, 2H); 13C NMR (100 MHz) δ 22.0, 26.3, 41.6, 69.3, 120.5, 125.8, 128.6, 129.6, 129.7, 130.7, 134.8, 135.4, 144.6, 146.3, 165.4, 172.9, 173.4. FT-IR (CH2Cl2) 1791, 1738, 1715, 1597, 1436, 1382, 1359, 1286, 1263, 1168, 1123, 1091, 1059, 702, 665 cm−1. HRMS calcd for C19H17N2O5S [M+H]+ 385.0853, found 385.0827.
4.13 3-Ethoxycarbonyl-3-ethoxycarbonylmethyl-5-methyl-2-tosylisoindolin-1-one (3n)
1H NMR (400 MHz, CDCl3) δ 0.77 (t, J = 7.2 Hz, 3H), 1.24 (t, J = 7.2 Hz, 3H), 2.40 (s, 3H), 2.43 (s, 3H), 3.52–3.62 (m, 2H), 3.66 (d, J = 17.6 Hz, 1H), 3.90 (d, J = 17.6 Hz, 1H), 4.12–4.22 (m, 1H), 4.28–4.36 (m, 1H), 7.20 (s, 1H), 7.28 (d, J = 8.0 Hz, 1H), 7.30 (d, J = 8.4 Hz, 2H), 7.68 (d, J = 8.0 Hz, 1H), 8.08 (d, J = 8.4 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 13.7, 14.0, 21.9, 22.4, 38.5, 60.7, 63.4, 70.2, 121.6, 125.0, 127.4, 129.2, 129.4, 131.2, 136.2, 144.0, 145.3, 145.7, 166.6, 168.0, 168.8. FT-IR (CH2Cl2) 2983, 1737, 1613, 1598, 1364, 1284, 1250, 1169, 1133, 1088, 1027, 853, 808, 704, 666 cm−1. HRMS calcd for C23H26NO7S [M+H]+ 460.1424, found 460.1413.
4.14 3-Ethoxycarbonyl-3-ethoxycarbonylmethyl-5-methoxy-2-tosylisoindolin-1-one (3o)
1H NMR (400 MHz, CDCl3) δ 0.80 (t, J = 7.2 Hz, 3H), 1.26 (t, J = 7.2 Hz, 3H), 2.42 (s, 3H), 3.54–3.64 (m, 2H), 3.66 (d, J = 17.6 Hz, 1H), 3.86 (s, 3H), 3.92 (d, J = 17.6 Hz, 1H), 4.14–4.24 (m, 1H), 4.30–4.38 (m, 1H), 6.86 (d, J = 2.0 Hz, 1H), 6.99 (dd, J = 2.0, 8.8 Hz, 1H), 7.31 (d, J = 8.4 Hz, 2H), 7.73 (d, J = 8.4 Hz, 1H), 8.09 (d, J = 8.4 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 13.7, 14.0, 21.9, 38.7, 56.1, 60.8, 63.5, 70.0, 105.8, 116.9, 122.2, 126.8, 129.1, 129.4, 136.3, 145.2, 146.1, 164.9, 166.2, 167.9, 168.7. FT-IR (CH2Cl2) 2982, 1738, 1604, 1495, 1362, 1343, 1291, 1254, 1168, 1126, 1085, 1026, 855, 659 cm−1. HRMS calcd for C23H26NO8S [M+H]+ 476.1374, found 476.1376.
4.15 3-Ethoxycarbonyl-3-ethoxycarbonylmethyl-5-fluoro-2-tosylisoindolin-1-one (3p)
1H NMR (400 MHz, CDCl3) δ 0.86 (t, J = 7.2 Hz, 3H), 1.27 (t, J = 7.2 Hz, 3H), 2.42 (s, 3H), 3.63 (q, J = 7.2 Hz, 2H), 3.65 (d, J = 17.6 Hz, 1H), 3.94 (d, J = 17.6 Hz, 1H), 4.18–4.26 (m, 1H), 4.30–4.39 (m, 1H), 7.12 (dd, J = 2.0, 7.6 Hz, 1H), 7.20 (ddd, J = 2.0, 8.4, 8.8 Hz, 1H), 7.33 (d, J = 8.4 Hz, 2H), 7.82 (dd, J = 4.8, 8.4 Hz, 1H), 8.08 (d, J = 8.4 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 13.7, 14.0, 21.9, 38.4, 60.9, 63.7, 70.1, 109.0 (d, JC-F = 25.0 Hz), 118.1 (d, JC-F = 23.4 Hz), 126.1 (d, JC-F = 2.2 Hz), 127.5 (d, JC-F = 9.9 Hz), 129.2, 129.4, 135.9, 145.5, 146.3 (d, JC-F = 10.0 Hz), 165.2, 166.8 (d, JC-F = 267 Hz), 167.7, 167.8. FT-IR (CH2Cl2) 2983, 1736, 1606, 1488, 1365, 1287, 1250, 1170, 1124, 1084, 1027, 853, 814, 655 2 cm−1. HRMS calcd for C22H23FNO7S [M+H]+ 464.1174, found 464.1158.
4.16 3-Ethoxycarbonyl-3-ethoxycarbonylmethyl-5-trifluoromethyl-2-tosylisoindolin-1-one (3q)
1H NMR (400 MHz, CDCl3) δ 0.87 (t, J = 7.2 Hz, 3H), 1.27 (t, J = 7.2 Hz, 3H), 2.43 (s, 3H), 3.63 (q, J = 7.2 Hz, 2H), 3.72 (d, J = 17.6 Hz, 1H), 3.98 (d, J = 17.6 Hz, 1H), 4.18–4.27 (m, 1H), 4.33–4.42 (m, 1H), 7.34 (d, J = 8.4 Hz, 2H), 7.69 (s, 1H), 7.78 (d, J = 8.0 Hz, 1H), 7.95 (d, J = 8.0 Hz, 1H), 8.10 (d, J = 8.4 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 13.8, 14.0, 21.9, 38.2, 61.0, 63.9, 70.6, 118.6 (q, JC-F = 3.8 Hz), 123.3 (q, JC-F = 272 Hz), 125.8, 127.3 (q, JC-F = 3.5 Hz), 129.2, 129.5, 133.3, 135.6, 136.0 (q, JC-F = 32.9 Hz), 144.2, 145.8, 165.3, 167.7, 167.9. FT-IR (CH2Cl2) 2986, 1739, 1369, 1329, 1259, 1171, 1133, 1099, 1028, 846, 696, 659 cm−1. HRMS calcd for C23H23F3NO7S [M+H]+ 514.1142, found 514.1158.
4.17 3-Ethoxycarbonyl-3-ethoxycarbonylmethyl-5-bromo-2-tosylisoindolin-1-one (3r)
1H NMR (400 MHz, CDCl3) δ 0.87 (t, J = 7.2 Hz, 3H), 1.28 (t, J = 7.2 Hz, 3H), 2.42 (s, 3H), 3.63 (q, J = 7.2 Hz, 2H), 3.65 (d, J = 17.6 Hz, 1H), 3.93 (d, J = 17.6 Hz, 1H), 4.16–4.26 (m, 1H), 4.32–4.42 (m, 1H), 7.33 (d, J = 8.4 Hz, 2H), 7.59 (s, 1H), 7.66 (dd, J = 8.0, 16.8 Hz, 2H), 8.08 (d, J = 8.4 Hz, 2H); 13 C NMR (100 MHz, CDCl3) δ 13.8, 14.0, 21.9, 38.3, 61.0, 63.8, 70.0, 124.7, 126.4, 129.0, 129.1, 129.2, 129.5, 133.6, 135.8, 145.3, 145.6, 165.7, 167.7, 168.1. FT-IR (CH2Cl2) 2983, 1737, 1605, 1593, 1367, 1278, 1247, 1170, 1131, 1090, 1028, 839, 664 cm−1. HRMS calcd for C22H23BrNO7S [M+H]+ 524.0373, found 524.0378.
4.18 3-Ethoxycarbonyl-3-ethoxycarbonylmethyl-2-tosyl-5,6-benzo isoindolin-1-one (3s)
1H NMR (400 MHz, CDCl3) δ 0.74 (t, J = 7.2 Hz, 3H), 1.24 (t, J = 7.2 Hz, 3H), 2.41 (s, 3H), 3.51 (q, J = 7.2, 2H), 3.82 (d, J = 18.0 Hz, 1H), 4.01 (d, J = 18.0 Hz, 1H), 4.14–4.20 (m, 1H), 4.30–4.38 (m, 1H), 7.32 (d, J = 8.4 Hz, 2H), 7.57 (dd, J = 7.6, 7.6 Hz, 1H), 7.62 (dd, J = 7.6, 7.6 Hz, 1H), 7.84 (s, 1H), 7.89 (d, J = 8.0 Hz, 1H), 7.99 (d, J = 8.0 Hz, 1H), 8.12 (d, J = 8.4 Hz, 2H), 8.36 (s, 1H); 13C NMR (100 MHz, CDCl3) δ 13.7, 14.0, 21.9, 39.1, 60.8, 63.5, 70.2, 120.5, 126.3, 127.4, 127.6, 128.7, 129.1, 129.2, 129.5, 130.1, 133.6, 136.1, 136.2, 138.3, 145.4, 166.6, 168.1, 169.1. FT-IR (CH2Cl2) 2983, 1736, 1365, 1252, 1180, 1163, 1130, 1086, 1027, 764, 664 cm−1. HRMS calcd for C26H26NO7S [M+H]+ 496.1424, found 496.1421.
4.19 3-Ethoxycarbonyl-3-ethoxycarbonylmethyl-6-methoxy-2-tosylisoindolin-1-one (3t)
1H NMR (400 MHz, CDCl3) δ 0.81 (t, J = 7.2 Hz, 3H), 1.23 (t, J = 7.2 Hz, 3H), 2.40 (s, 3H), 3.55–3.65 (m, 2H), 3.64 (d, J = 17.2 Hz, 1H), 3.80 (s, 3H), 3.87 (d, J = 17.2 Hz, 1H), 4.14–4.22 (m, 1H), 4.26–4.34 (m, 1H), 7.14 (dd, J = 2.4, 8.4 Hz, 1H), 7.24 (d, J = 2.4 Hz, 1H), 7.30 (d, J = 8.4 Hz, 1H), 7.31 (d, J = 8.0 Hz, 2H), 8.08 (d, J = 8.0 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 13.8, 14.0, 21.9, 38.3, 56.0, 60.7, 63.4, 70.2, 107.6, 122.4, 122.7, 129.2, 129.4, 131.3, 135.8, 136.0, 145.3, 161.3, 166.6, 168.0, 168.7. FT-IR (CH2Cl2) 2983, 1736, 1494, 1365, 1289, 1251, 1169, 1129, 1091, 1028, 664 cm−1. HRMS calcd for C23H26NO8S [M+H]+ 476.1374, found 476.1382.
4.20 3-Ethoxycarbonyl-3-ethoxycarbonylmethyl-7-fluoro-2-tosylisoindolin-1-one (3u)
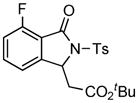
1H NMR (400 MHz, CDCl3) δ 0.86 (t, J = 7.2 Hz, 3H), 1.26 (t, J = 7.2 Hz, 3H), 2.43 (s, 3H), 3.56–3.68 (m, 2H), 3.67 (d, J = 17.6 Hz, 1H), 3.94 (d, J = 17.6 Hz, 1H), 4.16–4.26 (m, 1H), 4.30–4.38 (m, 1H), 7.14 (dd, J = 8.4, 8.4 Hz, 1H), 7.22 (d, J = 7.6 Hz, 1H), 7.32 (d, J = 8.4 Hz, 2H), 7.61 (ddd, J = 4.8, 7.6, 8.0 Hz, 1H), 8.09 (d, J = 8.4 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 13.7, 14.0, 21.9, 38.6, 60.9, 63.7, 70.1, 117.2 (d, JC-F = 4.2 Hz), 117.4 (d, JC-F = 18.8 Hz), 129.3, 129.5, 135.7, 136.4 (d, JC-F = 7.9 Hz), 145.6, 145.9 (d, JC-F = 2.3 Hz), 159.4 (d, JC-F = 274 Hz), 163.3 (d, JC-F = 2.6 Hz), 167.8, 168.2. FT-IR (CH2Cl2) 2984, 1740, 1622, 1483, 1367, 1256, 1236, 1196, 1171, 1122, 1090, 1073, 1035, 814, 691, 664 cm−1. HRMS calcd for C22H23FNO7S [M+H]+ 464.1174, found 464.1186.
4.21 3-Ethoxycarbonyl-3-ethoxycarbonylmethyl-4,6-dimethoxy-2-tosylisoindolin-1-one (3v)
1H NMR (400 MHz, CDCl3) δ 0.84 (t, J = 7.2 Hz, 3H), 1.24 (t, J = 7.2 Hz, 3H), 2.42 (s, 3H), 3.57–3.65 (m, 2H), 3.79 (d, J = 17.2 Hz, 1H), 3.81 (s, 3H), 3.83 (s, 3H), 3.90 (d, J = 17.2 Hz, 1H), 4.16–4.24 (m, 1H), 4.26–4.34 (m, 1H), 6.61 (d, J = 2.0 Hz, 1H), 6.87 (d, J = 2.0 Hz, 1H), 7.31 (d, J = 8.4 Hz, 2H), 8.07 (d, J = 8.4 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 13.8, 14.1, 21.9, 36.0, 56.0, 56.1, 60.5, 62.9, 69.3, 98.7, 105.1, 124.3, 129.1, 129.4, 132.5, 136.1, 145.3, 155.1, 162.9, 166.7, 167.8, 168.6. FT-IR (CH2Cl2) 2982, 1741, 1625, 1598, 1503, 1459, 1356, 1323, 1244, 1169, 1151, 1090, 1068, 1031, 827, 664 cm−1. HRMS calcd for C24H28NO9S [M+H]+ 506.1479, found 506.1458.
Supplementary Material
Acknowledgments
We thank the NIH (GM31278) and the Robert A. Welch Foundation (GL625910) for financial support.
Footnotes
NMR spectra. Supplementary data related to this article can be found online at
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.For recent reviews about C-H activation, see: Lewis JC, Bergman RG, Ellman JA. Acc Chem Res. 2008;41:1013. doi: 10.1021/ar800042p.Li BJ, Yang SD, Shi ZJ. Synlett. 2008:949.Chen X, Engle KM, Wang DH, Yu JQ. Angew Chem, Int Ed. 2009;48:5094. doi: 10.1002/anie.200806273.Ackermann L, Vicente R, Kapdi AR. Angew Chem, Int Ed. 2009;48:9792. doi: 10.1002/anie.200902996.Daugulis O, Do HQ, Shabashov D. Acc Chem Res. 2009;42:1074. doi: 10.1021/ar9000058.Giri R, Shi BF, Engle KM, Maugel N, Yu JQ. Chem Soc Rev. 2009;38:3242. doi: 10.1039/b816707a.Colby DA, Bergman RG, Ellman JA. Chem Rev. 2010;110:624. doi: 10.1021/cr900005n.Mkhalid IAI, Barnard JH, Marder TB, Murphy JM, Hartwig JF. Chem Rev. 2010;110:890. doi: 10.1021/cr900206p.Lyons TW, Sanford MS. Chem Rev. 2010;110:1147. doi: 10.1021/cr900184e.Ackermann L. Chem Commun. 2010;46:4866. doi: 10.1039/c0cc00778a.Jazzar R, Hitce J, Renaudat A, Sofack-Kreutzer J, Baudoin O. Chem Eur J. 2010;16:2654. doi: 10.1002/chem.200902374.Yeung CS, Dong VM. Chem Rev. 2011;111:1215. doi: 10.1021/cr100280d.McMurry L, O’Hara F, Gaunt MJ. Chem Soc Rev. 2011;40:1885. doi: 10.1039/c1cs15013h.Gutekunst WR, Baran PS. Chem Soc Rev. 2011;40:1976. doi: 10.1039/c0cs00182a.Hartwig JF. Chem Soc Rev. 2011;40:1992. doi: 10.1039/c0cs00156b.Wencel-Derord J, Dröge T, Liu F, Glorius F. Chem Soc Rev. 2011;40:4740. doi: 10.1039/c1cs15083a.Song G, Wang F, Li X. Chem Soc Rev. 2012;41:3651. doi: 10.1039/c2cs15281a.Zhu C, Wang R, Falck JR. Chem Asian J. 2012;7:1502. doi: 10.1002/asia.201200035.
- 2.(a) Wrobel J, Dietrich A, Woolson SA, Millen J, McCaleb M, Harrison MC, Hohman TC, Sredy J, Sullivan D. J Med Chem. 1992;35:4613. doi: 10.1021/jm00102a016. [DOI] [PubMed] [Google Scholar]; (b) Anzini M, Capelli A, Vomero S, Giorgi G, Langer T, Bruni G, Romero MR, Basile AS. J Med Chem. 1996;39:4275. doi: 10.1021/jm960325j. [DOI] [PubMed] [Google Scholar]; (c) Chang LC, Bhat KPL, Pisha E, Kennelly EJ, Fong HHS, Pezzuto JM, Kinghorn AD. J Nat Prod. 1998;61:1257. doi: 10.1021/np980162x. [DOI] [PubMed] [Google Scholar]; (d) Belliotti TR, Brink WA, Kesten SR, Rubin JR, Wustrow DJ, Zoski KT, Whetzel SZ, Corbin AE, Pugsley TA, Heffner TG, Wise LD. Bioorg Med Chem Lett. 1998;8:1499. doi: 10.1016/s0960-894x(98)00252-2. [DOI] [PubMed] [Google Scholar]; (e) Honma T, Hayashi K, Aoyama T, Hashimoto N, Machida T, Fukasawa K, Iwama T, Ikeura C, Ikuta M, Suzuki-Takahashi I, Iwasawa Y, Hayama T, Nishimura S, Morishima H. J Med Chem. 2001;44:4615. doi: 10.1021/jm0103256. [DOI] [PubMed] [Google Scholar]; (f) Sorbera LA, Leeson PA, Silvestre J, Castaner J. Drug Future. 2001;26:651. [Google Scholar]; (g) Stuk TL, Assink BK, Bates RC, Jr, Erdman DT, Fedij V, Jennings SM, Lassig JA, Smith RJ, Smith TL. Org Process Res Dev. 2003;7:851. [Google Scholar]; (h) Riedinger C, Endicott JA, Kemp SJ, Smyth LA, Watson A, Valeur E, Golding BT, Griffin RJ, Hardcastle IR, Noble ME, McDonnell JM. J Am Chem Soc. 2008;130:16038. doi: 10.1021/ja8062088. [DOI] [PubMed] [Google Scholar]
- 3.For selected examples, see: Pin F, Comesse S, Garrigues B, Marchalin S, Daich A. J Org Chem. 2007;72:1181. doi: 10.1021/jo062077x.Klumpp DA, Zhang Y, O’Connor MJ, Esteves PM, de Almeida LS. Org Lett. 2007;9:3085. doi: 10.1021/ol0711570.Guo S, Xie Y, Hu X, Xia C, Huang H. Angew Chem, Int Ed. 2010;49:2728. doi: 10.1002/anie.200907320.Sai KKS, O’Connor MJ, Klumpp DA. Tetrahedron Lett. 2010;52:2195. doi: 10.1016/j.tetlet.2010.11.164.Campbell JB, Dedinas RF, Trumbower-Walsh S. Synlett. 2010:3008.Shacklady-McAtee DM, Dasgupta S, Watson MP. Org Lett. 2011;13:3490. doi: 10.1021/ol201248c.
- 4.Zhu C, Falck JR. Org Lett. 2011;13:1214. doi: 10.1021/ol200093f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wrigglesworth JW, Cox B, Lloyd-Jones GC, Booker-Milburn KI. Org Lett. 2011;13:5326. doi: 10.1021/ol202187h. [DOI] [PubMed] [Google Scholar]
- 6.Li DD, Yuan TT, Wang GW. Chem Commun. 2011;47:12789. doi: 10.1039/c1cc15897j. [DOI] [PubMed] [Google Scholar]
- 7.Wang F, Song G, Li X. Org Lett. 2010;12:5430. doi: 10.1021/ol102241f. [DOI] [PubMed] [Google Scholar]
- 8.Patureau FW, Besset T, Glorius F. Angew Chem Int Ed. 2011;50:1064. doi: 10.1002/anie.201006222. [DOI] [PubMed] [Google Scholar]
- 9.Ackermann L, Wang L, Wolfram R, Lygin AV. Org Lett. 2012;14:728. doi: 10.1021/ol203251s. [DOI] [PubMed] [Google Scholar]
- 10.For a special review about [RhCl2Cp*]2, see: Satoh T, Miura M. Chem Eur J. 2010;16:11212. doi: 10.1002/chem.201001363.
- 11.For selected examples, see: Ueura K, Satoh T, Miura M. J Org Chem. 2007;72:5362. doi: 10.1021/jo070735n.Ueura K, Satoh T, Miura M. Org Lett. 2007;9:1407. doi: 10.1021/ol070406h.Stuart DR, Bertrand-Laperle M, Burgess KMN, Fagnou K. J Am Chem Soc. 2008;130:16474. doi: 10.1021/ja806955s.Shimizu M, Hirano K, Satoh T, Miura M. J Org Chem. 2009;74:3478. doi: 10.1021/jo900396z.Mochida S, Hirano K, Satoh T, Miura M. J Org Chem. 2009;74:6295. doi: 10.1021/jo901077r.Fukutani T, Umeda N, Hirano K, Satoh T, Miura M. Chem Commun. 2009:5141. doi: 10.1039/b910198e.Mochida S, Shimizu M, Hirano K, Satoh T, Miura M. Chem Asian J. 2010;5:847. doi: 10.1002/asia.200900639.Guimond N, Gouliaras C, Fagnou K. J Am Chem Soc. 2010;132:6908. doi: 10.1021/ja102571b.Hyster TK, Rovis T. J Am Chem Soc. 2010;132:10565. doi: 10.1021/ja103776u.Mochida S, Umeda N, Hirano K, Satoh T, Miura M. Chem Lett. 2010;39:744.Song G, Chen D, Pan CL, Crabtree RH, Li X. J Org Chem. 2010;75:7487. doi: 10.1021/jo101596d.Rakshit S, Patureau FW, Glorius F. J Am Chem Soc. 2010;132:9585. doi: 10.1021/ja104305s.Su Y, Zhao M, Han K, Song G, Li X. Org Lett. 2010;12:5462. doi: 10.1021/ol102306c.Rakshit S, Grohmann C, Besset T, Glorius F. J Am Chem Soc. 2011;133:2350. doi: 10.1021/ja109676d.Li X, Gong X, Zhao M, Song G, Deng J, Li X. Org Lett. 2011;13:5808. doi: 10.1021/ol2023856.Willwacher J, Rakshit S, Glorius F. Org Biomol Chem. 2011;9:4736. doi: 10.1039/c1ob05636k.
- 12.Zhu C, Falck JR. Chem Comm. 2012;48:1674. doi: 10.1039/c2cc16963k. [DOI] [PubMed] [Google Scholar]
- 13.Zhu C, Xie W, Falck JR. Chem Eur J. 2011;17:12591. doi: 10.1002/chem.201102475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.For selected reviews, see: Doyle MP, Forbes DC. Chem Rev. 1998;98:911. doi: 10.1021/cr940066a.Lebel H, Marcoux J, Molinaro C, Charette AB. Chem Rev. 2003;103:977. doi: 10.1021/cr010007e.Davies HML, Beckwith REJ. Chem Rev. 2003;103:2861. doi: 10.1021/cr0200217.
- 15.Callot HJ, Metz F, Piechocki C. Tetrahedron. 1982;38:2365. [Google Scholar]
- 16.During the review process of this manuscript, a transformation of C-H activation incorporating with diazo compounds in the use of same rhodium (III) catalyst has been reported. See: Chan WW, Lo SF, Zhou Z, Yu WY. J Am Chem Soc. 2012;134:13565. doi: 10.1021/ja305771y.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.