Abstract
Adrenergic hormones are essential for early heart development. To gain insight into understanding how these hormones influence heart development, we evaluated genomic expression changes in embryonic hearts from adrenergic-deficient and wild-type control mice. To perform this study, we used a mouse model with targeted disruption of the Dopamine β-hydroxylase (Dbh) gene, whose product is responsible for enzymatic conversion of dopamine into norepinephrine. Embryos homozygous for the null allele (Dbh−/−) die from heart failure beginning as early as embryonic day 10.5 (E10.5). To assess underlying causes of heart failure, we isolated hearts from Dbh−/− and Dbh+/+ embryos prior to manifestation of the phenotype and examined gene expression changes using genomic Affymetrix 430A 2.0 arrays, which enabled simultaneous evaluation of >22,000 genes. We found that only 22 expressed genes showed a significant twofold or greater change, representing ∼0.1% of the total genes analyzed. More than half of these genes are associated with either metabolism (31%) or signal transduction (22%). Remarkably, several of the altered genes encode for proteins that are directly involved in retinoic acid (RA) biosynthesis and transport. Subsequent evaluation showed that RA concentrations were significantly elevated by an average of ∼3-fold in adrenergic-deficient (Dbh−/−) embryos compared with controls, thereby suggesting that RA may be an important downstream mediator of adrenergic action during embryonic heart development.
Keywords: hormone, mouse, heart, catecholamine, microarray, ultrasound
adrenergic hormones play an essential yet unexplained role in the mammalian heart during embryonic/fetal development (17, 43, 44, 56, 99, 109). Norepinephrine (NE) and epinephrine (EPI) are the main adrenergic hormones/neurotransmitters in mammals. In adults, EPI and NE are primarily produced in the chromaffin cells of the adrenal medulla as well as in neurons in the sympathetic and central nervous systems. Numerous other sources of adrenergic hormones have also been found in a diverse array of tissues and organs including the organ of Zuckerkandl (78), retina (30), kidney (28, 52), spleen (28, 52, 77), testes (11), lungs (28, 52, 77), and heart (1, 14, 16, 18, 19, 44, 46, 47, 52, 74–76). In the mid-1990s, it was discovered that NE and/or EPI are vital for embryonic survival in utero since disruption of the genes encoding for the catecholamine biosynthetic enzymes, tyrosine hydroxylase (Th) or dopamine β-hydroxylase (Dbh), resulted in fetal and perinatal lethality due to apparent heart failure (55, 99, 109). In the absence of NE and EPI, embryos survive and appear relatively “normal” until approximately embryonic day 10.5 (E10.5) but then begin to die over the next several days with ∼90% of the Dbh−/− mice not surviving to birth. We showed that disruption of the EPI-specific biosynthetic enzyme, phenylethanolamine n-methyltransferase (Pnmt), results in a loss of EPI but does not display any obvious developmental phenotype (16). Since Pnmt−/− mice still produce NE, this hormone likely compensates, in part, for the loss of EPI. NE and EPI can both stimulate adrenergic receptors.
It is known that while both α- and β-adrenergic receptors are expressed and functional in the developing heart (21, 32, 42, 62, 66, 67, 70, 84, 102), it is primarily the β-receptors that are important for survival through birth and the early postnatal period (25, 49, 70, 79, 98, 100). Drug rescue experiments, for example, showed that the β-agonist isoproterenol could rescue Dbh−/− embryos when supplied in the maternal drinking water (98, 100). In contrast, the α-agonist l-phenylephrine had little effect when administered alone but appeared to slightly improve survival when used in combination with isoproterenol (79, 98, 100). Furthermore, targeted deletion of the β-adrenergic receptor kinase 1 (βARK-1) gene in mice resulted in embryonic lethality due to heart failure with many phenotypic characteristics similar to those described for Dbh−/− embryos (49). In addition, genetic knockout of β1- and β2-adrenergic receptors led to cardiovascular and metabolic defects (8, 85, 86). These studies in total indicate that NE stimulation of β-adrenergic receptors is crucial for heart development and embryonic survival. The downstream targets and molecular signaling mechanisms secondary to the stimulation of adrenergic receptors in the developing heart are not well-defined.
Recent reports suggest that hypoxia may be an important incoming signal that stimulates NE release, which, in turn, stimulates heart rate during hypoxic stress (79, 81). A hypothesis drawn from these studies is that adrenergic-deficient embryos are unable to respond to hypoxic stress by releasing NE and thus cannot properly compensate for lower O2 concentrations by increasing cardiac output. A prediction from this hypothesis is that adrenergic-deficient embryos would become increasingly hypoxic over time and ultimately perish as a result of inadequate blood flow. While there is good evidence supporting the hypoxia hypothesis, it does not appear to provide a full explanation for adrenergic action in the developing heart. Notably, the hypoxia experiments were primarily performed with isolated fetal hearts at E12.5 and later stages, whereas Dbh−/− embryos tend to begin dying a couple of days earlier. Furthermore, most of the supportive evidence stems from ex vivo cultures where conditions were carefully controlled and thus not completely reflective of the in vivo environment. It therefore remains unclear if hypoxia is a causative stimulus for adrenergic action in vivo, particularly at early embryonic stages.
To investigate underlying mechanisms that mediate adrenergic actions in early heart development, we utilized a Dbh−/− mouse model to evaluate physiological and genetic changes associated with the loss of NE and EPI during the early embryonic period extending from E9.5 to E12.5 (17). We employed a combination of noninvasive high-resolution ultrasound imaging and genome-wide microarray analyses to evaluate cardiac changes that occurred due to the absence of endogenous adrenergic hormones. These results suggest that the major categories of genes affected by the absence of adrenergic hormones are those involved in metabolism and signal transduction. In particular, we show that multiple retinoic acid (RA) synthesis and signaling genes display altered expression leading to significant increases in embryonic RA concentrations during a critical phase of early heart development.
MATERIALS AND METHODS
Animals.
All procedures and care of the mice were conducted in accordance with National Institutes of Health guidelines for use of vertebrate animals in research. All animal procedures were reviewed and approved by the appropriate Institutional Animal Care and Use Committees. Breeding and maintenance of the Dbh knockout mouse model were performed as previously described (99). Timed matings were performed with E0.5 defined as noon of the day of vaginal plug verification. All-trans RA (Sigma-Aldrich, St. Louis, MO) was diluted in peanut oil and administered on E10 via oral gavage (50 mg/kg) as previously described (9, 83). Vehicle-only controls received peanut oil only in the same volume as used for RA administration (0.1 ml per mouse).
Ultrasonography.
Embryonic heart rates were evaluated noninvasively in vivo using a Vevo 660 high-resolution ultrasound instrument (Visualsonics, Toronto, Canada). Briefly, pregnant mice were anesthetized with isoflurane and placed onto a heated stage. Maternal body temperature and heart rate were monitored via rectal thermometer and limb ECG leads, respectively. Abdominal hair was removed with Nair hair removal product, and ultrasound data were collected for each embryo using 40 or 55 MHz transducers. Embryonic heart rates were measured using instrument software and confirmed by visual inspection of the recordings. The dams were returned to their cages with heating pads to facilitate recovery. Recordings were performed daily beginning at E9.5.
Embryonic tissue collection.
The dams were killed and the embryos were collected for genotyping and gene expression analyses immediately after the last ultrasound recording session. In most cases, we could readily correlate the position of the embryo with the ultrasound recording. In some cases, however, it was not always clear, especially if the litter was large. Only those embryos that were clearly identified on ultrasound recordings at the time of isolation (i.e., prior to genotype determination) were used for further analysis. For microarray studies, Dbh+/− sire and dam were mated generating Dbh+/+, Dbh+/−, and Dbh−/− offspring collected at E10.5. Furthermore, only embryos that were viable with clearly beating hearts, bright red blood, and no signs of stunted or abnormal morphological development apparent upon visual inspection under a dissecting microscope were used for the microarray, RT-PCR, and RA bioassay assessments. The head was rapidly removed for DNA extraction and genotyping by PCR methods and primers as previously described (99). For the microarray and RT-PCR analyses, the heart was isolated and rapidly frozen for subsequent RNA extraction as described below. For the RA bioassay, the entire embryonic trunk, including the heart, was rapidly frozen following removal of the head for DNA extraction. Tissues were further processed for each assay as described below.
RNA isolation.
Hearts collected for mRNA expression analysis were rapidly frozen in prechilled microfuge tubes on dry ice and stored at −80°C for subsequent use. Frozen embryonic hearts were homogenized in TRIzol (Sigma Aldrich, St. Louis, MO), and total RNA was extracted by established methods as previously described (15). Total RNA quality and yield were assessed with NanoDrop 1000. Only total RNA samples showing an OD260/OD280 ratio between 1.8 and 2.1 were used for microarray and real-time PCR.
Microarray data analysis.
Mouse Genome 430A 2.0 Arrays (AffyMetrix, Santa Clara, CA) were used to analyze gene expression differences in Dbh+/+ and Dbh−/− hearts at E10.5. Purified total RNA was prepared from frozen embryonic hearts and delivered to Gene Logic (Gaithersburg, MD) to perform the microarray analysis. RNA quality-control results and basic statistical analysis of gene expression results were provided by Gene Logic. Access to these files as well as the raw microarray data files from this experiment is provided at the following web link: http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=rfghpyusacumoho&acc=GSE33906, These files include individual chip data, MAS 5 signal intensities, detection calls, and detection P values. Probe set annotations included in these files provide the gene name, ontology, pathway membership, and Unigene ID for each gene on the array. Additional information about the basic statistical analysis can be found below under Statistical methods. The networks and canonical pathways were generated through the use of Ingenuity Pathway Analysis (IPA) algorithms (Ingenuity Systems, http://www.ingenuity.com).
Real-time PCR analysis.
Genomic DNA was removed from the isolated RNA by digestion with RNase-free DNase (Promega, Madison, WI). The cDNA was prepared using Affinity Script QPCR cDNA synthesis kit (600559; Stratagene, Cedar Creek, TX). We used 0.4 μg total RNA for each cDNA synthesis reaction. Real-time PCR reactions were performed using IQ SYBR Green super mix (Bio-Rad Laboratories, Hercules, CA) and run in the Bio-Rad ICycler. Oligonucleotide sequences and annealing temperatures are shown in Table 1.
Table 1.
Primer information for RT-PCR analyses
| Transcript Name | Sequence | Amplicon Size, bp | Anealing Temp., °C |
|---|---|---|---|
| Neurofibromin 1 (1) | F: 5′GCATTGCCAATCATGTACTGT3′ | ||
| R: 5′CTGGTAAGGTTAAGGCTGGACCAG3′ | 356 | 60 | |
| Neurofibromin 1 (2) | F: 5′CCGGAGCACAAGCCTGTGGC3′ | ||
| R: 5′CTGGTAAGGTTAAGGCTGGACCAG3′ | 53 | 63 | |
| Retinol binding protein 1 | F: 5′CGCTTTCTGTCCAGTGCATA3′ | ||
| R: 5′CAGGTTTGCTAGCGTCATCA3′ | 191 | 56 | |
| Fibromodulin | F: 5′GCACTTGGAGAGGCTGTACT3′ | ||
| R: 5′AGGCCCTCCAAAGCATTGTT3′ | 134 | 57 | |
| Fgf 20 | F: 5′ATGGCTCCCTTGACCGAAGTCGG3′ | ||
| R: 5′GGCGGCGCAGGATGCCGTCAGG3′ | 200 | 59 | |
| Aldh1A2 | F: 5′CGCTGAGCAGACACCGCT3′ | ||
| R: 5′CCAGCTGCTTCTTGAATAAGC3′ | 210 | 55 | |
| Bcmo1 | SUPERARRAY-PPM32381A | ||
| SUPERARRAY-PPM32381A | 130 | 60 | |
| Gapdh | F: 5′AGAGATGATGACCCTTTTGGC3′ | ||
| R: 5′CCATCACCATCTTCCAGGAGCG3′ | 149 | 56 | |
| VegfA | F: 5′CGACAGAAGGAGAGCAGAAGTCCC3′ | ||
| R: 5′TGGCTTTGGTGAGGTTTGATCCGC3′ | 256 | 65 |
RA bioassay.
F9-RARE-LacZ cells were kindly provided by Drs. Michael Wagner and Alexander Moise (SUNY Downstate Medical Center, Brooklyn, NY) (104). These cells were maintained in L-15 Leibovitz media containing 20% heat inactivated, charcoal-stripped fetal bovine serum and 1% PenStrep (vol/vol) as previously described (104). We used a modification of previous assay methods for quantification of β-galactosidase activity (91, 104, 108) as described below. The cells were grown to confluence (80–90%) on 100 mm gelatin-coated dishes then plated at 60% confluence on 24-well dishes and allowed to settle overnight. Immediately before the assay individual embryonic trunks were homogenized via 5 s sonication twice in F9-RARE-LacZ cell culture assay media (passage media plus 1.6 g/l d-glucose) under dim lighting. The homogenate was centrifuged at 8,000 g for 5 min to remove cell debris before transfer to the F9-RARE-LacZ cells. Immediately prior to adding the homogenate, we removed the normal passage media from the F9-RARE-LacZ cells and added 0.9 ml of assay media followed by 100 μl of the embryonic trunk homogenate. The cells were then incubated overnight at 37°C and 5% CO2. For the detection of β-galactosidase activity using the fluorescein di-β-d-galactopyranoside (FDG) substrate, cocultured F9-RARE-lacZ cells were lysed in 100μl of Z-buffer (0.8 g Na2HPO4, 0.28 g NaH2PO4, 0.5 ml 1 M KCL, 0.05 ml 1 M MgSO4, and 0.135 ml β-mercaptoethanol, all dissolved in pure water at a final volume of 50 ml) via 5 s sonication, and added to a 96-well plate with the addition of 2 μl of 1 mM FDG. Light production was measured with an EnVision Multilabel plate reader (PerkinElmer).
Statistical methods.
Data are presented as means ± SE, except where specified otherwise. Student's t-test (two-sample comparisons) or one-way analysis of variance (three or more sample comparisons) was performed with P < 0.05 required to reject the null hypothesis. For post hoc testing, the Bonferroni transformation was used to assess statistical significance of differences between specific groups. The basic statistical analysis of gene expression data from the microarray provides the mean, standard deviation, and percent present (P) values for each gene. For pairwise statistical comparison between Dbh+/+ and Dbh−/− E10.5 gene expression data (n = 4 each), fold-change and t-test P values are indicated for each gene on the array. For canonical pathway analysis, Fisher's exact test was used to determine significance (P < 0.05) (Ingenuity Systems, http://www.ingenuity.com).
RESULTS
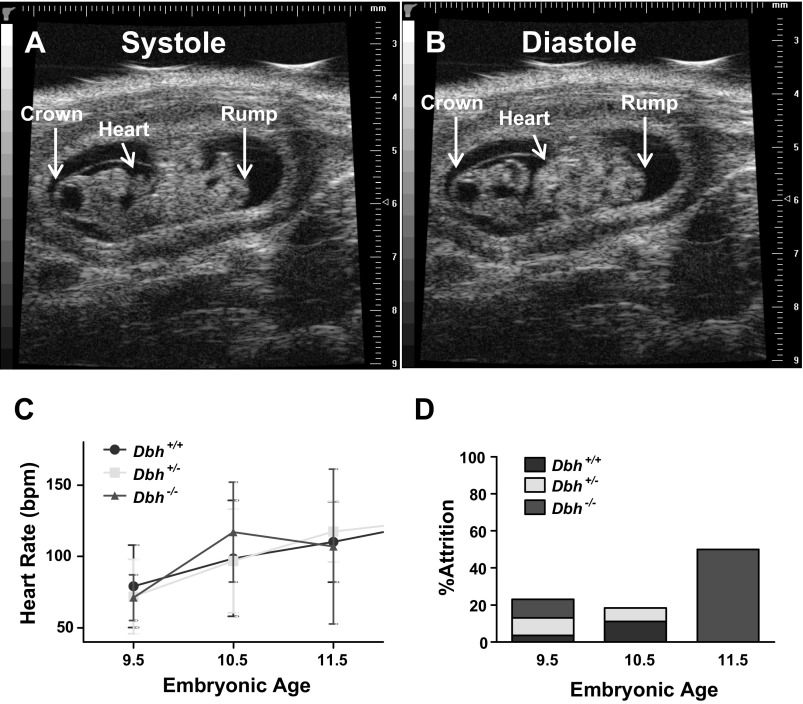
To investigate how adrenergic deficiency leads to embryonic heart failure and death in utero, we used high-resolution ultrasound imaging to evaluate cardiac performance in adrenergic-deficient (Dbh−/−) and adrenergic-competent (Dbh+/+ and Dbh+/−) mouse hearts in vivo. Representative images from this analysis are shown in Fig. 1, A and B, where sagittal views of an E10.5 mouse illustrate the ability to detect cardiac contractions and ability to measure crown-rump length as an estimation of embryonic age (51). Heart rates were assessed on viable E9.5, E10.5, and E11.5 embryos. Embryos were subsequently collected for genotyping and mRNA expression profiling as described in materials and methods. No significant differences were observed in mean heart rates from adrenergic-deficient mouse embryos compared with controls at any of these ages (Fig. 1C).
Fig. 1.
Phenotypic analysis of adrenergic-deficient (Dbh−/−) mouse embryonic development. In vivo ultrasound image of a wild-type embryonic day 10.5 (E10.5) mouse embryo in systole (A) and diastole (B). Crown-rump lengths were estimated as indicated. C: heart rates (means ± SE) for the corresponding embryonic ages, as assessed by high-resolution ultrasound imaging. D: attrition rates by genotype between E9.5 and E11.5.
Overall attrition rates were similar between genotypes at E9.5 and E10.5 but increased dramatically in the Dbh−/− group at E11.5, with roughly half of the adrenergic-deficient population failing to survive to this stage of development (Fig. 1D). These findings are consistent with the original reports that most Dbh−/− mice survive through E10.5 but then steadily decline in numbers over the next few days with >80% dead before birth in heterozygous (Dbh+/−) dams (99). The most notable differences were observed in the heart rate and rhythm of the Dbh−/− embryos shortly before they died after E10.5. Having recorded heart rates from the same sets of embryos on successive developmental days, we were able to witness this phenomenon directly. Heart rates would appear essentially normal for all genotypes at E9.5, E10.5, and E11.5, but occasionally a few Dbh−/− hearts that were beating well at E9.5, E10.5, or even E11.5 began to develop slow, labored contractions sometimes with associated asynchronous and arrhythmic activity. An example of one such recording of a Dbh−/− heart at E10.5 is shown in Supplemental Video 1.1 The heart rate of this Dbh−/− embryo had been ∼60 beats/min (bpm) at E9.5, similar to that of the adrenergic-competent embryos (Fig. 1C), but had declined to ∼34 bpm by E10.5, and stopped sometime between E10.5 and E11.5, as no heart beat was detected on the final round of ultrasound measurements for this particular litter. Other Dbh−/− embryos within the same litter survived with little or no observable difference in heart rates through E11.5. In other cases, the slow labored irregular contractile activity first appeared at E11.5 or E12.5, typically followed by death within 24 h. Consequently, it appears that beating rates develop normally during the early phase of heart development but then start to decline beginning around E10.5 in some Dbh−/− hearts but not in others. There appears to be a window that extends for a few days where the heart is vulnerable to the absence of adrenergic hormones, but this does not always begin at E10.5.
We took advantage of our echocardiographic assessments to identify viable hearts of various genotypes (Dbh+/+, Dbh+/−, and Dbh−/−) that were beating at normal physiological rates. These hearts were immediately collected for analysis of gene expression changes at E10.5, near the beginning of the developmental period where embryos show greatest vulnerability to adrenergic deficiency (99, 109). The objective of this experiment was to determine if there were underlying alterations in gene expression profiles that precede the physiological decline and ultimate death of Dbh−/− embryos in utero. To minimize confounding variables related to downstream gene expression changes incurred as a consequence of heart failure in general, we sought to obtain early specimens that were asymptomatic. The criteria that we used included assessment of heart rates, crown-rump lengths, and general morphological/structural appearance at time of dissection. The embryos collected for this analysis were indistinguishable from E10.5 wild-type controls in terms of these criteria. Notably, all appeared to be in relatively good health with adequate cardiac function at the time of isolation at E10.5.
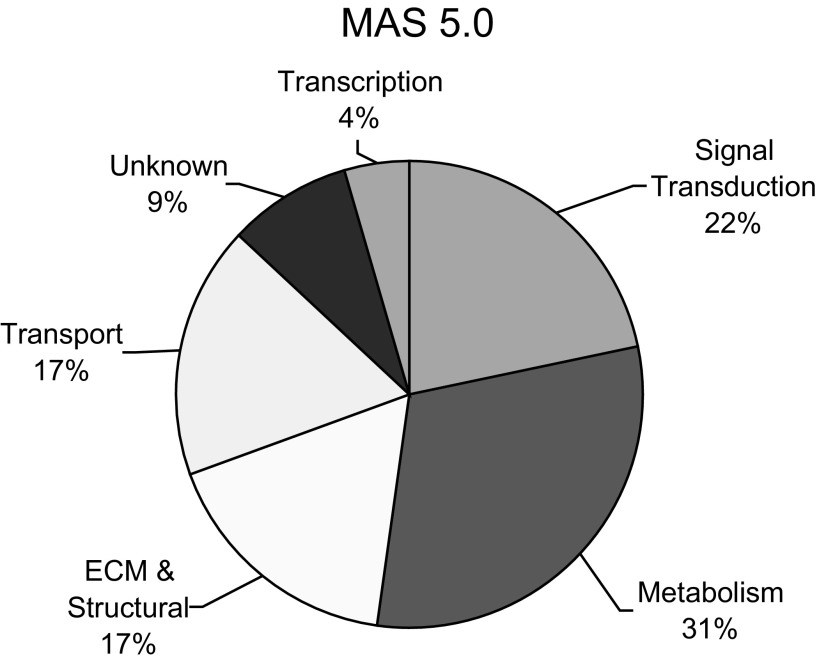
We identified four viable Dbh−/− (experimental) and four Dbh+/+ (control) age- and size-matched E10.5 mouse hearts for microarray analysis. To assess changes in gene expression between these two groups, we extracted RNA and analyzed the samples using Mouse Genome 430A 2.0 Arrays (Affymetrix, Santa Clara, CA). These arrays permit simultaneous evaluation of >22,000 annotated genes in the mouse genome. The microarray data were analyzed using the Affymetrix MAS5.0 software to compare the two groups according to the following criteria: 1) P value < 0.05, 2) ≥2-fold change, and 3) detection call and signal must be present (P) in at least half of the samples from either group. This yielded a list of 22 genes (23 transcripts) that were significantly (P < 0.05) altered by a factor of two or more between E10.5 Dbh−/− and Dbh+/+ hearts (Table 2). Nine of these genes were downregulated in Dbh−/− hearts relative to Dbh+/+ hearts, with the RA biosynthetic enzyme, β-carotene 15,15′-monooxygenase (Bcmo1), and phospholipase A2, group IID (Pla2g2d) genes displaying the greatest decreases (-2.7-fold). Twelve genes showed marked increase in expression, with fibroblast growth factor 20 (Fgf20) commanding the greatest increase (+5.3-fold). One gene, fibromodulin (Fmod), was identified twice (from two different target sequences on the array) as a gene displaying increased expression (+2.2-fold and +2.7-fold) in the adrenergic-deficient hearts. An analysis of general ontology categories of the altered genes identified from the microarray data shows that most changes were found in metabolic (31%) or signal transduction (22%) genes (Fig. 2). Other categories showing significant expression changes included those encoding for extracellular matrix (ECM) and structural proteins (17%), transport proteins (17%), transcription factors (4%), and proteins of unknown function (9%).
Table 2.
Genes identified as significantly changed on microarray by MAS 5.0 analysis
| Gene Symbol | Gene Name | Gene Function | Fold Change | P Value |
|---|---|---|---|---|
| Bcmo1 | beta-carotene 15,15′-monooxygenase | RA metabolism | −2.7 | 0.042 |
| Pla2 g2d | phospholipase A2, group IID | lipid catabolism | −2.7 | 0.043 |
| Ptgfr | prostaglandin F receptor | signal transduction | −2.6 | 0.009 |
| Otub1 | OTU domain, ubiquitin aldehyde binding 1 | ubiquitin cycle | −2.4 | 0.012 |
| Cacna2d2 | calcium channel, voltage-dependent, alpha 2/delta subunit 2 | calcium ion transport | −2.3 | 0.036 |
| Nelf | nasal embryonic LHRH factor | biological process unknown | −2.2 | 0.033 |
| Nf1 | neurofibromatosis 1 | Ras signaling, proto-oncogene | −2.2 | 0.039 |
| Pcdhac2 | protocadherin alpha subfamily C, 2 | cell adhesion | −2.1 | 0.014 |
| Jak3 | Janus kinase 3 | protein amino acid phosphorylation | −2.1 | 0.034 |
| Rdh12 | retinol dehydrogenase 12 | RA synthesis | 2.1 | 0.040 |
| Ndufb2 | NADH dehydrogenase (ubiquinone) 1 beta subcomplex 2 | oxidative metabolism | 2.1 | 0.046 |
| Cdk5rap2 | CDK5 regulatory subunit associated protein 2 | biological process unknown | 2.1 | 0.050 |
| Galnt2 | UDP-N-acetyl-alpha-d-galactosamine:polypeptide N-acetylgalactosaminyltransferase 2 | protein amino acid O-linked glycosylation | 2.1 | 0.033 |
| Fmod | fibromodulin | ECM | 2.2 | 0.043 |
| Pqlc2 | PQ loop repeat containing 2 | signal transduction | 2.3 | 0.030 |
| Mgp | matrix Gla protein | regulation of bone mineralization | 2.4 | 0.027 |
| Col9a3 | procollagen, type IX, alpha 3 | cell adhesion | 2.5 | 0.040 |
| Fmod | fibromodulin | ECM | 2.7 | 0.019 |
| Ebf2 | early B-cell factor 2 | transcription | 2.7 | 0.043 |
| Kif2c | kinesin family member 2C | microtubule-based movement | 3.1 | 0.048 |
| Kcnj6 | potassium inwardly-rectifying channel, subfamily J, 6 | transport | 3.4 | 0.048 |
| C1ql3 | C1q-like 3 | phosphate transport | 3.9 | 0.030 |
| Fgf20 | fibroblast growth factor 20 | signal transduction | 5.3 | 0.001 |
Boldface indicates most significantly altered genes.
Fig. 2.
Distribution of significantly altered gene expression from adrenergic-deficient E10.5 mouse hearts based on ontological category.
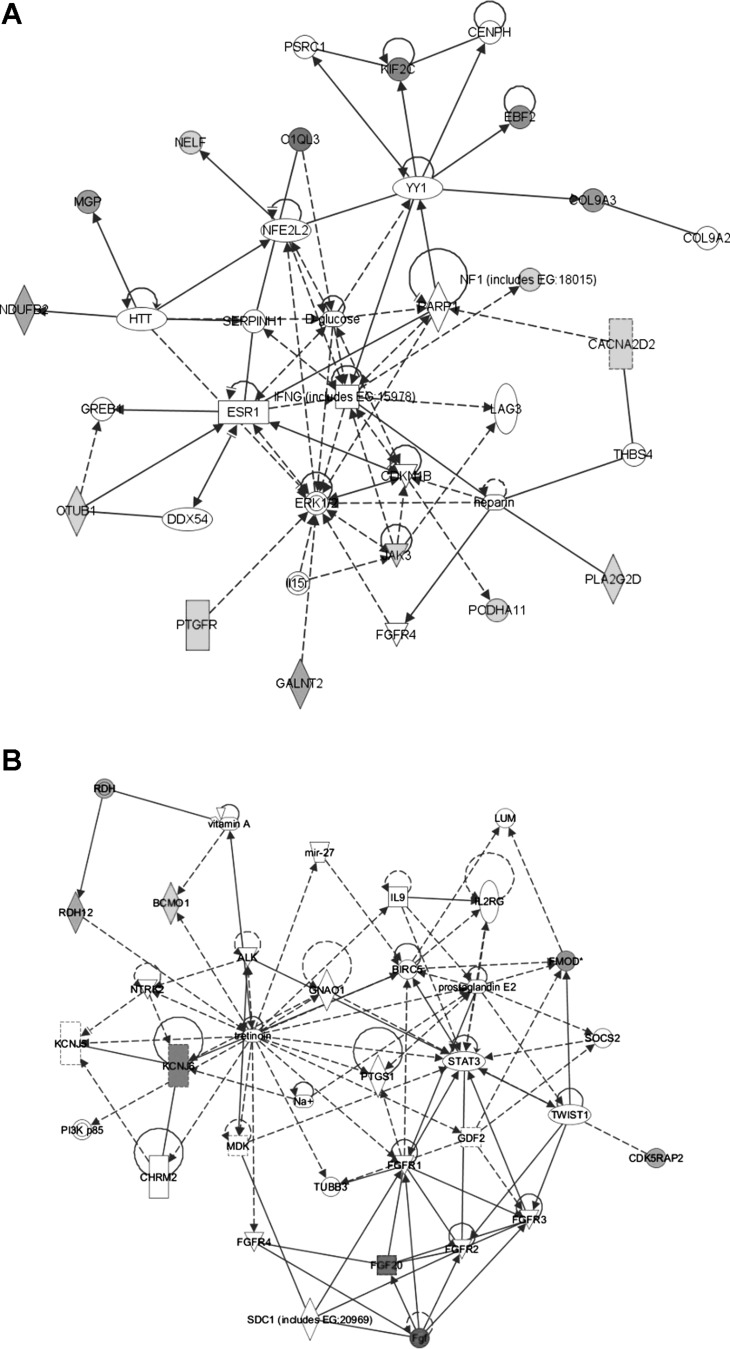
To gain insight about how these various alterations in gene expression may be connected, we performed pathway analysis using the Ingenuity Systems Knowledge Database (http://www.ingenuity.com). The top networks identified were: amino acid metabolism, molecular transport, and small molecule biochemistry (Fig. 3A) and cellular development, nervous system development, and developmental disorders (Fig. 3B). The top biological functions and canonical pathways associated with the altered genes are highlighted in Table 3.
Fig. 3.
Top gene networks associated with the expression changes observed in adrenergic-deficient mouse hearts and highlighted in Table 2. A: amino acid synthesis, molecular transport, and small molecule biochemistry network (score = 40). B: cellular development, nervous system development and function, and developmental disorder network (score = 13). This analysis was generated with Ingenuity Systems software (http://www.ingenuity.com).
Table 3.
Ingenuity Pathway Analysis of the 22 genes showing significant alterations in adrenergic-deficient E10.5 mouse hearts (from Table 1)
| Top Biological Functions | |
|---|---|
| P Value Range | |
| Diseases and Disorders | |
| Developmental disorder | 5.65E-04-4.93E-02 |
| Endocrine System disorders | 5.65E-04-4.02E-02 |
| Reproductive system dsease | 5.65E-04-3.49E-02 |
| Cancer | 1.26E-03-3.89E-02 |
| Cardiovascular disease | 1.36E-03-4.02E-02 |
| Molecular and Cellular Function | |
| Carbohydrate metabolism | 1.36E-03-8.16E-03 |
| Cell cycle | 1.36E-03-4.15E-02 |
| Cell-to-cell signaling and interaction | 1.36E-03-4.02E-02 |
| Cellular assembly and organization | 1.36E-03-4.15E-02 |
| Cellular development | 1.36E-03-4.41E-02 |
| Physiological System and Development and Function | |
| Behavior | 1.36E-03-2.30E-02 |
| Cell-mediated Immune Response | 1.36E-03-8.16E-03 |
| Connective Tissue Development and Function | 1.36E-03-4.28E-02 |
| Endocrine System Development and Function | 1.36E-03-3.23E-02 |
| Hematological System Development and Function | 1.36E-03-4.41E-02 |
| Top Canonical Pathways | |
| P Value | |
| Retinol metabolism | 1.62E-03 |
| Eicosanoid signaling | 2.8E-03 |
| IL-15 production | 3.62E-02 |
| O-glycan biosynthesis | 4.15E-02 |
| MIF-mediated glucocorticoid regulation | 4.41E-02 |
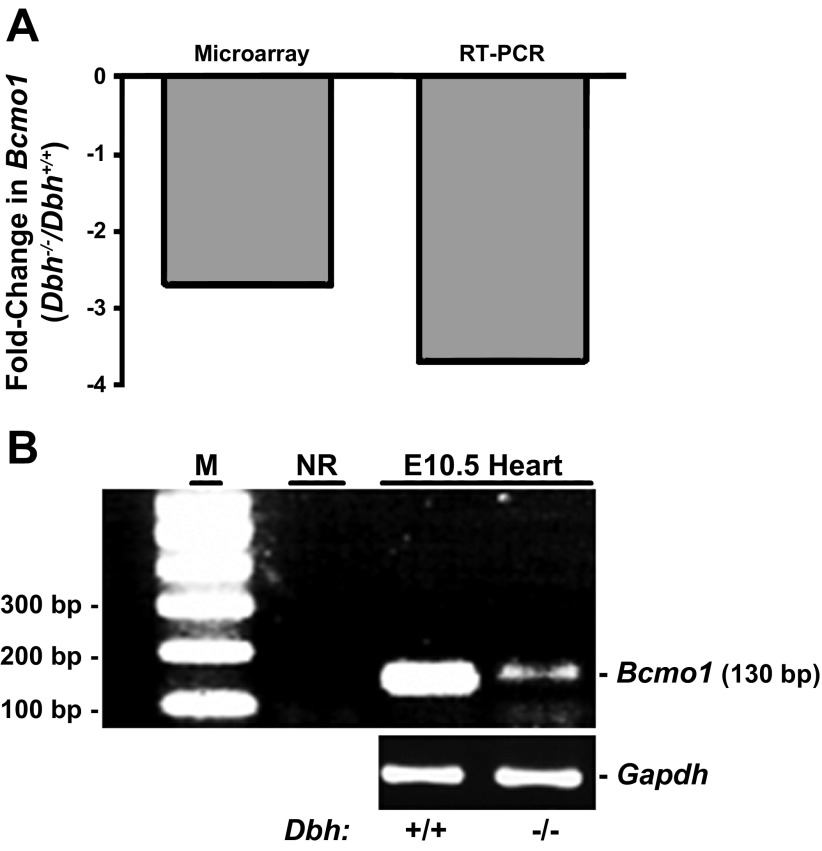
Of particular note, two of the most significantly altered genes are directly involved in RA metabolism (Bcmo1 and Rdh12: see boldfaced items in Table 2 and the top canonical pathways in Table 3). A representative comparison of changes observed from the microarray data with independent assessments of gene expression by RT-PCR is shown for the Bcmo1 gene in Fig. 4, where it can be seen that similar data were obtained by either method. The RT-PCR results showed a slightly larger 3.7-fold decrease in Bcmo1 gene expression compared with the 2.7-fold decrease obtained from the microarray data (Fig. 4A). Verification of the accuracy of the RT-PCR amplification with the Bcmo1 primers is shown by agarose gel electrophoresis in Fig. 4B at the termination of the RT-PCR reactions. These data support the finding that Bcmo1 mRNA expression is decreased in adrenergic-deficient E10.5 mouse hearts relative to controls.
Fig. 4.
Comparison of microarray and RT-PCR results from E10.5 mouse hearts. A: fold-change in expression of Bcmo1 gene expression from Dbh−/− E10.5 mouse hearts relative to Dbh+/+ littermate controls. B: sample agarose gel picture of RT-PCR results confirming expression of Bcmo1 in E10.5 mouse hearts. Lanes: M, markers (100 bp ladder); NR, no RNA input control; E10.5 Heart, RNA input from Dbh+/+ and Dbh−/− hearts as indicated. Bcmo1 primers were used to amplify the samples shown in the NR and E10.5 Heart lanes. Gapdh product was amplified from the same samples.
We independently evaluated a sample of additional genes from the microarray analysis using RT-PCR methods. These genes include key RA indicator genes such as Aldehyde dehydrogenase family 1, subfamily a2 (Aldh1a2, also known as Retinaldehyde dehydrogenase 2 or Raldh2) and Retinol-binding protein 1 (Rbp1). The results are summarized in Table 4. The Aldh1a2 gene encodes for the principal rate-limiting enzyme for RA biosynthesis and is essential for heart development and embryonic survival (72). Changes in Rbp1 expression generally parallel changes in RA concentration, and thus expression of this gene is often used as an indicator of increased or decreased RA concentrations (22, 45, 105). We also measured Retinol dehydrogenase 12 (Rdh12) expression using RT-PCR but could not precisely determine fold-difference for this gene because expression was undetectable in 75% of the wild-type control heart samples (data not shown). Rdh12 expression was detected in 50% of the Dbh−/− hearts both by RT-PCR and microarray analyses, suggesting that its expression was likely induced in adrenergic-deficient E10.5 mouse hearts relative to wild-type controls.
Table 4.
Comparison of expression results for sample genes as measured by microarray and RT-PCR analysis methods
| Gene Symbol | Gene Name | Microarray | RT-PCR |
|---|---|---|---|
| Bcmo1 | β-carotene 15,15′-monooxygenase | −2.7 | −3.7 |
| Nf1 | neurofibromatosis 1 | −2.2 | 1.2 |
| Rbp1 | retinol-binding protein 1 | 1.4 | 3.7 |
| Aldh1a2 (Raldh2) | aldehyde dehydrogenase family 1, subfamily A2 | 1.5 | 1.3 |
| Fmod | fibromodulin | 1.7/2.2/2.7* | 1.9 |
| Fgf20 | fibroblast growth factor 20 | 5.3 | 2.2 |
The different values listed for Fmod represent detection from 3 different positions for this gene on the microarray (see Supplemental Data File 1).
To facilitate comparison of the microarray and RT-PCR results, we performed correlation analysis with linear regression and 95% confidence intervals for each of the target genes shown in Table 4. While the RT-PCR and microarray results showed similar degree and direction of change in most cases, some exceptions were also observed. For example, we did not observe good correlation for Neurofibromin 1 (Nf1). The microarray results suggested 2.2-fold decreased expression for Nf1 in Dbh−/− hearts, but the RT-PCR results indicated a 1.2-fold increase. To investigate this discrepancy, we tried an alternative RT-PCR primer set for Nf1 as there are several splice variants of this gene; however, this still did not reproduce the decreased expression of Nf1 observed in the microarray dataset. With the exception of Nf1, all other targets examined showed similarity between the microarray and RT-PCR results, both in terms of the magnitude and direction of the change. From this subset of genes, all lie within the 95% confidence intervals for linear regression analysis (data not shown). In general, these results validate the findings of the microarray dataset.
Since previous studies had suggested that hypoxia may be an important physiological inducer of adrenergic action in the developing heart (79–81, 101), we evaluated potential expression changes in well-known hypoxia-sensitive genes such as hypoxia-inducible factor 1α (Hif1α) and the vascular endothelial growth factor A (VegfA), but no significant differences were observed (see following web link for access to full microarray dataset and basic statistical analysis: http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=rfghpyusacumoho&acc=GSE33906). To examine this further, we performed RT-PCR analysis on VegfA expression in adrenergic-deficient and control mouse hearts across multiple ages including E9.5, E10.5, and E11.5, but VegfA expression was remarkably consistent in both groups during this period (Fig. 5). These data suggest that adrenergic-deficient embryos are not in hypoxic distress during this early period of embryonic development.
Fig. 5.
Gene expression analysis of Vegf for evaluation of hypoxia in the embryonic mouse heart at midgestation. A–C: VegfA gene expression analysis shows no statistical difference between Dbh+/− (adrenergic-competent) and Dbh−/− (adrenergic-deficient) through the midgestational period from E9.5 to E11.5 as determined by real-time RT-PCR analysis (n = 4 per group).
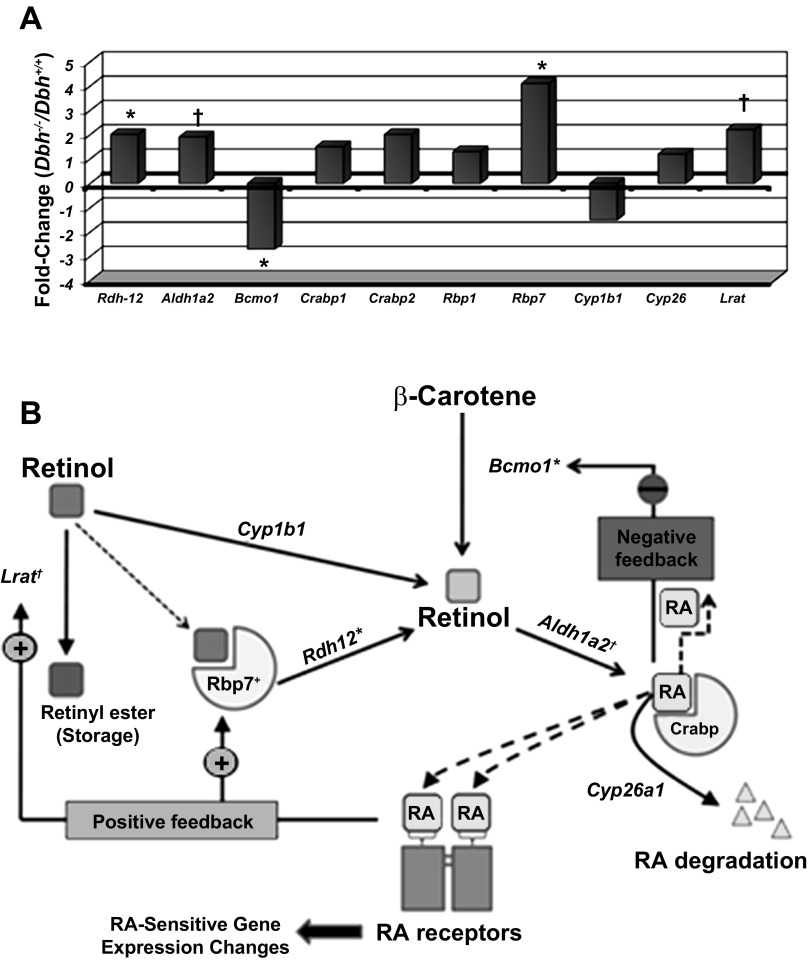
As indicated above, there were a number of RA metabolic and other genes whose expression was significantly altered on the microarray (Table 2), and several of these were verified by independent RT-PCR analysis (Table 4). These results prompted us to probe the microarray data further to determine if other genes involved with RA synthesis and signaling were also altered in adrenergic-deficient hearts. Closer examination of the microarray data for RA-related genes revealed that many of them were altered in adrenergic-deficient mouse hearts. For example, a notably altered gene involved in RA synthesis was Aldh1a2 (1.5-fold increase, P = 0.089), and a gene involved in RA storage was lecithin retinol acetyltransferase (Lrat) (2.2-fold increase, P = 0.078) (33, 54, 73). Although these genes did not meet the arbitrary conventional threshold for significance (P < 0.05), they nevertheless displayed consistent changes in a relatively small sample size (n = 4 for each genotype, Dbh+/+ and Dbh−/−). Similarly, several other genes involved in RA metabolism also displayed increased expression in Dbh−/− E10.5 mouse hearts relative to control Dbh+/+ hearts (Fig. 6A). Although some of these gene expression changes were not found to be “significant,” they appeared to be indicative of a trend toward increased production of RA in adrenergic-deficient hearts. We have included a schematic diagram to show how each of the products of these genes is involved in RA metabolism (Fig. 6B).
Fig. 6.
Retinoic acid (RA) gene expression changes associated with adrenergic-deficient (Dbh−/−) E10.5 mouse embryos. A: display of RA-related gene expression changes from the Affymetrix 430A mouse genome chip data. Significant differences in fold-change relative to wild-type (Dbh+/+) controls (n = 4 each) are designated as follows: *P < 0.05; †P < 0.1. B: schematic representation of RA metabolism and the observed significant changes (marked with an asterisk) in individual genes in adrenergic-deficient E10.5 mouse hearts. Solid-line arrows indicate enzymatic conversion steps. Dashed-line arrows indicate hypothetical movement/transport of retinol and RA molecules. Aldh1a2, acetaldehyde dehydrogenase 1a2 (Retinaldehyde dehydrogenase); Bcmo1, β-carotene 15,15′-monooxygenase; Crabp, cellular retinoic acid binding protein (multiple isoforms - e.g., Crabp1 and Crabp2); Cyp1b1, Cytochrome P4501b1; Cyp26a1, Cytochrome P45026a1; Lrat, lecithin retinol acetyl transferase; RA, retinoic acid; Rbp7, retinol binding protein 1 (multiple isoforms - e.g., Rbp1 and Rbp4); Rdh12, retinol dehydrogenase 12 (also multiple isoforms).
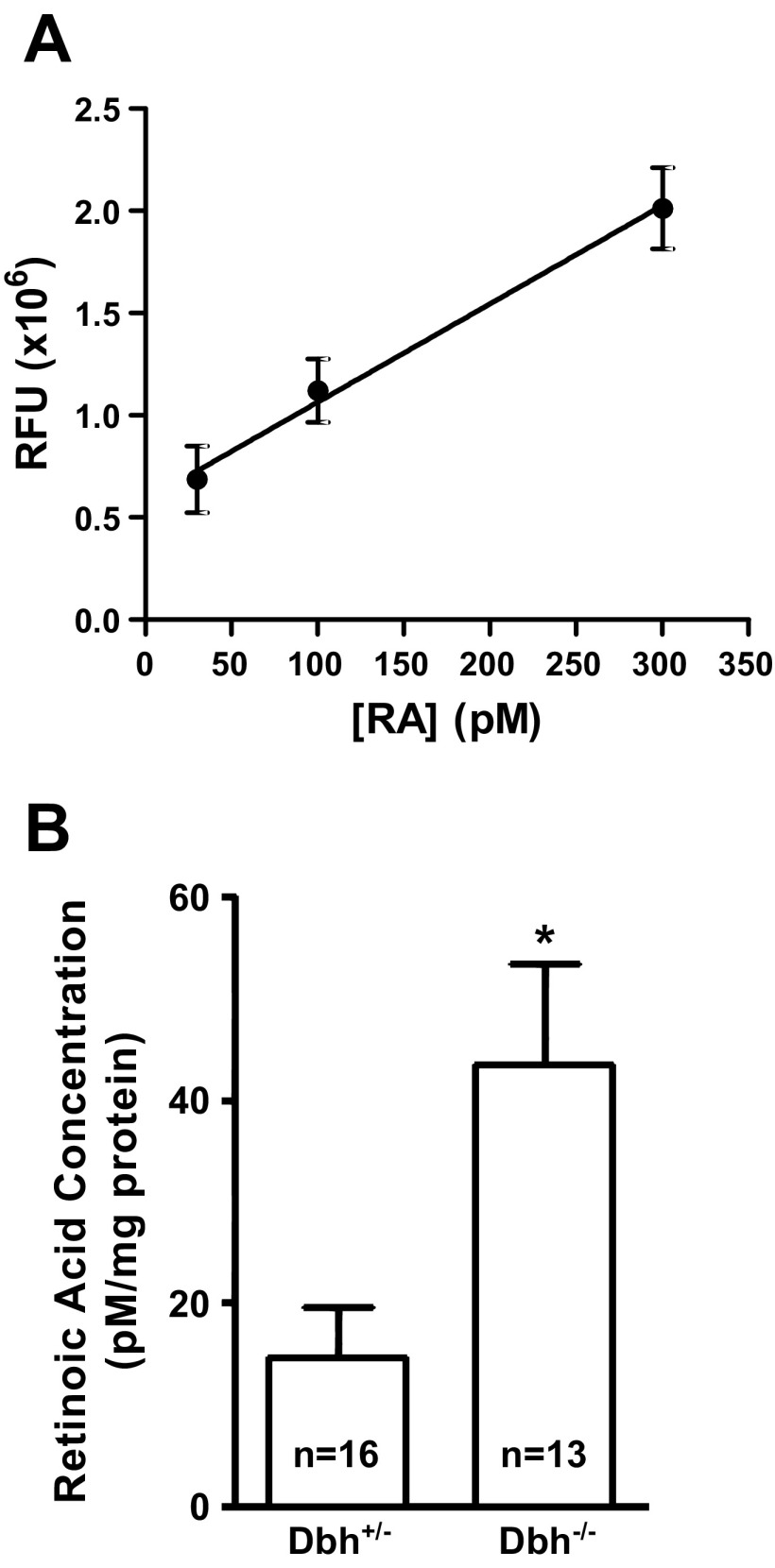
To determine if RA concentrations were actually altered in concert with the observed gene expression changes in RA metabolic genes in adrenergic-deficient mouse embryos, we employed a sensitive bioassay (91, 104, 108) to assess RA concentrations in both adrenergic-deficient (Dbh−/−) and adrenergic-competent (Dbh+/−) embryos. Using this approach, we could accurately measure RA concentrations between 30 and 300 pM as shown by the nearly linear (r2 = 0.97) standard curve over this range of concentrations (Fig. 7A). Embryonic RA concentrations fell within this range. When normalized to the total amount of protein per sample, the average RA concentration in wild-type embryos was 14.6 ± 4.8 pM/mg total protein. Quantitative comparison with Dbh−/− embryos showed that RA concentrations were significantly elevated (P < 0.05) by nearly threefold (43.4 ± 10.1 pM/mg total protein) compared with Dbh+/− controls (Fig. 7B). These results demonstrate that RA concentrations were sharply increased in adrenergic-deficient E10.5 mouse embryos during a critical period of development when the heart is exquisitely sensitive to hormonal fluctuations (13, 29, 51, 68, 84, 88, 94, 99, 106, 109).
Fig. 7.
Quantification of RA in Dbh+/− and Dbh−/− embryos. Quantification was performed using a sensitive bioassay described in materials and methods. A: standard curve (r2 = 0.97) of the RA bioassay showing sensitivity to all-trans RA concentrations as low as 30 pM. B: assessment of absolute tissue RA concentrations shows a significant increase in RA in the Dbh−/− embryos compared with the age-matched Dbh+/− mice (n = 16 and, 13 respectively). *P < 0.05.
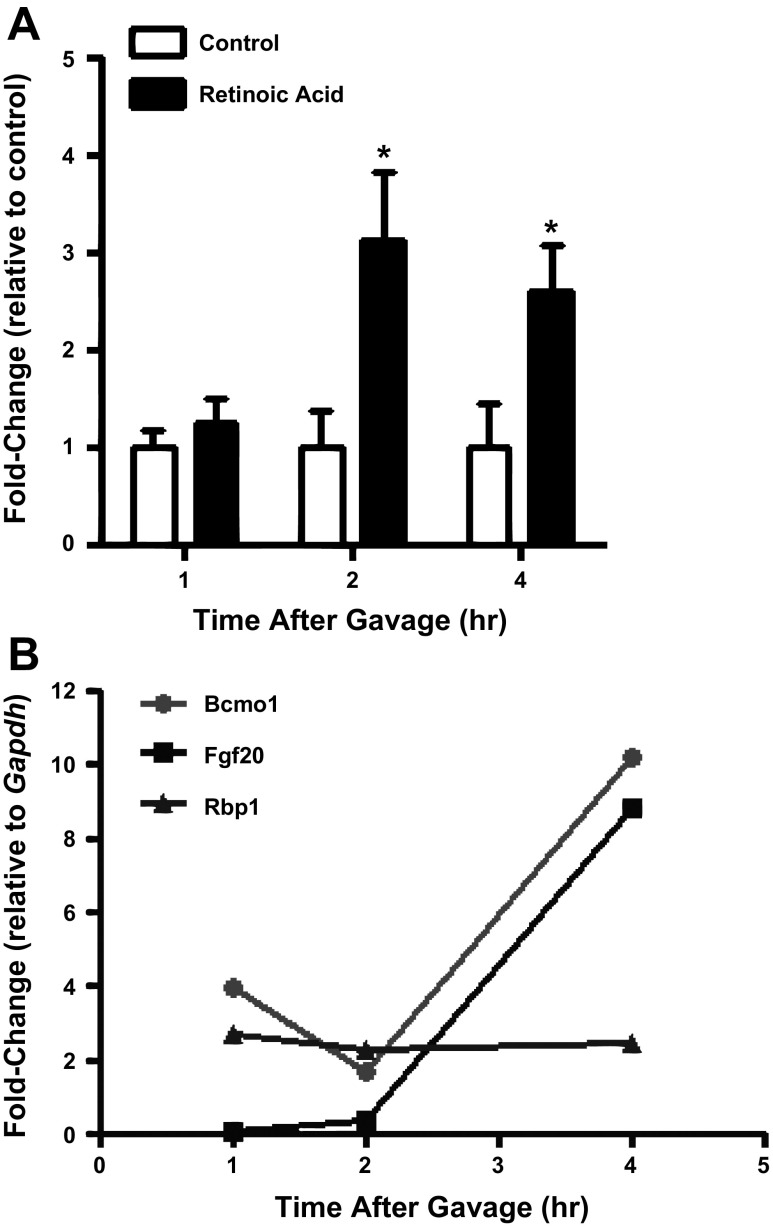
If the gene expression changes observed on the microarray were due to increases in embryonic RA concentrations resulting from adrenergic deficiency, then one might predict that administration of exogenous RA should produce similar effects. To test this idea, we administered RA (50 mg/kg) to the pregnant dams and then measured embryonic RA concentrations at 1, 2, and 4 h from samples collected at E10.5. Little change was observed at 1 h, but RA concentrations increased >3-fold above control (vehicle-only gavage) by 2 h and remained at similar though slightly declining levels through 4 h (Fig. 8A). In parallel, we also measured gene expression changes for a few representative RA-associated genes in isolated E10.5 hearts from these embryos. As shown in Fig. 8B, both Bcmo1 and Fgf20 were highly responsive to RA administration, with peak expression levels observed at 4 h. In contrast, Rbp1 expression was mildly increased similar to that observed in the microarray analysis (Fig. 6) and did not appear to fluctuate much over this time course. These results demonstrate that cardiac Rbp1, Bcmo1, and Fgf20 were responsive to increases in embryonic RA concentrations at E10.5.
Fig. 8.
Evaluation of embryonic RA concentrations and gene expression changes following administration of maternal RA (50 mg/kg) on E10. A: fold-change in embryonic RA concentrations at 1, 2, and 4 h. *P < 0.05. B: fold-change in gene expression for representative target genes as measured by RT-PCR at 1, 2, and 4 h.
DISCUSSION
In this study, we have examined in vivo heart rates and cardiac gene expression changes associated with adrenergic deficiency in a mouse model (Dbh−/−) of embryonic heart failure. Our results show that embryonic heart rates were remarkably similar in adrenergic-deficient and adrenergic-competent hearts until shortly before the onset of heart failure and death. Physiological signs of heart failure included bradycardia with associated arrhythmia and asynchrony. When these signs appeared, the embryos were typically found dead within 24 h. Surprisingly little change was observed in hypoxia-sensitive genes, suggesting that adrenergic-deficient embryos may not necessarily experience abnormal or excessive hypoxic stress during this critical early period of heart development (E9.5–E11.5) in mouse embryos. Our results thus suggest that adrenergic deficiency may lead to heart failure and death due to mechanisms other than those induced by hypoxia.
The use of noninvasive high-resolution ultrasound imaging provided a glimpse into the physiological characteristics of cardiac performance in utero, thereby enabling us to assess the effects of adrenergic deficiency on embryonic heart rates compared with control embryos vivo. This extends earlier work that evaluated heart rates in explanted E12.5 Th−/− embryos at room temperature, where a slight but significant decrease of ∼15% was observed in heart rates from the adrenergic-deficient group (109). More recent studies showed similar heart rates for adrenergic-deficient (Th−/−) and control hearts in ex vivo cultures at physiological temperature under normoxic conditions but significantly slower rates for the adrenergic-deficient hearts under hypoxic conditions (79). An additional study also showed similar heart rates for Th−/− and Th+/+ embryos in vivo following acutely administered oxygen with the animals under anesthesia (81). We did not specifically examine hypoxia in the present study, but our results are consistent with the previous findings, indicating that there is little or no difference in heart rates in adrenergic-deficient embryos compared with controls under normal conditions. Our results are in alignment with previous findings of in vivo studies performed with Th−/− embryos by specifically showing that Dbh−/− embryos exhibit heart rates indistinguishable from Dbh+/+ and Dbh+/− controls until shortly before death when physiological signs of heart failure become evident.
Measurement of genome-wide changes in gene expression in asymptomatic hearts prior to the onset of heart failure revealed a limited population of 22 genes from a total of >22,000 total genes that showed significant expression changes of twofold or greater magnitude. More than half of the 22 altered genes are involved in either metabolism or signal transduction pathways, including two genes that directly participate in RA synthesis, Bcmo1 and Rdh12. Interestingly, both Bcmo1 and Rdh12 encode for enzymes that produce retinal; however, they utilize different substrates. Retinol is the substrate for Rdh12, and β-carotene is the substrate for Bcmo1. In the case of Rdh12, there are multiple isoforms that comprise the retinol dehydrogenase family of enzymes, and based on our microarray data it is clear that several of these are expressed much more robustly than Rdh12 in the embryonic heart (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=rfghpyusacumoho&acc=GSE33906). No significant differences in expression of the other isoforms of the Rdh gene were observed from the microarray analysis of adrenergic-deficient and control embryonic mouse hearts. Despite the apparently minor role for Rdh12 under these circumstances, induction of its expression in adrenergic-deficient hearts during embryonic development appears to serve as an indicator of perturbations in RA metabolism.
In contrast, the Bcmo1 gene product is the primary enzyme and only isoform characterized to generate two molecules of retinal for each molecule of β-carotene (4, 27, 82). Although the Bcmo1 gene does not appear to be essential for mouse development when vitamin A is sufficient in the diet (38), its role becomes much more important when vitamin A intake is limited (53, 61). Bcmo1 is a highly conserved gene found in animals as diverse as fruit flies, chickens, and mammals (3). Moreover, Bcmo1 is critical for embryogenesis in zebrafish (59). Expression of Bcmo1 is known to be robust in early mammalian embryogenesis (82), consistent with our observation that it is expressed in the embryonic heart. These results suggest that appropriate Bcmo1 expression and regulation are important for animal development. In our study, Bcmo1 expression was significantly decreased roughly threefold in Dbh−/− hearts at E10.5, coincident with the observed elevations in RA concentrations measured in Dbh−/− embryos at this stage of development. Interestingly, however, its expression increased dramatically in response to maternal RA administration. These data imply that the decreased expression of Bcmo1 in Dbh−/− hearts may not solely be due to the observed increase in RA concentrations but likely involve other regulatory components. Indeed, previous examination of the Bcmo1 promoter identified a RA-responsive element (RARE) and a putative cAMP-responsive element immediately adjacent to the RARE, as well as a number of other transcriptional regulatory elements (27). The fact that Bcmo1 expression is responsive to RA in mouse embryos is consistent with the RARE in its promoter and earlier studies showing that this gene is sensitive to transcriptional regulation in the presence of RA in other systems (4, 97). An intriguing hypothesis generated from our results is that the cAMP-responsive element in the Bcmo1 promoter plays an important role in stimulating Bcmo1 expression in the embryonic heart during this formative period of early heart development. Future experiments aim to test this and other hypotheses that will explain how Bcmo1 gene expression is influenced by developmental hormones. It is nonetheless clear that Bcmo1 expression is responsive to hormonal influences in the embryonic heart, thus providing one of the first tangible pieces of evidence indicating that there may be functional cross talk between adrenergic and RA signaling pathways in the embryonic heart.
Changes in gene expression such as those noted above provide strong circumstantial evidence that RA synthesis and signaling are awry in adrenergic-deficient hearts, but perhaps the most convincing direct evidence comes from our demonstration that RA concentrations themselves were significantly elevated in adrenergic-deficient mouse embryos. This finding is consistent with the observed increased expression of RA biosynthesis genes such as Aldh1a2 and Rdh12, as well as retinol-binding proteins, Rbp1 and Rbp7, and RA-binding proteins, Crabp1 and Crabp2 (69, 87, 92). Increased expression of the gene encoding the RA storage enzyme, Lrat, is also consistent with a previous report showing that Lrat is transcriptionally stimulated by RA (110). Interestingly, published reports have shown that Lrat and Bcmo1 appear to be reciprocally expressed during early mouse development (53), as well as in obese adult rats (96), in a manner that appears not entirely dissimilar to the inverse expression profiles for Lrat and Bcmo1 observed in Dbh−/− embryonic mouse hearts.
The hypothesis that RA synthesis was perturbed in adrenergic-deficient hearts is further supported by closer examination of other genes involved in RA metabolism, many of which displayed altered expression patterns. Notable among these were Aldh1a2 and Lrat, whose products play critical roles in RA synthesis (72) and storage (33, 54, 73), respectively. Additional supporting evidence is provided by the microarray results showing that several known RA-responsive genes also showed significantly altered gene expression in adrenergic-deficient embryonic mouse hearts compared with controls. These include the Pla2g2d (20), Nf 1 (41, 64), Mgp (7), and Col9a3 (107) genes (Table 2). This hypothesis is also supported by the pathway analysis shown in Fig. 3B, where tretinoin, all-trans RA, is seen to be at the center of several connected pathways and thus may serve to coordinate and integrate expression of many of the associated genes shown.
One of these genes, Fgf20, appears to merit special attention because it was the most strongly and significantly induced (5.3-fold increase, P < 0.001, n = 4/group) gene in Dbh−/− hearts from the entire microarray analysis (see Table 2). Moreover, it is clear that various Fgf isoforms serve as important mediators of RA actions during development (12, 71). Of particular relevance is a study by Lavine et al. (60) that showed Fgf20 expression in E10.5 mouse hearts where it was primarily found in ventricular (left and right) epicardium and endocardium, as well as in the outflow tract region. Fgf20 is closely related to Fgf9 and Fgf16 in terms of sequence homology, cardiac spatio-temporal expression patterns, and Fgf receptor selectivity (31, 48, 60). Although RA regulation of Fgf20 has not been previously examined directly, Lavine et al. (60) showed that RA treatment of embryonic mouse hearts significantly induced Fgf9. These findings are consistent with the results from the present study showing that Fgf20 expression was strongly induced when embryonic RA concentrations were elevated following maternal administration of RA and in Dbh−/− mouse embryos at E10.5. Taken together, these findings suggest that Fgf20 expression is stimulated by RA in E10.5 mouse hearts and that the increased expression of Fgf20 in adrenergic-deficient hearts may be a direct result of the elevated RA concentrations observed in these embryos. Further testing will be required to confirm this hypothesis and determine what consequence(s) may result from transient increases in Fgf20 expression in the developing heart.
Several additional genes and pathways from the microarray analysis described here appear likely to play a role in the observed cardiac phenotypes found in adrenergic-deficient mouse embryos. For example, two of the 22 significantly altered genes from Table 2 are ion channel subunits. One encodes for a calcium channel subunit (Cacna2d2), and the other for a potassium channel (Kcnj6). The Cacna2d2 subunit has previously been shown to be expressed in the heart, and it appears to increase the size of both L- and T-type calcium currents when expressed in a heterologous system (23). Other studies have shown that pace-making and atrial tissue express higher levels of this subunit than the ventricles (37, 65). In contrast, the Kcnj6 gene encodes for an ATP-sensitive inward-rectifying K+ channel (89). We did not perform electrophysiological characterization in the present study but recently reported slowed atrial-ventricular conduction, decreased connexin 43 expression, and increased propensity for cardiac arrhythmias in adrenergic-deficient embryonic mouse hearts (2). It is certainly conceivable that altered expression of Kcnj6 and/or Cacna2d2 could contribute to the observed electrophysiological impairments in these animals, though further experimental evaluation is needed to determine if this is true. At present, there is no indication that either of these ion channel genes are regulated by RA, but several independent reports suggest that elevated RA can lead to delayed ventricular activation (103), decreased connexin 43 expression (103), diminished growth of atrioventricular tissue (5), and accelerated expression of atrial calcium and potassium currents in differentiating cardiomyocytes (24). Thus, the elevated RA concentrations in adrenergic-deficient embryos may indeed contribute to the observed electrophysiological phenotypes, thereby providing additional fodder for future experimentation in this area.
One of the more curious findings stemming from the microarray analysis is that at least two of the genes identified have been shown to be involved in bone formation. The Mgp gene is involved in regulation of bone mineralization and has previously been shown to be regulated by RA (7). The Ebf2 gene, on the other hand, has not been shown to be sensitive to RA, but it apparently “regulates osteoblast-dependent differentiation of osteoclasts” (41). While it is entirely unclear why expression of these genes is altered in adrenergic-deficient hearts, it is intriguing to note that adrenergic-deficient mice display impaired leptin-dependent bone formation (95). Consequently, Mgp and/or Ebf2 could represent novel molecular intermediaries for adrenergic influence on bone formation, thereby opening the door for another new area of investigation.
Study limitations.
The present evidence shows that RA concentrations are increased in adrenergic-deficient embryos, but further study is required to determine if the observed RA fluctuations contribute to embryonic/fetal heart failure in adrenergic-deficient mice. We also recognize that significant gene expression changes observed in our analysis of the microarray data represent genes that appear to be unrelated to RA synthesis or signaling. We were initially excited about Nf1 as a potential target since earlier studies had shown that it is also essential for embryonic heart development (6, 26, 57), but we were unable to confirm altered Nf1 expression with independent RT-PCR assays. We tried two different primer sets targeted to different known splice variants of the Nf1 gene, but there are many other variants that would need to be evaluated before a definitive conclusion can be reached about whether or not this gene is truly influenced by adrenergic hormones in the developing heart. We have not yet systematically verified all the significant gene alterations observed from the microarray data with independent RT-PCR and other methods, but this dataset should prove useful for subsequent investigations into the underlying mechanisms of adrenergic action in the developing heart.
The elevations in embryonic RA concentrations likely contribute to the heart failure phenotype observed in Dbh−/− mice, though this is probably not the whole story since earlier studies have shown that maternal administration of excess RA or vitamin A does not necessarily produce heart failure and embryonic death (83, 87, 90). Nevertheless, overexpression of RA receptors has been reported to cause dilated cardiomyopathy and lead to congestive heart failure in adult mice (10, 93). On the other hand, it is well-established that too much or too little RA can lead to congenital cardiac malformations similar to those seen in human patients (34, 35, 58). In this context, it is potentially interesting to note that excess β-adrenergic stimulation has also been shown to produce similar types of congenital cardiac defects when applied during an analogous window of development in chick embryos (39, 40). It is therefore possible that teratogenic influences of adrenergic stimulation may be mediated, in part, through RA-dependent mechanisms. Additional experiments are required to test this hypothesis.
Study conclusions.
The major conclusion from this report is that adrenergic deficiency results in significant alterations in RA synthesis in the developing mouse embryo near midgestation (E10.5). Although it has been known for many years that adrenergic and RA hormones are each essential for heart development, the present study is the first report to show that disruption of adrenergic signaling significantly influences RA metabolism in the embryonic mouse heart. Interestingly, recent studies have shown that the Th gene is regulated by RA and expressed early in the developing heart (50, 63). The Th gene encodes for the enzyme that catalyzes conversion of L-tyrosine to L-DOPA, which is generally considered the rate-limiting step in adrenergic hormone production. Furthermore, the adrenal gland appears to be a major site of RA production during mouse fetal development (36). Thus, there is mounting evidence suggesting that adrenergic and RA signaling pathways are functionally interconnected during a critical phase of heart development, and perhaps beyond.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01HL-78716 to S. N. Ebert.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.O., D.W., and S.N.E. conception and design of research; K.O., C.N.B., H.-L.N., C.M., D.W., and S.N.E. performed experiments; K.O., C.N.B., H.-L.N., C.M., and S.N.E. analyzed data; K.O., H.-L.N., D.W., and S.N.E. interpreted results of experiments; K.O., C.N.B., C.M., and S.N.E. prepared figures; K.O. drafted manuscript; K.O., C.N.B., H.-L.N., C.M., D.W., and S.N.E. edited and revised manuscript; K.O., C.N.B., H.-L.N., C.M., D.W., and S.N.E. approved final version of manuscript.
ACKNOWLEDGMENTS
Current address for K. Osuala: Wayne State Univ., School of Medicine, 540 E. Canfield, Detroit, MI 48201 (e-mail: kosuala@med.wayne.edu).
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1. Axelrod J. Purification and properties of phenylethanolamine n-methyltransferase. J Biol Chem 237: 1657–1660, 1962. [PubMed] [Google Scholar]
- 2. Baker C, Taylor DG, Osuala K, Natarajan A, Molnar PJ, Hickman J, Alam S, Moscato B, Weinshenker D, Ebert SN. Adrenergic deficiency leads to impaired electrical conduction and increased arrhythmic potential in the embryonic mouse heart. Biochem Biophys Res Commun 423: 536–541, 2012. [DOI] [PubMed] [Google Scholar]
- 3. Biesalski HK, Chichili GR, Frank J, von Lintig J, Nohr D. Conversion of beta-carotene to retinal pigment. Vitam Horm 75: 117–130, 2007. [DOI] [PubMed] [Google Scholar]
- 4. Boulanger A, McLemore P, Copeland NG, Gilbert DJ, Jenkins NA, Yu SS, Gentleman S, Redmond TM. Identification of beta-carotene 15, 15′-monooxygenase as a peroxisome proliferator-activated receptor target gene. FASEB J 17: 1304–1306, 2003. [DOI] [PubMed] [Google Scholar]
- 5. Bouman HG, Broekhuizen ML, Baasten AM, Gittenberger-de Groot AC, Wenink AC. Diminished growth of atrioventricular cushion tissue in stage 24 retinoic acid-treated chicken embryos. Dev Dyn 213: 50–58, 1998. [DOI] [PubMed] [Google Scholar]
- 6. Brannan CI, Perkins AS, Vogel KS, Ratner N, Nordlund ML, Reid SW, Buchberg AM, Jenkins NA, Parada LF, Copeland NG. Targeted disruption of the neurofibromatosis type-1 gene leads to developmental abnormalities in heart and various neural crest-derived tissues. Genes Dev 8: 1019–1029, 1994. [DOI] [PubMed] [Google Scholar]
- 7. Cancela ML, Price PA. Retinoic acid induces matrix Gla protein gene expression in human cells. Endocrinology 130: 102–108, 1992. [DOI] [PubMed] [Google Scholar]
- 8. Chruscinski AJ, Rohrer DK, Schauble E, Desai KH, Bernstein D, Kobilka BK. Targeted disruption of the beta2 adrenergic receptor gene. J Biol Chem 274: 16694–16700, 1999. [DOI] [PubMed] [Google Scholar]
- 9. Chung SS, Wang X, Roberts SS, Griffey SM, Reczek PR, Wolgemuth DJ. Oral administration of a retinoic acid receptor antagonist reversibly inhibits spermatogenesis in mice. Endocrinology 152: 2492–2502, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Colbert MC, Hall DG, Kimball TR, Witt SA, Lorenz JN, Kirby ML, Hewett TE, Klevitsky R, Robbins J. Cardiac compartment-specific overexpression of a modified retinoic acid receptor produces dilated cardiomyopathy and congestive heart failure in transgenic mice. J Clin Invest 100: 1958–1968, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davidoff MS, Ungefroren H, Middendorff R, Koeva Y, Bakalska M, Atanassova N, Holstein AF, Jezek D, Pusch W, Muller D. Catecholamine-synthesizing enzymes in the adult and prenatal human testis. Histochem Cell Biol 124: 313–323, 2005. [DOI] [PubMed] [Google Scholar]
- 12. Duester G. Retinoic acid synthesis and signaling during early organogenesis. Cell 134: 921–931, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dyson E, Sucov HM, Kubalak SW, Schmid-Schonbein GW, DeLano FA, Evans RM, Ross J, Jr, Chien KR. Atrial-like phenotype is associated with embryonic ventricular failure in retinoid X receptor alpha -/- mice. Proc Natl Acad Sci USA 92: 7386–7390, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ebert SN, Baden JM, Mathers LH, Siddall BJ, Wong DL. Expression of phenylethanolamine n-methyltransferase in the embryonic rat heart. J Mol Cell Cardiol 28: 1653–1658, 1996. [DOI] [PubMed] [Google Scholar]
- 15. Ebert SN, Balt SL, Hunter JP, Gashler A, Sukhatme V, Wong DL. Egr-1 activation of rat adrenal phenylethanolamine N-methyltransferase gene. J Biol Chem 269: 20885–20898, 1994. [PubMed] [Google Scholar]
- 16. Ebert SN, Rong Q, Boe S, Thompson RP, Grinberg A, Pfeifer K. Targeted insertion of the Cre-recombinase gene at the phenylethanolamine n-methyltransferase locus: A new model for studying the developmental distribution of adrenergic cells. Dev Dyn 231: 849–858, 2004. [DOI] [PubMed] [Google Scholar]
- 17. Ebert SN, Taylor DG. Catecholamines and development of cardiac pacemaking: an intrinsically intimate relationship. Cardiovasc Res 72: 364–374, 2006. [DOI] [PubMed] [Google Scholar]
- 18. Ebert SN, Thompson RP. Embryonic epinephrine synthesis in the rat heart before innervation: association with pacemaking and conduction tissue development. Circ Res 88: 117–124, 2001. [DOI] [PubMed] [Google Scholar]
- 19. Ellison JP, Hibbs RG. Catecholamine-containing cells of the guinea pig heart: an ultrastructural study. J Mol Cell Cardiol 6: 17–26, 1974. [DOI] [PubMed] [Google Scholar]
- 20. Farooqui AA, Antony P, Ong WY, Horrocks LA, Freysz L. Retinoic acid-mediated phospholipase A2 signaling in the nucleus. Brain Res Brain Res Rev 45: 179–195, 2004. [DOI] [PubMed] [Google Scholar]
- 21. Fujinaga M, Scott JC. Gene expression of catecholamine synthesizing enzymes and b adrenoceptor subtypes during rat embryogenesis. Neurosci Lett 231: 108–112, 1997. [DOI] [PubMed] [Google Scholar]
- 22. Furr HC. Analysis of retinoids and carotenoids: problems resolved and unsolved. J Nutr 134: 281S–285S, 2004. [DOI] [PubMed] [Google Scholar]
- 23. Gao B, Sekido Y, Maximov A, Saad M, Forgacs E, Latif F, Wei MH, Lerman M, Lee JH, Perez-Reyes E, Bezprozvanny I, Minna JD. Functional properties of a new voltage-dependent calcium channel alpha(2)delta auxiliary subunit gene (CACNA2D2). J Biol Chem 275: 12237–12242, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gassanov N, Er F, Zagidullin N, Jankowski M, Gutkowska J, Hoppe UC. Retinoid acid-induced effects on atrial and pacemaker cell differentiation and expression of cardiac ion channels. Differentiation 76: 971–980, 2008. [DOI] [PubMed] [Google Scholar]
- 25. Ginsberg BA, Gallardo HF, Rasalan TS, Adamow M, Mu Z, Tandon S, Bewkes BB, Roman RA, Chapman PB, Schwartz GK, Carvajal RD, Panageas KS, Terzulli SL, Houghton AN, Yuan JD, Wolchok JD. Immunologic response to xenogeneic gp100 DNA in melanoma patients: comparison of particle-mediated epidermal delivery with intramuscular injection. Clin Cancer Res 16: 4057–4065, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gitler AD, Zhu Y, Ismat FA, Lu MM, Yamauchi Y, Parada LF, Epstein JA. Nf1 has an essential role in endothelial cells. Nat Genet 33: 75–79, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gong X, Tsai SW, Yan B, Rubin LP. Cooperation between MEF2 and PPARgamma in human intestinal beta,beta-carotene 15,15′-monooxygenase gene expression. BMC Mol Biol 7: 7, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Greenberg RE, Lind J. Catecholamines in tissues of the human fetus. Pediatrics 904–911, 1962. [PubMed] [Google Scholar]
- 29. Gruber PJ, Kubalak SW, Pexieder T, Sucov HM, Evans RM, Chien KR. RXR alpha deficiency confers genetic susceptibility for aortic sac, conotruncal, atrioventricular cushion, and ventricular muscle defects in mice. J Clin Invest 98: 1332–1343, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hadjiconstantinou M, Cohen J, Neff NH. Epinephrine: a potential neurotransmitter in retina. J Neurochem 41: 1440–1444, 1983. [DOI] [PubMed] [Google Scholar]
- 31. Hajihosseini MK, Heath JK. Expression patterns of fibroblast growth factors-18 and -20 in mouse embryos is suggestive of novel roles in calvarial and limb development. Mech Dev 113: 79–83, 2002. [DOI] [PubMed] [Google Scholar]
- 32. Hall E. Acetylcholine and epinephrine effects on the embryonic rat heart. J Cell Comp Physiol 49: 187–200, 1957. [DOI] [PubMed] [Google Scholar]
- 33. Harrison EH. Mechanisms of digestion and absorption of dietary vitamin A. Annu Rev Nutr 25: 87–103, 2005. [DOI] [PubMed] [Google Scholar]
- 34. Hart RC, McCue PA, Ragland WL, Winn KJ, Unger ER. Avian model for 13-cis-retinoic acid embryopathy: demonstration of neural crest related defects. Teratology 41: 463–472, 1990. [DOI] [PubMed] [Google Scholar]
- 35. Hart RC, Winn KJ, Unger ER. Avian model for 13-cis-retinoic acid embryopathy: morphological characterization of ventricular septal defects. Teratology 46: 533–539, 1992. [DOI] [PubMed] [Google Scholar]
- 36. Haselbeck RJ, Ang HL, Deltour L, Duester G. Retinoic acid and alcohol/retinol dehydrogenase in the mouse adrenal gland: a potential endocrine source of retinoic acid during development. Endocrinology 138: 3035–3041, 1997. [DOI] [PubMed] [Google Scholar]
- 37. Hatano S, Yamashita T, Sekiguchi A, Iwasaki Y, Nakazawa K, Sagara K, Iinuma H, Aizawa T, Fu LT. Molecular and electrophysiological differences in the L-type Ca2+ channel of the atrium and ventricle of rat hearts. Circ J 70: 610–614, 2006. [DOI] [PubMed] [Google Scholar]
- 38. Hessel S, Eichinger A, Isken A, Amengual J, Hunzelmann S, Hoeller U, Elste V, Hunziker W, Goralczyk R, Oberhauser V, von Lintig J, Wyss A. CMO1 deficiency abolishes vitamin A production from beta-carotene and alters lipid metabolism in mice. J Biol Chem 282: 33553–33561, 2007. [DOI] [PubMed] [Google Scholar]
- 39. Hodach RJ, Gilbert EF, Fallon JF. Aortic arch anomalies associated with the administration of epinephrine in chick embryos. Teratology 9: 203–209, 1974. [DOI] [PubMed] [Google Scholar]
- 40. Hodach RJ, Hodach AE, Fallon JF, Folts JD, Bruyere HJ, Gilbert EF. The role of beta-adrenergic activity in the production of cardiac and aortic arch anomalies in chick embryos. Teratology 12: 33–45, 1975. [DOI] [PubMed] [Google Scholar]
- 41. Holzel M, Huang S, Koster J, Ora I, Lakeman A, Caron H, Nijkamp W, Xie J, Callens T, Asgharzadeh S, Seeger RC, Messiaen L, Versteeg R, Bernards R. NF1 is a tumor suppressor in neuroblastoma that determines retinoic acid response and disease outcome. Cell 142: 218–229, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hsu FY. The effect of adrenaline and acetylcholine on the heart rate of the chick embryo. Chin J Physiol VII: 243–252, 1933. [Google Scholar]
- 43. Huang MH, Bahl JJ, Wu Y, Hu F, Larson DF, Roeske WR, Ewy GA. Neuroendocrine properties of intrinsic cardiac adrenergic cells in fetal rat heart. Am J Physiol Heart Circ Physiol 288: H497–H503, 2005. [DOI] [PubMed] [Google Scholar]
- 44. Huang MH, Friend DS, Sunday ME, Singh K, Haley K, Austen KF, Kelly RA, Smith TW. An intrinsic adrenergic system in mammalian heart. J Clin Invest 98: 1298–1303, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Husmann M, Hoffmann B, Stump DG, Chytil F, Pfahl M. A retinoic acid response element from the rat CRBPI promoter is activated by an RAR/RXR heterodimer. Biochem Biophys Res Commun 187: 1558–1564, 1992. [DOI] [PubMed] [Google Scholar]
- 46. Ignarro LJ, Shideman FE. Appearance and concentrations of catecholamines and their biosynthesis in the embryonic and developing chick. J Pharmacol Exp Ther 159: 38–48, 1968. [PubMed] [Google Scholar]
- 47. Ignarro LJ, Shideman FE. Norepinephrine and epinephrine in the embryo and embryonic heart of the chick: uptake and subcellular distribution. J Pharmacol Exp Ther 159: 49–58, 1968. [PubMed] [Google Scholar]
- 48. Itoh N, Ornitz DM. Fibroblast growth factors: from molecular evolution to roles in development, metabolism and disease. J Biochem 149: 121–130, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jaber M, Koch WJ, Rockman H, Smith B, Bond RA, Sulik KK, Ross J, Jr, Lefkowitz RJ, Caron MG, Giros B. Essential role of beta-adrenergic receptor kinase 1 in cardiac development and function. Proc Natl Acad Sci USA 93: 12974–12979, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jeong H, Kim MS, Kim SW, Kim KS, Seol W. Regulation of tyrosine hydroxylase gene expression by retinoic acid receptor. J Neurochem 98: 386–394, 2006. [DOI] [PubMed] [Google Scholar]
- 51. Kaufman MH. The Atlas of Mouse Development. San Diego, CA: Harcourt Brace Jovanovich, 1992. [Google Scholar]
- 52. Kennedy B, Bigby TD, Ziegler MG. Nonadrenal epinephrine-forming enzymes in humans. Characteristics, distribution, regulation, and relationship to epinephrine levels. J Clin Invest 95: 2896–2902, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kim YK, Wassef L, Chung S, Jiang H, Wyss A, Blaner WS, Quadro L. beta-Carotene and its cleavage enzyme beta-carotene-15,15′-oxygenase (CMOI) affect retinoid metabolism in developing tissues. FASEB J 25: 1641–1652, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kim YK, Wassef L, Hamberger L, Piantedosi R, Palczewski K, Blaner WS, Quadro L. Retinyl ester formation by lecithin: retinol acyltransferase is a key regulator of retinoid homeostasis in mouse embryogenesis. J Biol Chem 283: 5611–5621, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kobayashi K, Morita S, Sawada H, Mizuguchi T, Yamada K, Nagatsu I, Hata T, Watanabe Y, Fujita K, Nagatsu T. Targeted disruption of the tyrosine hydroxylase locus results in severe catecholamine depletion and perinatal lethality in mice. J Biol Chem 270: 27235–27243, 1995. [DOI] [PubMed] [Google Scholar]
- 56. Kvetnansky R, Sabban EL, Palkovits M. Catecholaminergic systems in stress: structural and molecular genetic approaches. Physiol Rev 89: 535–606, 2009. [DOI] [PubMed] [Google Scholar]
- 57. Lakkis MM, Epstein JA. Neurofibromin modulation of ras activity is required for normal endocardial-mesenchymal transformation in the developing heart. Development 125: 4359–4367, 1998. [DOI] [PubMed] [Google Scholar]
- 58. Lammer EJ, Chen DT, Hoar RM, Agnish ND, Benke PJ, Braun JT, Curry CJ, Fernhoff PM, Grix AW, Jr, Lott IT, Richard JM, Sun SC. Retinoic acid embryopathy. N Engl J Med 313: 837–841, 1985. [DOI] [PubMed] [Google Scholar]
- 59. Lampert JM, Holzschuh J, Hessel S, Driever W, Vogt K, von Lintig J. Provitamin A conversion to retinal via the beta,beta-carotene-15,15′-oxygenase (bcox) is essential for pattern formation and differentiation during zebrafish embryogenesis. Development 130: 2173–2186, 2003. [DOI] [PubMed] [Google Scholar]
- 60. Lavine KJ, Yu K, White AC, Zhang X, Smith C, Partanen J, Ornitz DM. Endocardial and epicardial derived FGF signals regulate myocardial proliferation and differentiation in vivo. Dev Cell 8: 85–95, 2005. [DOI] [PubMed] [Google Scholar]
- 61. Lindshield BL, King JL, Wyss A, Goralczyk R, Lu CH, Ford NA, Erdman JW., Jr Lycopene biodistribution is altered in 15,15′-carotenoid monooxygenase knockout mice. J Nutr 138: 2367–2371, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lipshulz S, Shanfeld J, Chacko S. Emergence of b-adrenergic sensitivity in the developing chicken heart. Proc Natl Acad Sci USA 78: 288–292, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lopez-Sanchez C, Bartulos O, Martinez-Campos E, Ganan C, Valenciano AI, Garcia-Martinez V, De Pablo F, Hernandez-Sanchez C. Tyrosine hydroxylase is expressed during early heart development and is required for cardiac chamber formation. Cardiovasc Res 88: 111–120, 2010. [DOI] [PubMed] [Google Scholar]
- 64. Mantani A, Wakasugi S, Yokota Y, Abe K, Ushio Y, Yamamura K. A novel isoform of the neurofibromatosis type-1 mRNA and a switch of isoforms during murine cell differentiation and proliferation. Gene 148: 245–251, 1994. [DOI] [PubMed] [Google Scholar]
- 65. Marionneau C, Couette B, Liu J, Li H, Mangoni ME, Nargeot J, Lei M, Escande D, Demolombe S. Specific pattern of ionic channel gene expression associated with pacemaker activity in the mouse heart. J Physiol 562: 223–234, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Markowitz C. Response of explanted embryonic cardiac tissue to epinephrine and acetylcholine. Am J Physiol 97: 271–275, 1931. [Google Scholar]
- 67. Martin S, Levey BA, Levey GS. Development of the cardiac beta adrenergic receptor in fetal rat heart. Biochem Biophys Res Commun 54: 949–954, 1973. [DOI] [PubMed] [Google Scholar]
- 68. Merki E, Zamora M, Raya A, Kawakami Y, Wang J, Zhang X, Burch J, Kubalak SW, Kaliman P, Belmonte JC, Chien KR, Ruiz-Lozano P. Epicardial retinoid X receptor alpha is required for myocardial growth and coronary artery formation. Proc Natl Acad Sci USA 102: 18455–18460, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Napoli JL, Boerman MH, Chai X, Zhai Y, Fiorella PD. Enzymes and binding proteins affecting retinoic acid concentrations. J Steroid Biochem Mol Biol 53: 497–502, 1995. [DOI] [PubMed] [Google Scholar]
- 70. Natarajan AR, Rong Q, Katchman AN, Ebert SN. Intrinsic cardiac catecholamines help maintain beating activity in neonatal rat cardiomyocyte cultures. Pediatr Res 56: 411–417, 2004. [DOI] [PubMed] [Google Scholar]
- 71. Niederreither K, Dolle P. Retinoic acid in development: towards an integrated view. Nat Rev Genet 9: 541–553, 2008. [DOI] [PubMed] [Google Scholar]
- 72. Niederreither K, Vermot J, Messaddeq N, Schuhbaur B, Chambon P, Dolle P. Embryonic retinoic acid synthesis is essential for heart morphogenesis in the mouse. Development 128: 1019–1031, 2001. [DOI] [PubMed] [Google Scholar]
- 73. O'Byrne SM, Wongsiriroj N, Libien J, Vogel S, Goldberg IJ, Baehr W, Palczewski K, Blaner WS. Retinoid absorption and storage is impaired in mice lacking lecithin:retinol acyltransferase (LRAT). J Biol Chem 280: 35647–35657, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Osuala K, Telusma K, Khan SM, Wu S, Shah M, Baker C, Alam S, Abukenda I, Fuentes A, Seifein HB, Ebert SN. Distinctive left-sided distribution of adrenergic-derived cells in the adult mouse heart. PLoS One 6: e22811, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Papka RE. A study of catecholamine-containing cells in the hearts of fetal and postnatal rabbits by fluorescence and electron microscopy. Cell Tissue Res 154: 471–484, 1974. [DOI] [PubMed] [Google Scholar]
- 76. Pappano AJ. Ontogenetic development of autonomic neuroeffector transmission and transmitter reactivity in embryonic and fetal hearts. Pharmacol Rev 29: 3–33, 1977. [PubMed] [Google Scholar]
- 77. Pendleton RG, Gessner G, Sawyer J. Studies on the distribution of phenylethanolamine N-methyltransferase and epinephrine in the rat. Res Commun Chem Pathol Pharmacol 21: 315–325, 1978. [PubMed] [Google Scholar]
- 78. Peters LL, Wood BG. The prenatal development of the organ of Zuckerkandl in rats. Life Sci 41: 1355–1359, 1987. [DOI] [PubMed] [Google Scholar]
- 79. Portbury AL, Chandra R, Groelle M, McMillian MK, Elias A, Herlong JR, Rios M, Roffler-Tarlov S, Chikaraishi DM. Catecholamines act via a beta-adrenergic receptor to maintain fetal heart rate and survival. Am J Physiol Heart Circ Physiol 284: H2069–H2077, 2003. [DOI] [PubMed] [Google Scholar]
- 80. Ream M, Ray AM, Chandra R, Chikaraishi DM. Early fetal hypoxia leads to growth restriction and myocardial thinning. Am J Physiol Regul Integr Comp Physiol 295: R583–R595, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ream MA, Chandra R, Peavey M, Ray AM, Roffler-Tarlov S, Kim HG, Wetsel WC, Rockman HA, Chikaraishi DM. High oxygen prevents fetal lethality due to lack of catecholamines. Am J Physiol Regul Integr Comp Physiol 295: R942–R953, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Redmond TM, Gentleman S, Duncan T, Yu S, Wiggert B, Gantt E, Cunningham FX., Jr Identification, expression, and substrate specificity of a mammalian beta-carotene 15,15′-dioxygenase. J Biol Chem 276: 6560–6565, 2001. [DOI] [PubMed] [Google Scholar]
- 83. Robinson JF, Verhoef A, Pennings JL, Pronk TE, Piersma AH. A comparison of gene expression responses in rat whole embryo culture and in vivo: time-dependent retinoic acid-induced teratogenic response. Toxicol Sci 126: 242–254, 2012. [DOI] [PubMed] [Google Scholar]
- 84. Roeske WR, Wildenthal K. Responsiveness to drugs and hormones in the murine model of cardiac ontogenesis. Pharmacol Ther 14: 55–66, 1981. [DOI] [PubMed] [Google Scholar]
- 85. Rohrer DK, Chruscinski A, Schauble EH, Bernstein D, Kobilka BK. Cardiovascular and metabolic alterations in mice lacking both beta1- and beta2-adrenergic receptors. J Biol Chem 274: 16701–16708, 1999. [DOI] [PubMed] [Google Scholar]
- 86. Rohrer DK, Desai KH, Jasper JR, Stevens ME, Regula DPJ, Barsh GS, Bernstein D, Kobilka BK. Targeted disruption of the mouse beta1-adrenergic receptor gene: developmental and cardiovascular effects. Proc Natl Acad Sci USA 93: 7375–7380, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ross SA, McCaffery PJ, Drager UC, De Luca LM. Retinoids in embryonal development. Physiol Rev 80: 1021–1054, 2000. [DOI] [PubMed] [Google Scholar]
- 88. Ruiz-Lozano P, Smith SM, Perkins G, Kubalak SW, Boss GR, Sucov HM, Evans RM, Chien KR. Energy deprivation and a deficiency in downstream metabolic target genes during the onset of embryonic heart failure in RXRalpha-/- embryos. Development 125: 533–544, 1998. [DOI] [PubMed] [Google Scholar]
- 89. Sakura H, Bond C, Warren-Perry M, Horsley S, Kearney L, Tucker S, Adelman J, Turner R, Ashcroft FM. Characterization and variation of a human inwardly-rectifying-K-channel gene (KCNJ6): a putative ATP-sensitive K-channel subunit. FEBS Lett 367: 193–197, 1995. [DOI] [PubMed] [Google Scholar]
- 90. Sinning AR. Role of vitamin A in the formation of congenital heart defects. Anat Rec 253: 147–153, 1998. [DOI] [PubMed] [Google Scholar]
- 91. Sonneveld E, van den Brink CE, van der Leede BJ, Maden M, van der Saag PT. Embryonal carcinoma cell lines stably transfected with mRARbeta2-lacZ: sensitive system for measuring levels of active retinoids. Exp Cell Res 250: 284–297, 1999. [DOI] [PubMed] [Google Scholar]
- 92. Stachurska E, Loboda A, Niderla-Bielinska J, Szperl M, Juszynski M, Jozkowicz A, Dulak J, Ratajska A. Expression of cellular retinoic acid-binding protein I and II (CRABP I and II) in embryonic mouse hearts treated with retinoic acid. Acta Biochim Pol 58: 19–29, 2011. [PubMed] [Google Scholar]
- 93. Subbarayan V, Mark M, Messadeq N, Rustin P, Chambon P, Kastner P. RXRalpha overexpression in cardiomyocytes causes dilated cardiomyopathy but fails to rescue myocardial hypoplasia in RXRalpha-null fetuses. J Clin Invest 105: 387–394, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sucov HM, Dyson E, Gumeringer CL, Price J, Chien KR, Evans RM. RXR alpha mutant mice establish a genetic basis for vitamin A signaling in heart morphogenesis. Genes Dev 8: 1007–1018, 1994. [DOI] [PubMed] [Google Scholar]
- 95. Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell 111: 305–317, 2002. [DOI] [PubMed] [Google Scholar]
- 96. Takitani K, Miyazaki H, Fukunishi S, Takaya R, Yoden A, Higuchi K, Tamai H. Altered expression of both beta-carotene 15,15′ monooxygenase and lecithin:retinol acyltransferase in obese Zucker rats. J Nutr Sci Vitaminol (Tokyo) 57: 108–113, 2011. [DOI] [PubMed] [Google Scholar]
- 97. Takitani K, Zhu CL, Inoue A, Tamai H. Molecular cloning of the rat beta-carotene 15,15′-monooxygenase gene and its regulation by retinoic acid. Eur J Nutr 45: 320–326, 2006. [DOI] [PubMed] [Google Scholar]
- 98. Thomas SA, Marck BT, Palmiter RD, Matsumoto AM. Restoration of norepinephrine and reversal of phenotypes in mice lacking dopamine beta-hydroxylase. J Neurochem 70: 2468–2476, 1998. [DOI] [PubMed] [Google Scholar]
- 99. Thomas SA, Matsumoto AM, Palmiter RD. Noradrenaline is essential for mouse fetal development. Nature 374: 643–646, 1995. [DOI] [PubMed] [Google Scholar]
- 100. Thomas SA, Palmiter RD. Examining adrenergic roles in development, physiology, and behavior through targeted disruption of the mouse dopamine beta-hydroxylase gene. Adv Pharmacol 42: 57–60, 1998. [DOI] [PubMed] [Google Scholar]
- 101. Tian H, Hammer RE, Matsumoto AM, Russell DW, McKnight SL. The hypoxia-responsive transcription factor EPAS1 is essential for catecholamine homeostasis and protection against heart failure during embryonic development. Genes Dev 12: 3320–3324, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Tseng YT, Kopel R, Stabila JP, McGonnigal BG, Nguyen TT, Gruppuso PA, Padbury JF. Beta-adrenergic receptors (betaAR) regulate cardiomyocyte proliferation during early postnatal life. FASEB J 15: 1921–1926, 2001. [DOI] [PubMed] [Google Scholar]
- 103. van Veen TA, van Rijen HV, Wiegerinck RF, Opthof T, Colbert MC, Clement S, de Bakker JM, Jongsma HJ. Remodeling of gap junctions in mouse hearts hypertrophied by forced retinoic acid signaling. J Mol Cell Cardiol 34: 1411–1423, 2002. [DOI] [PubMed] [Google Scholar]
- 104. Wagner M, Han B, Jessell TM. Regional differences in retinoid release from embryonic neural tissue detected by an in vitro reporter assay. Development 116: 55–66, 1992. [DOI] [PubMed] [Google Scholar]
- 105. Wei LN, Blaner WS, Goodman DS, Nguyen-Huu MC. Regulation of the cellular retinoid-binding proteins and their messenger ribonucleic acids during P19 embryonal carcinoma cell differentiation induced by retinoic acid. Mol Endocrinol 3: 454–463, 1989. [DOI] [PubMed] [Google Scholar]
- 106. Wildenthal K. Maturation of responsiveness to cardioactive drugs. Differential effects of acetylcholine, norepinephrine, theophylline, tyramine, glucagon, and dibutyryl cyclic AMP on atrial rate in hearts of fetal mice. J Clin Invest 52: 2250–2258, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Yasui N, Benya PD, Nimni ME. Coordinate regulation of type IX and type II collagen synthesis during growth of chick chondrocytes in retinoic acid or 5-bromo-2′-deoxyuridine. J Biol Chem 261: 7997–8001, 1986. [PubMed] [Google Scholar]
- 108. Zhang YZ, Naleway JJ, Larison KD, Huang ZJ, Haugland RP. Detecting lacZ gene expression in living cells with new lipophilic, fluorogenic beta-galactosidase substrates. FASEB J 5: 3108–3113, 1991. [DOI] [PubMed] [Google Scholar]
- 109. Zhou QY, Quaife CJ, Palmiter RD. Targeted disruption of the tyrosine hydroxylase gene reveals that catecholamines are required for mouse fetal development. Nature 374: 640–643, 1995. [DOI] [PubMed] [Google Scholar]
- 110. Zolfaghari R, Ross AC. An essential set of basic DNA response elements is required for receptor-dependent transcription of the lecithin:retinol acyltransferase (Lrat) gene. Arch Biochem Biophys 489: 1–9, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]