Abstract
Adeno-associated virus type 3b (AAV3b) has been largely ignored by gene therapists because of the inability of vectors based on this serotype to transduce target tissues efficiently. Here we describe a phenomenon unique to AAV3b in that vectors based on this serotype mediate enhanced transduction in the presence of heparin. Among the many biological functions attributed to heparin, its interaction with, and ability to regulate, several growth factors (GFs) and growth factor receptors (GFRs) has been well characterized. Using GFR-overexpressing cell lines, soluble GFs and heparins, as well as specific GFR inhibitors, we have demonstrated a requirement for fibroblast growth factor receptor-2 (FGFR2) and FGF1 in the heparin-mediated augmentation of AAV3b vector transduction. In contrast to AAV2, we establish that heparin can be used as an adjunct with AAV3b to further increase transduction in a variety of cells and target tissues, additionally suggesting that AAV3b may be an attractive viral vector for clinical use during procedures in which heparin is used. In summary, AAV3b exhibits FGFR2-dependent, markedly enhanced transduction efficiency in the presence of heparin and FGFs, which could make it a useful vector for gene therapy in a variety of human diseases.
Using growth factor receptor (GFR)-overexpressing cell lines, soluble growth factors, and heparins, Messina and colleagues demonstrate a requirement for fibroblast growth factor receptor-2 (FGFR2) and FGF1 in the heparin-mediated augmentation of AAV3b vector transduction. Heparin can increase transduction of AAV3b in a variety of cells and target tissues.
Introduction
Adeno-associated viruses (AAVs) have been developed as vectors for gene therapy. Because AAV does not cause disease, relatively little research has focused on defining the infectious process of the 12 AAV serotypes (or hundreds of AAV strains or laboratory-generated variants). As with any biological intended for human use, a complete understanding of the mechanisms of AAV vector transduction is essential for ensuring safety and efficacy. Increased knowledge of the AAV transduction process may lead to more successful gene therapy applications.
AAV serotype 2 (AAV2) and the closely related AAV3 are both derived from human sources (Hoggan et al., 1966). AAV2 is the best characterized AAV at all levels of the infectious process and infects a wide variety of cell types using ubiquitously expressed heparan sulfate proteoglycan (HSPG) as a primary attachment molecule (Summerford and Samulski, 1998). Initially deposited into the American Type Culture Collection (ATCC, Manassas, VA) as AAV3 strain H (VR-681; ATCC), two distinct AAV3 isolates (AAV3a and AAV3b) differing by only six amino acids have been subsequently cloned (Muramatsu et al., 1996; Rutledge et al., 1998). Both appear to bind to heparin and the infectivity of both is inhibited by soluble heparin, albeit at much higher doses than for AAV2 (Handa et al., 2000; Rabinowitz et al., 2004; Lerch and Chapman, 2012). In comparison with vectors based on other AAV serotypes, AAV3 vectors inefficiently transduce most cell types with the exceptions of cochlear inner hair cells (Chang et al., 2005) and human liver cancer cells (Glushakova et al., 2009).
Similar to what has been discovered for unrelated viruses, coreceptors are necessary for efficient AAV entry. Fibroblast growth factor receptor-1 (FGFR1), hepatocyte growth factor receptor (HGFR), αvβ5 integrin, and α5β1 integrin have all been implicated as coreceptors for AAV2 (Qing et al., 1999; Summerford et al., 1999; Kashiwakura et al., 2005; Asokan et al., 2006). FGFR1 has been described as an AAV3h coreceptor (Blackburn et al., 2006) and HGFR has been reported as a coreceptor for AAV3 (Ling et al., 2010). It is noteworthy that all aforementioned receptors have well-described interactions with heparin or HSPG, which are in many cases critical for receptor functioning (Ornitz, 2000). Receptors, both primary and coreceptors, for some serotypes have been identified that do not appear to involve HSPG. Sialic acid moieties are primary receptors for AAV1, AAV4, AAV5, and AAV6 (Kaludov et al., 2001; Walters et al., 2001; Wu et al., 2006); platelet-derived growth factor receptor (PDGFR) has been recognized as a receptor for AAV5 (Di Pasquale et al., 2003); and a laminin receptor has been reported used by AAV2, AAV3, AAV8, and AAV9 (Akache et al., 2006).
In cell culture-based experiments, the glycosaminoglycan heparin has been used as a substitute or competitive inhibitor for HSPG. Infectivity of certain viruses including AAV2 is inhibited in the presence of soluble heparin (Summerford and Samulski, 1998; Dechecchi et al., 2000; Hilgard and Stockert, 2000). Presumably, heparin attaches to heparin-binding sites on the viral particle, directly blocking infection. It is still unclear what influences heparin may exert on AAV2 transduction in vivo, and the effect may vary with the type or form of heparin studied (Schuettrumpf et al., 2006). For example, the presence of heparin serving a positive effect in vivo has been seen after coinjection of AAV2 and heparin in the brain (Nguyen et al., 2001; Mastakov et al., 2002). Apparently, immediate binding to cell surface HSPG at the site of injection was blocked by the presence of soluble heparin, thereby allowing the vector to spread further from the injection site.
When heparin is used experimentally in cell culture as a competitive inhibitor to block infection, AAV3b is more resilient than AAV2 (Rabinowitz et al., 2004). This is believed to occur because the AAV3b capsid interacts with less affinity to heparin than AAV2 (Rabinowitz et al., 2002; Lerch and Chapman, 2012), a trait ascribed to its lack of high-affinity heparin-binding sites that exist on the closely related AAV2 capsid (Opie et al., 2003).
We decided to further explore this heparin-resistant phenotype of AAV3b for two reasons. First, surgical or percutaneous vector delivery methodology often necessitates reversible and transient anticoagulation; AAV3b or similar vectors containing the heparin resistance phenotype might be advantageous for cardiovascular gene therapy. Second, known coreceptors of AAV2 and AAV3b have well-described interactions with either heparin or HSPG. It is generally accepted that heparin plays an essential role in FGFR signaling by direct association with both FGF and FGFR on the cell surface (reviewed in Ornitz, 2000; Ornitz and Itoh, 2001). Studying the AAV3b heparin-resistant phenotype might reveal more about AAV receptor interactions and subsequent signaling events.
Here we report that AAV3b-based vectors possess the unique and favorable property of significantly enhanced transduction in the presence of soluble heparin. We have undertaken studies to understand both why this occurs and whether this effect can be translated from a cell culture observation to an in vivo model. Our results suggest that heparin modulates an effect at AAV3b vector binding or entry through the use of FGFR2 and fibroblast growth factors (FGFs). Further inquiries indicate that signaling through FGFRs confers a cellular environment more conducive to infectivity of all tested AAV serotypes; it is not unique just to AAV3b. These observations suggest that AAV vector transduction in general may be optimal under conditions in which FGFR signaling is enhanced, and that additional improvements on AAV vector transduction can be made by directly targeting cellular pathways influenced by FGFR signaling.
Materials and Methods
Cell lines
293, 911, A431, HEp-2, HeLa, and COS-7 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) and penicillin (100 U/ml)–streptomycin (100 μg/ml). 293G cells were maintained in DMEM containing 10% FBS, 0.1 mM nonessential amino acids, G418 (600 μg/ml), and penicillin (100 U/ml)–streptomycin (100 μg/ml). CHO K1, CHO pgsD, PC3, and A549 cells were maintained in Ham's F-12 containing 10% FBS. SK-N-AS and SK-N-FI cells were maintained in DMEM containing 10% FBS and 0.1 mM nonessential amino acids. LNCaP cells were grown in RPMI 1640 medium containing 2 mM l-glutamine, NaHCO3 (2 g/liter), 10% FBS, 10 mM HEPES, 1 mM sodium pyruvate, glucose (4.5 g/liter), and penicillin (100 U/ml)–streptomycin (100 μg/ml). DU145 cells were maintained in DMEM, 10% FBS, 1 mM nonessential amino acids, 1 mM sodium pyruvate, and penicillin (100 U/ml)–streptomycin (100 μg/ml). SUM-52 and SUM-44 cells (generously donated by S. Ethier, University of Michigan Comprehensive Cancer Center, Ann Arbor, MI) were grown according to their instructions. H16N2 pNG cells were plated in Ham's F-12 containing 2% FBS, insulin (5 μg/ml), hydrocortisone (1 μg/ml), epidermal growth factor (EGF, 10 ng/ml), and G418 (100 μg/ml), and maintained in the same without serum. H16N2 C1 and C3 cells were split in Ham's F-12 containing 2% FBS, insulin (5 μg/ml), hydrocortisone (1 μg/ml), and G418 (100 μg/ml), and maintained in the same without serum. H16N2 cells and variants were also provided by S. Ethier.
PCR
RNA was isolated from cell and vein samples, using an RNeasy midi kit (Qiagen, Valencia, CA) as per the manufacturer's instructions. cDNA was generated with a Transcriptor first-strand cDNA synthesis kit (Roche, Basel, Switzerland) according to the manufacturer's instructions. PCR was performed with a Platinum Taq DNA polymerase kit (Invitrogen, Carlsbad, CA). PCRs were performed in a PTC-200 thermo cycler (MJ Research, Waltham, MA). Primer sequences and conditions for the FGFRs and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) PCR amplifications were published by Tartaglia and colleagues (2001).
AAV production
All recombinant AAV vectors were produced via the standard triple transfection method (reviewed in Grieger et al., 2006), using the XX6-80 adenoviral helper plasmid with a packaging plasmid (either pxr1-3 or the SASTG modified plasmid) and an ITR plasmid carrying the luciferase transgene. The plasmids were transfected into 293 cells with polyethylenimine (Polysciences, Warrington, PA) and harvested 72 hr later. Cell suspensions were subjected to five freeze–thaw cycles, followed by two CsCl gradients. The isolated virus was then desalted on Econo-Pac 10DG columns (Bio-Rad, Hercules, CA) and eluted in sterile phosphate-buffered saline (PBS). Virus was quantified by nonradioactive dot blot, using a protocol described by Grieger and colleagues (2006). Viruses that were to be directly compared were evaluated on the same blot.
Luminometer assays
Luminometry was performed with a Veritas luminometer from Turner Biosystems (Sunnyvale, CA). Cells were plated at 10,000 cells per well in BD Falcon tissue culture-treated, 96-well, black with clear bottom plates (Becton Dickinson, Franklin Lakes, NJ). The cells were infected 24 hr later in medium containing 2% FBS, unless otherwise stated. Other growth factors, inhibitors, and glycosaminoglycans were not used except where specifically stated. Cells were incubated with virus (1000 particles/cell unless otherwise stated in the text or figure legends) for 24 hr at 37°C and subsequently lysed with passive lysis buffer (Promega, Madison, WI) for 10 min. Within the luminometer, wells were injected with 100 μl of luciferase assay reagent (Promega) and, after a delay of 2 sec, a measurement was taken for 10 sec. Each figure shows results of experiments repeated at least twice with each data point in triplicate or quadruplicate.
Glycosaminoglycan, FGF, and FGFR inhibitor experiments
Twenty-four hours after plating, cells were pretreated with 100 μl of respective medium containing 2% FBS, unless otherwise stated, and either no glycosaminoglycan (GAG), or a dilution of GAG ranging from 1000 to 0.32 μg/ml (specific range stated in figure legend). AAV vector was mixed with medium containing no GAG or a dilution of the same GAG. Pretreatment medium was then removed and replaced with the same respective medium containing virus (1000 viral genome-containing particles [VG]/cell unless otherwise stated) in a total volume of 100 μl. Cells were maintained at 37°C before and after infection, and during pretreatment. GAGs used included chondroitin sulfate A, chondroitin sulfate C, and heparin and were purchased from Sigma-Aldrich (St. Louis, MO). Chondroitin sulfate B was purchased from US Biological (Swampscott, MA). Heparin disaccharide and tetrasaccharide were purchased from Neoparin (Alameda, CA). In experiments with growth factors, cells were infected in serum-free medium containing FGF7 (KGF, 25 ng/ml) or FGF1 (αFGF, 50 ng/ml). Growth factor was included in growth media after plating, pretreatment and infection media. FGF7 was obtained from Biovision (Mountain View, CA) and FGF1 was obtained from Sigma-Aldrich. The FGFR inhibitor PD173074 (Calbiochem, San Diego, CA) was resuspended in dimethyl sulfoxide (DMSO). When PD173074 was used, a DMSO-only control was also included. The inhibitor was used in the pretreatment of cells and during infection at a concentration of 8×10–3 μmol/ml. In the inhibitor experiment, a titer of 10,000 VG/cell was used for AAV1.
FGF pull-down assays
AAV1, AAV2, or SASTG luciferase vector (3.8×1011 VG) was mixed with 30 ng of FGF1 and 100 μg of heparin in a total volume of 1 ml of cold PBS. Two micrograms of FGF1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) was mixed with 50 μl of A/G agarose slurry (Santa Cruz Biotechnology) in a 1-ml volume of PBS. Mixtures were rocked for 3 hr before the agarose mixture was spun down at 7000 rpm and the beads were washed twice in cold PBS. Virus mixture was then added to the beads and the mixture was rocked for an additional 2 hr. Mixtures were spun down at 7000 rpm, supernatant was removed, and the beads were washed in cold PBS. All of the above-described steps were performed at 4°C. At room temperature, beads were resuspended in 2× Laemmli buffer with 50 mM dithiothreitol (DTT) and heated to 85°C for 20 min. Twenty-five microliters of the supernatant was run on a 10–20% Tris-glycine gel (Invitrogen). After transfer and blocking, blots were probed with anti-AAV B1 antibody (American Research Products, Waltham, MA) at a 1:333 dilution.
Mouse leg injections in vivo imaging
Animals were maintained and treated in accordance with the Institutional Animal Care and Use Committee (IACUC) at Duke University (Chapel Hill, NC). All care and procedures were in accordance with the Guide for the Care and Use of Laboratory Animals (DHHS Publication No. [NIH] 85-23), and all procedures received prior approval by Duke University Institutional Animal Care and Usage Committees. Mice were anesthetized and sedation was maintained with isoflurane. Virus (1×1010 genome-containing viral particles) was premixed with heparin in PBS so that the final concentration of heparin was 1.95, 31.2, or 260 μg/ml. The animal's right gastrocnemius was injected with the mixture of heparin and virus, while the left was injected only with virus in an equal injection volume. To image expression, mice were anesthetized and sedation was maintained with isoflurane. One hundred microliters of luciferin was delivered via intraperitoneal injection. The animals were imaged 10 min after injection, using the Xenogen IVIS imaging system (Caliper Life Sciences, Hopkinton, MA) and luminescence was measured.
Vein experiments
After the Duke University Medical Center Institutional Review Board provided exempted status, discarded saphenous vein segments were obtained from patients undergoing coronary artery bypass grafting. Vector delivery was achieved by bathing pieces of the vein segments in vector and applying nondistending pressure to the entire suspension.
Assessment of green fluorescent protein expression in infected cells by flow cytometry analysis
911 cells were infected with SASTG-GFP (green fluorescent protein) virus as described previously. Three days postinfection, cells were washed in PBS and fixed with 1% paraformaldehyde. Cells were assessed with a FACSAria flow cytometer (BD Biosciences, San Jose, CA) and FlowJo software (Tree Star, Ashland, OR).
Cardiomyocyte isolation
All procedures for tissue procurement were performed in compliance with institutional guidelines for an approved IACUC protocol at Duke University Medical Center. Briefly, porcine hearts were arrested with cold cardioplegia. The myocardium was perfused by selective arterial cannulation of the left anterior descending artery (LAD) or left circumflex arteries on a Langendorff apparatus. A modified Krebs (Sigma-Aldrich) solution (25 mM NaHCO3, 10 mM taurine; pH 7.4) was oxygenated and circulated at 37°C for 10 min at 30 ml/min. The oxygenated collagenase solution (Krebs [Sigma-Aldrich], type II collagenase [120 units/ml; Worthington, Lakewood, NJ], 25 mM NaHCO3, 20 mM taurine, 20 mM 2,3-butanedione 2-monoxime [BDM], 0.05 mM CaCl2; pH 7.4) was then circulated at 37°C for 20 min. The heart was then perfused with oxygenated rinse solution (Krebs [Sigma-Aldrich], 25 mM NaHCO3, 10 mM taurine, 20 mM 2,3-BDM, 0.1 mM CaCl2, 10 ml/liter penicillin [100 U/ml]–streptomycin [100 μg/ml]; pH 7.4) at 37°C for 10 min. The heart was then split at mid-myocardium and the myocytes were gently scraped and rinsed into a Petri dish, using the rinse solution. Cells were filtered and allowed to settle for 15 min. Part of the supernatant was removed, the remaining volume was diluted 1:1 with sterile low-calcium medium (Joklik's MEM with glutamine, 0.1 mM CaCl2, 1% bovine serum albumin [BSA], 15 mM HEPES, 10 ml/liter penicillin [100 U/ml]–streptomycin [100 μg/ml]; pH 7.4), and the cells were resuspended and allowed to settle for 15 min. Cells were then subjected to calcium tolerance by increasing the CaCl2 concentration by 0.1 mM every 5 min to 0.5 mM. Cells were plated on Matrigel (BD Biosciences) and incubated at 37°C in culture medium (Joklik's MEM with glutamine, 0.5 mM CaCl2, 1% BSA, 5% FBS, 15 mM HEPES, 10 ml/liter penicillin [100 U/ml]–streptomycin [100 μg/ml]; pH 7.4).
Results
Soluble heparin augments the ability of AAV3b-based vectors to transduce tissue culture cells
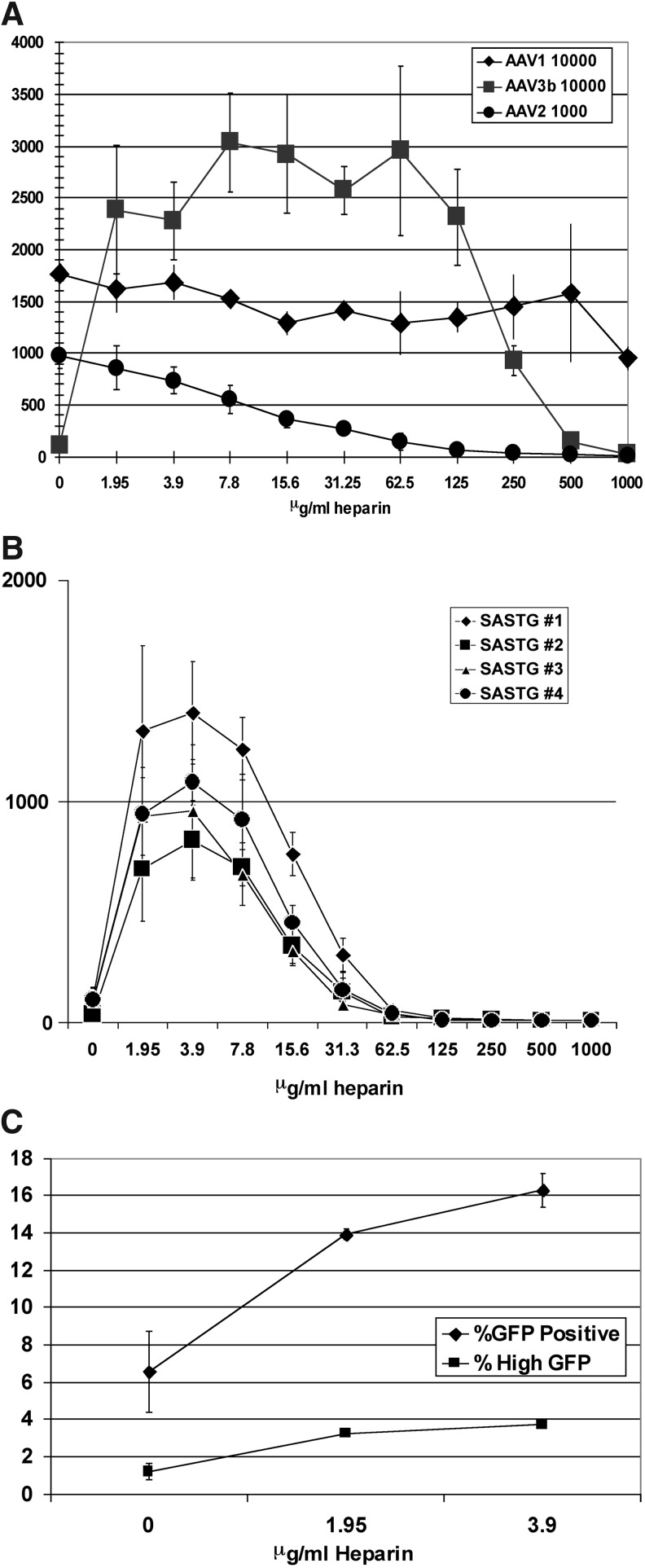
Both AAV3a and AAV3b have been reported to be inherently more resistant to inhibition by heparin than AAV2 (Handa et al., 2000; Rabinowitz et al., 2004). The heparin inhibition profiles of several AAVs (AAV1, AAV2, and AAV3b) were further compared in a dose–response experiment using 911 cells (Fig. 1A). As expected, AAV2 transduction was inhibited by heparin in a dose-dependent manner, exhibiting 50% reduction at 15.6 μg/ml and complete inhibition at higher doses (Summerford and Samulski, 1998). In contrast, AAV1 was virtually unaffected by heparin. Unexpectedly, as low a concentration as 1.95 μg/ml of heparin boosted AAV3b transduction by approximately 22-fold. This level of enhancement was maintained and increased even further as the heparin concentration was raised over a 2-fold range until a concentration of 250 μg/ml heparin was reached, which diminished the enhancement to 8.7-fold over baseline. Higher concentrations of heparin eventually inhibited AAV3b transduction. This clearly demonstrates a significant and dramatic increase in transduction of the AAV3b vector by heparin. We have extended our studies to examine AAV6 and AAV9 (data not shown); the cell culture transduction patterns of both are not enhanced by heparin.
FIG. 1.
AAV1, AAV2, and AAV3b exhibit different transduction profiles in 911 cells in the presence of increasing amounts of soluble heparin. (A) Direct comparison of luciferase expression between serotypes AAV1, AAV2, and AAV3b in response to increasing concentrations of soluble heparin. 911 cells were transduced with 10,000 particles per cell of AAV1 or AAV3 and only 1000 particles per cell of AAV2, all bearing the luciferase transgene. All transduction experiments described were performed in the absence of an adenoviral helper to eliminate compounding effects of a second virus. The use of 10 times more AAV1 and AAV3 in this experiment compared with AAV2 was done to obtain reliable signals and is consistent with work performed previously. The vectors were applied to 911 cells in a 96-well format. Infections were performed in the presence of increasing amounts of heparin ranging from 0 to 1000 μg/ml in 2-fold serial dilutions. Light emitted from the infections was assayed by luminometer the next day. These experiments in 911 cells were repeated a total of three times and data similar to that shown in Fig. 1 were observed. Data represent means±SD. (B) Response of AAV3b daughter vector, SASTG, to soluble heparin. Four independently generated preparations of the SASTG-luciferase vector gave similar results in the presence of increasing amounts of heparin in 911 cells. SASTG vectors (1000 particles/cell) were applied to 911 cells (10,000 per well) in a 96-well format. Infections were performed in the presence of increasing amounts of heparin ranging from 0 to 1000 μg/ml in 2-fold serial dilutions. Light emitted from the infections was assayed by luminometer the next day. Data represent means±SD. (C) FACS assay demonstrating the alteration of SASTG GFP expression in 911 cells with low concentrations of heparin. Infections were performed in the presence of heparin (1.95 or 3.9 μg/ml). GFP-positive cells were counted 5 days postinfection via flow cytometry. Similar results were observed in three independent experiments. Data are presented as percent GFP-positive cells and represent means±SD.
Soluble heparin augments the ability of SASTG-based vectors to transduce tissue culture cells
We have shown that replacing critical amino acids in the AAV2 and AAV3b capsid with the corresponding amino acids from AAV1 confers enhanced skeletal and cardiac muscle transduction (Bowles et al., 2012; Piacentino et al., 2012). In the next set of experiments we evaluated the influence of heparin on the more efficient version of the AAV3b capsid, termed SASTG (Piacentino et al., 2012). The response of AAV3b to heparin was conserved in SASTG as judged by transduction experiments performed in 911 cells with four independently generated preparations of SASTG-luc vector in the presence of increasing amounts of heparin (Fig. 1B). Because SASTG transduced target cells significantly more efficiently than wild-type AAV3b, we chose to use the SASTG variant in the remainder of the experiments in this study.
Regarding heparin-mediated augmentation of SASTG, this phenomenon was observed whether luminometry was performed at either 17 or 24 hr postinfection (Fig. 1B) or 48 hr postinfection (Supplementary Fig. S1A; supplementary data are available online at www.liebertpub.com/hum). A similar boost was observed when SASTG transduction was assessed in the setting of either porcine heparin (from two separate preparations) or with bovine heparin (Supplementary Fig. S1B and C).
To determine whether the observed heparin-mediated enhancement was due to an increase in the number of infected cells, or due to alterations in postentry events that increase the level of transgene expression, 911 cells were infected with SASTG-GFP vector in the absence or the presence of two low doses (1.95 and 3.9 μg/ml) of heparin (Fig. 1C). Flow cytometric analysis revealed both an increase in the percentage of GFP-expressing cells as well as an increase in the percentage of brighter cells at both doses of heparin. These results suggest that heparin is influencing the transduction process of SASTG at the level of vector binding and entry, and possibly influencing postentry events.
The heparin-mediated augmentation correlates with FGFR2 expression
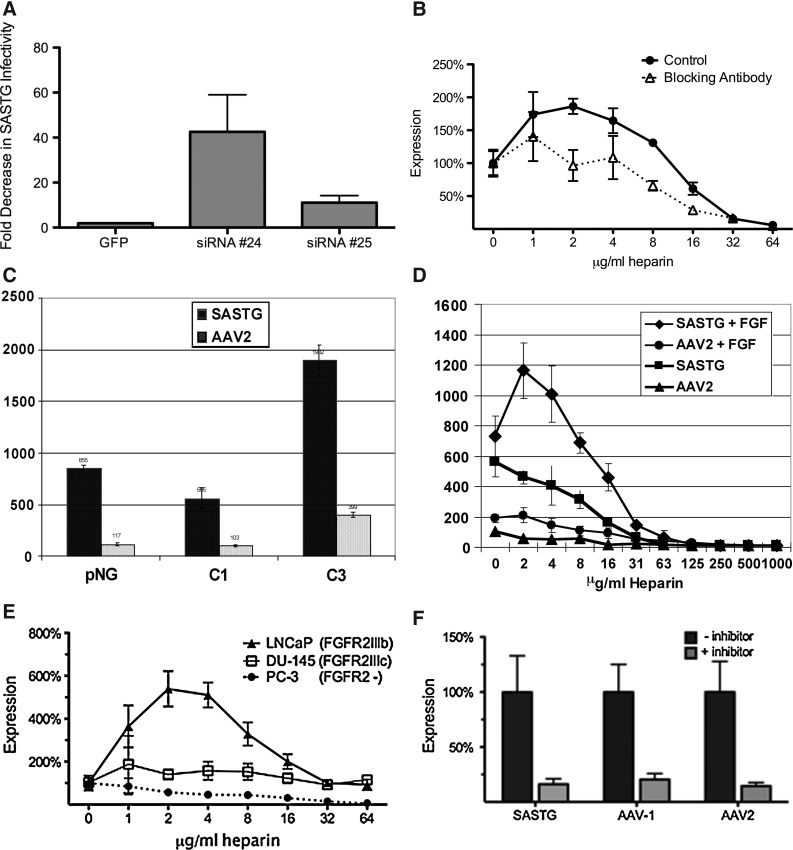
Because results from the flow cytometry study indicated an involvement of vector binding and entry and because an AAV coreceptor (FGFR1) was known to interact with heparin, a panel of cell lines (Table 1) was examined for mRNA expression of the five FGFR family members. From this we gleaned a positive correlation between the presence of FGFR2 and heparin-mediated augmentation of SASTG transduction. Transduction by SASTG requires the presence of the FGFR2 receptor, as two different FGFR2-specific small hairpin RNAs (shRNAs) decreased the ability of SASTG to transduce 911 cells by 11-fold (shRNA 25) or 45-fold (shRNA 24) (Fig. 2A). Treatment of cells with an FGFR2-specific antibody before and during infection with the SASTG vector blunted, but did not completely eliminate, the heparin-mediated transduction boost (Fig. 2B). The requirement for FGFR2 was further confirmed when the effects of heparin on SASTG transduction were examined in H16N2 pNG cells, which do not express the FGFR2 receptor, and in two derivative cell lines (C1 and C3) expressing an FGF1-sensitive form of FGFR2 (C1 cells) or expressing a constitutively active FGFR2 (C3 cells) (Moffa et al., 2004; Moffa and Ethier, 2007) (Table 1, and Fig. 2C and D). SASTG transduced all cell lines and the presence of the FGF1-sensitive form of FGFR2 did not confer any enhancement over the pNG cells, in the absence of heparin. However, the presence of the constitutively active form of FGFR2 translated to a 4-fold increase in expression over C1 cells for both SASTG and AAV2. In the presence of heparin and FGF1, no enhancement in SASTG expression was observed in either the pNG or C3 cells (Supplementary Fig. S2). However, a significant enhancement in SASTG transduction was seen in C1 cells with the presence of both FGF1 (50 ng/ml) and low levels of heparin (Fig. 2D).
Table 1.
Cell Lines and Tissues Used in Study
| |
FGFR RNA |
|
||||
|---|---|---|---|---|---|---|
| Cell line | 1 | 2 | 3 | 4 | 5 | Heparin augmentation |
| 293G | + | + | + | + | + | Yes |
| 911 | + | + | + | + | + | Yes |
| Cos-7 | + | + | – | – | + | Yes |
| HF1 (293) | + | + | + | + | + | Yes |
| SK-N-AS | + | + | – | + | + | Yes |
| SK-N-FI | + | + | – | – | + | Yes |
| HeLa | + | – | + | + | No | |
| + | ||||||
| C2 C12 | – | – | – | – | – | No |
| CHOK1 | – | – | – | – | – | No |
| pqsD | – | – | – | – | – | No |
| A431 | – | – | – | – | – | No |
| A549 | – | – | – | – | – | No |
| HEp-2 | + | – | + | – | + | No |
| SUM-52 PE | FGFR2 over expressed | Yes | ||||
| SUM-44 PE | FGFR2 under expressed | No | ||||
| H16N2 pNG | No FGFR2 | No | ||||
| H16N2 pNG C1 | FGFR2 inducible | Yes | ||||
| H16N2 pNG C3 | FGFR2 constitutively activated | No | ||||
FIG. 2.
Examination of the importance of fibroblast growth factor receptor-2 (FGFR2) in SASTG infection. (A) SASTG requires FGFR2 for infectivity. 911 cells transfected with either a control GFP plasmid or plasmids expressing shRNA (shRNA 24 or shRNA 25) specific for FGFR2 were infected 3 days posttransfection with SASTG-luc vector. Results are expressed as fold decrease in SASTG infectivity relative to infections performed on untransfected cells. (B) Anti-FGFR2 antibody blunts heparin-mediated SASTG transduction. Results are expressed as percent expression relative to infections performed in the absence of heparin. (C) Infection of H16N2 cell lines (pNG, no FGFR2; C1, FGF1-sensitive FGFR2; C3, constitutively active FGFR2) with SASTG-luc and AAV2-luc (1000 particles/cell) without heparin. Data represent means±SD. (D) Infection of H16N2 C1 cells (10,000 per well) with SASTG-luc or AAV2-luc (1000 particles/cell) in the presence and absence of FGF (50 ng/ml), over a range of heparin concentrations. Data represent means±SD. (E) Influence of heparin on SASTG transduction of three prostate cancer cell lines expressing different isoforms of FGFR2. Respective cell lines (10,000 cells per well) were infected with SASTG-luc (1000 particles/cell) in the presence of increasing amounts of heparin (ranging from 1 to 64 μg/ml). Results are expressed as percent expression relative to infections performed in the absence of heparin. Data represent means±SD. (F) Infection of 911 cells with an inhibitor of the tyrosine kinase domain of FGFR2, PD173074 (8×10–3 μmol/ml), without heparin. The inhibitor was administered during pretreatment of the cells and during infection. A titer of 10,000 VG/cell was used for AAV1 and 1000 VG/cell for AAV2 and SASTG. Data are normalized to infections performed without inhibitor and are shown as means±SD.
Studies that compared the heparin effect in the SUM-52 PE breast cancer cell line, which overexpresses nine splice variants of FGFR2, with SUM-44 PE cells, which do not overexpress FGFR2 (Tannheimer et al., 2000; Moffa et al., 2004; Moffa and Ethier, 2007) (Table 1), confirmed the requirement for FGFR2. Studies in prostate cancer cell lines LNCaP and DU145, which exclusively express the IIIb and IIIc isoforms of the FGFR2 receptor, respectively, suggest that the heparin-mediated enhancement is due to the presence of FGFR2IIIb (Fig. 2E). Non-FGFR2-expressing prostate cancer cells (PC3) further reinforced the trend, exhibiting no heparin-mediated enhancement of transduction (Fig. 2E). To examine the effect of FGFR2 phosphorylation, we used an FGFR family inhibitor, PD173074, which targets the tyrosine kinase domain of FGFR. A reduction of expression was observed with AAV1, AAV2, and SASTG vectors in response to the addition of this inhibitor (Fig. 2F). Together, these data suggest that activation and signaling from the FGFR2 receptor allow for more efficient infectivity of AAV vectors in general.
Growth factors are necessary for the heparin effect
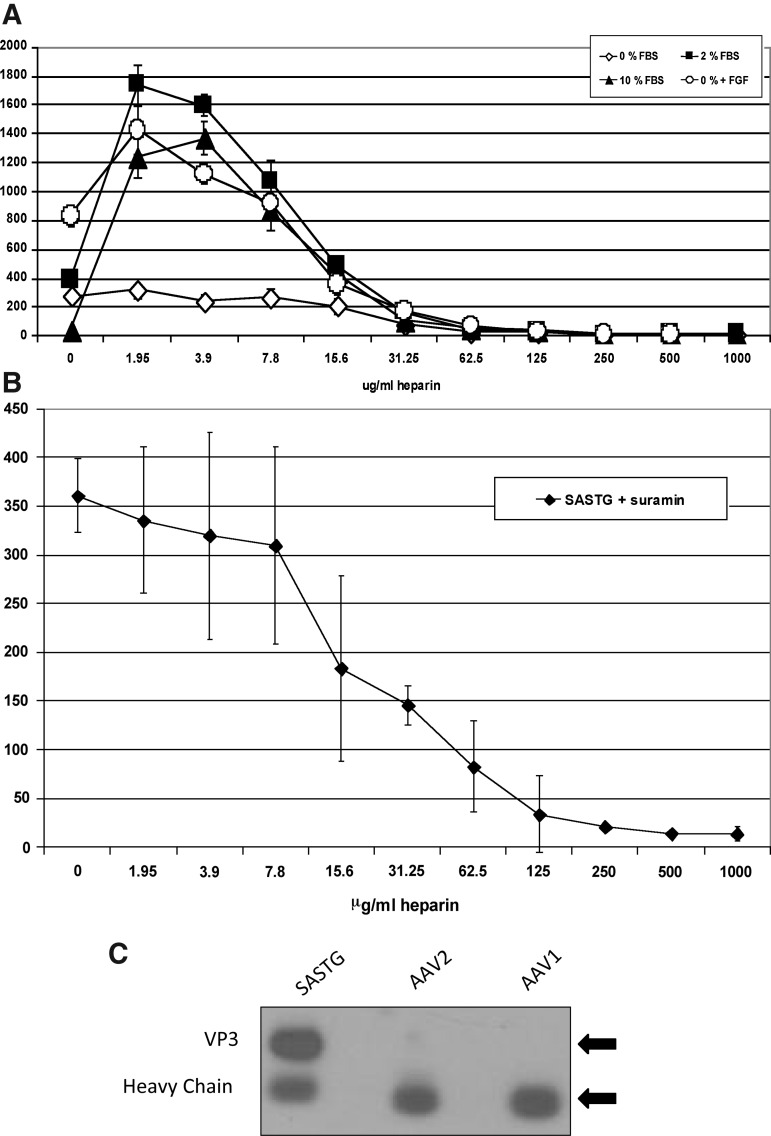
The interactions of heparin with many growth factors (GFs) including the ligands for FGFR2, the fibroblast growth factors (FGFs), have been well characterized (Ornitz et al., 1996; Ornitz, 2000; Ornitz and Itoh, 2001; Zhang et al., 2006). Therefore, we decided to examine the role that GFs may play in the heparin-mediated augmentation of SASTG by first altering the source of these GFs, the fetal bovine serum, and second, by blocking GFs from interacting with their receptors and heparin. 911 cells were grown in various concentrations of sera (0, 2, or 10%). SASTG infections were then performed in medium at each of the serum concentrations (Fig. 3A). Interestingly, in the absence of heparin, SASTG transduction was highest at 0 and 2% serum, whereas transduction in 10% serum was severely limited. In the presence of heparin, an enhancement was observed in both 2 and 10% serum, but significantly, not in 0% serum. However, the enhancement effect in 0% serum was recapitulated by the addition of FGF1. When infection was performed in 2% serum with the addition of suramin (Fig. 3B), a molecule that binds and inhibits the activity of many of the heparin-binding growth factors (Moscatelli and Quarto, 1989; Ganesh et al., 2005), the heparin enhancement was abrogated, reinforcing the need for the presence of growth factors to achieve the heparin augmentation effect.
FIG. 3.
Importance of growth factors in the heparin-mediated augmentation of SASTG. (A) Response of SASTG to heparin under various serum concentrations. 911 cells (10,000 cells per well) were infected with SASTG-luc (1000 VG/cell) in the presence of various serum concentrations (0, 2, 10, or 0% supplemented with FGF [50 ng/ml]) across a range of heparin concentrations. Data represent means±SD. (B) Alteration of the response of SASTG to heparin with the addition of suramin (100 μg/ml). Data represent means±SD. (C) Coimmunoprecipitation of SASTG with FGF1 in the presence of heparin and FGF1. AAV1, AAV2, or SASTG-luciferase vector (3.8×1011 VG) was mixed with 30 ng of FGF1 and 100 μg of heparin and subjected to immunoprecipitation with 2 μg of FGF1 antibody (Santa Cruz Biotechnology). Twenty-five microliters of the immunoprecipitation was electrophoresed on a 10–20% Tris-glycine gel (Invitrogen), transferred to nitrocellulose, and probed with anti-AAV B1 antibody (American Research Products) at a 1:333 dilution.
To examine whether the SASTG vector physically interacted with a growth factor or whether the heparin served to cross-link the vector to the growth factor, in vitro pull-down experiments were performed (Fig. 3C). Control immunoprecipitation using anti-FGF1 antibodies performed on SASTG vector incubated with either FGF1 or heparin, alone, did not result in substantial vector pull-down (data not shown). In contrast, of the three vectors examined (AAV3b [SASTG] and AAV2 and AAV1) (Fig. 3C, lanes 1 to 3, respectively) only the AAV3b-based capsids (lane 1) incubated with FGF1 and heparin were pulled down by anti-FGF1 antibody, demonstrating the specificity of this phenomenon to AAV3b and suggesting that the heparin cross-links the AAV3b vector to the growth factor.
The type and size of the glycosaminoglycan (GAG) are important for the heparin-mediated effect
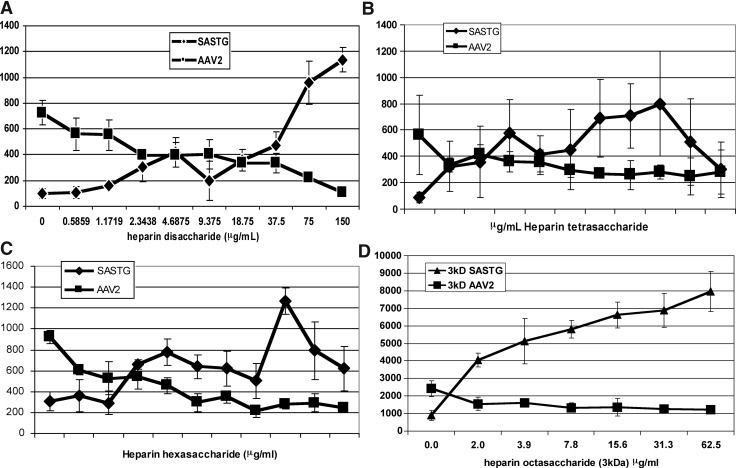
To further characterize the heparin-mediated augmentation of SASTG transduction, the ability of heparins of various sizes and related GAGs to mediate this process was examined (Fig. 4 and Supplementary Fig. S3). In all previously described studies (Figs. 1–3), a heterogeneous heparin solution composed of decasaccharides, undecasaccharides, and dodecasaccharides was used. When smaller, homogeneous heparins such as a disaccharide (Fig. 4A), a tetrasaccharide (Fig. 4B), or a hexasaccharide (Fig. 4C) were used, enhancement of expression was observed at comparably higher doses. When a low molecular mass (3-kDa) heparin with a calculated size of an octasaccharide was used, low doses of this were sufficient for heparin-mediated augmentation of transduction (Fig. 4D). Of the other heparin-related molecules tested, chondroitin sulfate A did not affect SASTG whereas chondroitin sulfate B and heparan sulfate elicited a small to moderate enhancement (Supplementary Fig. S3). This corresponds to studies demonstrating that longer oligosaccharide lengths, or more highly sulfated GAGs, are required to efficiently activate FGFR2 (Mohammadi et al., 2005).
FIG. 4.
Evaluation of heparins in their ability to enhance SASTG expression and inhibit AAV2. 911 cells (10,000 cells per well) were infected with SASTG-luc or AAV2-luc (1000 VG/cell) in the presence of (A) a small heparin disaccharide, (B) a tetrasaccharide, (C) a hexasaccharide, and (D) an octasaccharide over a range of heparin concentrations. Data represent means±SD.
SASTG transduction in primary cells and tissues, and in vivo can be enhanced by coadministration of heparin
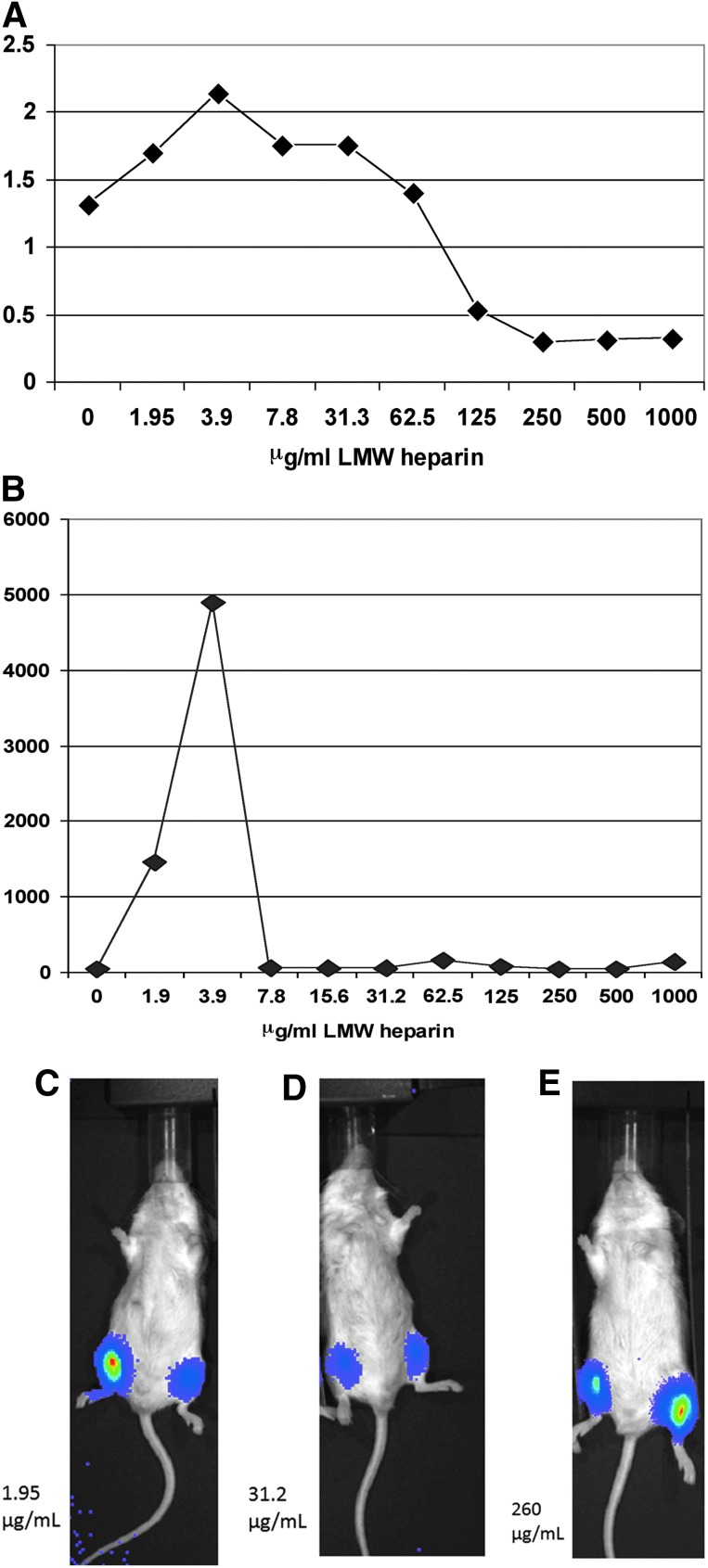
Porcine cardiac myocytes and human saphenous vein grafts were transduced in the setting of increasing heparin concentrations (Fig. 5A and B). In each of these instances, low doses of heparin served to augment SASTG transduction, mimicking what was observed in the studies using immortalized cell lines.
FIG. 5.
SASTG-luciferase expression in response to heparin in primary cell culture, tissue, and in vivo. (A) Primary porcine cardiomyocytes plated in 6-well dishes were infected with SASTG-luc and increasing amounts of heparin. Two days postinfection, cells were harvested, lysed, and subjected to luminometry. Results are expressed as relative light units (RLUs) normalized to milligrams of total protein. (B) Human saphenous vein transduced with SASTG and heparin. (C–E) Evaluation of in vivo skeletal muscle transduction of SASTG plus heparin, using in vivo biophotonic imaging of hind leg injections. Image showing SASTG expression with heparin injected into the (animal's) right leg and SASTG without heparin in the (animal's) left leg. Various doses of heparin were used (low, 1.95 μg/ml; medium, 31.2 μg/ml; and high, 260 μg/ml). Increasing wavelength reflects greater luminescence. Color images available online at www.liebertpub.com/hum
To assess the SASTG response to heparin in vivo, BALB/c mice were injected with SASTG and increasing amounts of heparin in the right leg and with SASTG alone in the left (Fig. 5C–E). Mimicking the cell and tissue culture conditions, the injections performed in the presence of the lowest amount of heparin (Fig. 5C) exhibited an enhancement in transduction compared with the contralateral leg. In contrast, intermediate doses of heparin (31.2 μg/ml; Fig. 5D) coinjected with SASTG had no influence on transduction efficiency. Higher doses of heparin (260 μg/ml; Fig. 5E) minimized SASTG transduction. These initial experiments demonstrate the potential for positive use of heparin in vivo with AAV3b-based vectors.
Discussion
Heparin-mediated enhancement of AAV3b-based vectors
We report that AAV3b vectors have a unique property in that their transduction ability is significantly enhanced in the presence of low concentrations of heparin. Only one other virus has been reported to be positively influenced by the presence of heparin, a neuropathogenic PVC-211 murine leukemia virus (Jinno-Oue et al., 2001). In anticipation of potential human applications, variables that alter the transduction behavior of AAV vectors, even modestly, may become critical. Here, we have carried out studies to understand both how this heparin-mediated augmentation occurs, and whether this effect can be translated from observations in established cell lines to primary cells and tissues and to an in vivo mouse model.
Components of the heparin-mediated enhancement effect
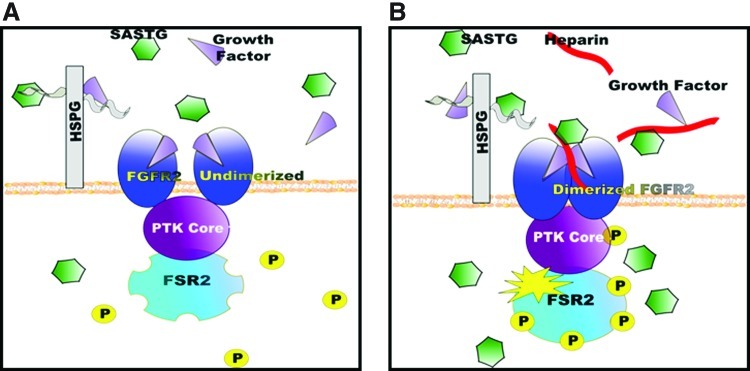
Our model for explaining the AAV3b heparin-mediated enhancement is shown in Fig. 6. This model requires three components: heparin, the presence of FGFR2, and an FGF, either provided during infection or produced by the cell. To the best of our knowledge, this scenario is unique to vectors based on AAV3b. The first component, heparin (or cellular HSPG), interacts with AAV3b. When one considers the role of the GAG in the heparin-mediated augmentation, it is important to note that an octasaccharide heparin is the minimal length for FGF1 and FGFR2-induced dimerization and subsequent activation (Mohammadi et al., 2005). In our studies, a heparin molecule of a similar size exhibited heparin-mediated augmentation at lower heparin doses. Molecules smaller than eight sugars in length exhibited heparin enhancement only at extremely high concentrations. Increasing the size of the heparin to 10–12 saccharides consistently achieved higher transduction than the 8-mer. Given that longer heparin oligosaccharides can result in the formation of multiple FGF–FGFR complexes on the same oligosaccharide, these long GAGs exhibit more biological activity (Faham et al., 1998; Harmer, 2006; Harmer et al., 2006). We anticipate that increasing the size of the heparin will further increase SASTG transduction efficiency.
FIG. 6.
Theoretical model of interactions between FGFR2, FGF, heparin, and vector in the absence of soluble heparin. (A) SASTG inefficiently binds to the undimerized form of FGFR2 and fewer viruses enter the cell. (B) With the addition of soluble heparin, SASTG is able to interact with the dimerized FGFR2 and enter the cell. Color images available online at www.liebertpub.com/hum
The second component of the model is the presence of FGFR2 on cells. Both AAV2 and AAV3b have been reported to interact with GFRs and to possibly use these as a means of cellular entry (Qing et al., 1999; Kashiwakura et al., 2005; Blackburn et al., 2006). It seemed likely that these or similar receptors were involved in the heparin-mediated augmentation, given that GFRs and their ligands are known to interact with heparin and/or HSPG. Using a panel of 18 cell lines with established FGFR expression patterns, we performed experiments to examine the interactions between SASTG and heparin. A strong, positive correlation was clearly seen between the presence of FGFR2 and a heparin-mediated boost of expression. It is important to point out the complexity of the FGFR family of receptors (reviewed in Ornitz, 2000; Eswarakumar et al., 2005; Mohammadi et al., 2005). There are five known members of this family (FGFR1–5) as well as a multitude of isoforms generated by tissue-specific splicing. Interestingly, RNA specific for FGFR5, a newly discovered member of this family (Sleeman et al., 2001), was detected in the majority of the cell lines examined (Table 1), to the extent that we were never able to identify a cell line that expressed only FGFR2 and not FGFR5. Our attempts to knock down FGFR5 in these cells, using shRNA, have been unsuccessful, presumably due to the high level of expression of these transcripts in all cell types tested. However, to date, we have consistent data that the FGFR2 receptor is definitely involved, and we have suggestive data that the involvement is specific for the FGFR2IIIb isoform and not the FGFR2IIIc version (Fig. 2E). It is unclear whether a combination of FGFR2 and FGFR5 might be needed for the maximal effect, or even whether unique heterodimers of these FGFRs could be conferring this effect.
The third component of the heparin-mediated effect is the presence of growth factor. This was evidenced by the observation that the addition of growth factor to the medium augmented SASTG transduction in the absence of heparin (Fig. 3A) and by the establishment that SASTG incubated in the presence of FGF1 and heparin can be pulled down by anti-FGF1 antibodies. This observation was specific for SASTG and was not observed with other vectors tested. It has been reported that FGF1 blocks AAV2 from entry by competing for FGFR1 (Qing et al., 1999). A simple explanation for the observed enhancement is that heparin is binding to the GFs in the medium, and thereby preventing them from interfering with AAV3b viral entry. This explanation may play a role under high-GF conditions (i.e., in the presence of 10% FBS; Fig. 3A) but fails to explain the full phenomenon. Low to moderate levels of GF (which may more closely reflect an in vivo scenario) are essential, not detrimental, to the enhancement. Removing them from the infection either through performing the infections under serum-free conditions, or by using suramin (a drug that blocks the biological activity of heparin-binding growth factors) (Moscatelli and Quarto, 1989; Ganesh et al., 2005), abolishes the affect. Addition of the growth factor back to the cells reconstitutes this effect. Thus we propose that the addition of soluble heparin allows AAV3b-based vectors to interact with the dimerized active FGFR2:FGF complex, thereby enhancing virus transduction through increased binding and entry and FGFR2 signaling.
Understanding AAV–receptor interactions
To add to the base knowledge of AAV receptor interactions, we have determined that components of the FGFR signaling complex are critical in the heparin-mediated augmentation of SASTG transduction. In cells that express FGFR2, AAV3b and SASTG infect with less efficiency when heparin is absent (Fig. 6A), but under optimal heparin conditions that augment FGFR dimerization, SASTG infects more efficiently (Fig. 6B). In addition, relatively little is known regarding subsequent signaling events arising from AAV vector interactions with receptors. Whether the receptors internalize with the vector or whether the binding of the virus influences normal receptor signaling is unclear. Our results using an FGFR inhibitor, as well as cell lines expressing different forms of the FGFR2 receptor, reveal that signaling via FGFR is important for AAV infectivity in general. This is in direct contrast to the study by Qing and colleagues (1999). There they report that FGFR inhibitors have no influence on AAV2 transgene expression. The disparity between our results and theirs may be due to differences in the type of inhibitor used in the two studies, although crystallographic studies show that both SU5402 (one of the inhibitors used in the Qing et al. studies) and PD173074 (used in our studies) bind in the ATP-binding cleft of the FGFR1 tyrosine kinase domain between the two lobes of the kinase (Mohammadi et al., 1997). Alternatively, differences in cell lines or amounts of inhibitor used may explain the differences in results.
Clinical situations in which SASTG plus heparin would be beneficial
There are several clinical situations in which SASTG and heparin might be useful. For example, most surgical procedures require anticoagulation, usually with heparin, and gene therapy performed in the context of such a surgery would therefore potentially expose the viral vector to heparin. In addition, AAV vectors are being developed for treatments of vein grafts in the prevention of vein graft failure. One of the primary causes of vein graft failure is vascular smooth muscle cell (VSMC) proliferation. Studies in cell culture and animal models have shown that heparin is capable of inhibiting vascular smooth muscle proliferation, and human clinical trials have suggested that low molecular weight forms of heparin can inhibit intimal hyperplasia associated with vein graft failure (Kiesz et al., 2001; Wilensky et al., 2000). Perhaps an SASTG vector codelivered with heparin may synergistically confer higher levels of gene expression and prevent initial onset of intimal hyperplasia.
A highly efficient viral vector such as SASTG, which uses FGFR2, shows unique potential for targeted gene delivery to breast and prostate tumors. FGFR2 was deemed highly associated with sporadic postmenopausal human breast cancer (Easton et al., 2007; Hunter et al., 2007). In addition, in human models of prostate cancer, changes in the expression patterns of FGFR2 isoforms seem to play a role in the development of prostate cancer. This isoform switch often correlates with androgen insensitivity and a more aggressive prostate cancer phenotype (Carstens et al., 1997). Potentially, by further understanding SASTG–FGFR2 interactions, the SASTG vector could be exploited in vivo to precisely target cells expressing specific isoforms of FGFR2.
In adults, the FGFRs and FGFs function in tissue repair and wound healing. FGFRs and their FGF ligands are up-regulated during many types of tissue injury. Further study is required to determine whether SASTG may be a useful gene therapy vector in the context of injured or diseased tissue. Understanding the exact relationship between SASTG and FGFR/cofactor/heparin requirements will facilitate this process.
Supplementary Material
Acknowledgments
This work was supported by American Heart Association grant 0630218N to D.E.B. Flow cytometry was performed in the Duke Human Vaccine Institute Flow Cytometry Core Facility, which is supported by National Institutes of Health award AI-51445. The authors are grateful to John F. Whitesides and Zoie Holzknecht for invaluable assistance in flow cytometry and to Aravind Asokan for critical review of the manuscript.
Author Disclosure Statement
No competing financial interests exist.
References
- Akache B. Grimm D. Pandey K., et al. The 37/67-kilodalton laminin receptor is a receptor for adeno-associated virus serotypes 8, 2, 3, and 9. J. Virol. 2006;80:9831–9836. doi: 10.1128/JVI.00878-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asokan A. Hamra J.B. Govindasamy L., et al. Adeno-associated virus type 2 contains an integrin α5β1 binding domain essential for viral cell entry. J. Virol. 2006;80:8961–8969. doi: 10.1128/JVI.00843-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn S.D. Steadman R.A. Johnson F.B. Attachment of adeno-associated virus type 3H to fibroblast growth factor receptor 1. Arch Virol. 2006;151:617–623. doi: 10.1007/s00705-005-0650-6. [DOI] [PubMed] [Google Scholar]
- Bowles D.E. McPhee S.W. Li C., et al. Phase 1 gene therapy for Duchenne muscular dystrophy using a translational optimized AAV vector. Mol. Ther. 2012;20:443–455. doi: 10.1038/mt.2011.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstens R.P. Eaton J.V. Krigman H.R., et al. Alternative splicing of fibroblast growth factor receptor 2 (FGF-R2) in human prostate cancer. Oncogene. 1997;15:3059–3065. doi: 10.1038/sj.onc.1201498. [DOI] [PubMed] [Google Scholar]
- Chang S.H. Liu C.H. Wu M.T. Hla T. Regulation of vascular endothelial cell growth factor expression in mouse mammary tumor cells by the EP2 subtype of the prostaglandin E2 receptor. Prostaglandins Other Lipid Mediat. 2005;76:48–58. doi: 10.1016/j.prostaglandins.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Dechecchi M.C. Tamanini A. Bonizzato A. Cabrini G. Heparan sulfate glycosaminoglycans are involved in adenovirus type 5 and 2–host cell interactions. Virology. 2000;268:382–390. doi: 10.1006/viro.1999.0171. [DOI] [PubMed] [Google Scholar]
- Di Pasquale G. Davidson B.L. Stein C.S., et al. Identification of PDGFR as a receptor for AAV-5 transduction. Nat. Med. 2003;9:1306–1312. doi: 10.1038/nm929. [DOI] [PubMed] [Google Scholar]
- Easton D.F. Pooley K.A. Dunning A.M., et al. Genome-wide association study identifies novel breast cancer susceptibility loci. Nature. 2007;447:1087–1093. doi: 10.1038/nature05887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eswarakumar V.P. Lax I. Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Faham S. Linhardt R.J. Rees D.C. Diversity does make a difference: Fibroblast growth factor–heparin interactions. Curr. Opin. Struct. Biol. 1998;8:578–586. doi: 10.1016/s0959-440x(98)80147-4. [DOI] [PubMed] [Google Scholar]
- Ganesh V.K. Muthuvel S.K. Smith S.A., et al. Structural basis for antagonism by suramin of heparin binding to vaccinia complement protein. Biochemistry. 2005;44:10757–10765. doi: 10.1021/bi050401x. [DOI] [PubMed] [Google Scholar]
- Glushakova L.G. Lisankie M.J. Eruslanov E.B., et al. AAV3-mediated transfer and expression of the pyruvate dehydrogenase E1α subunit gene causes metabolic remodeling and apoptosis of human liver cancer cells. Mol. Genet. Metab. 2009;98:289–299. doi: 10.1016/j.ymgme.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieger J.C. Choi V.W. Samulski R.J. Production and characterization of adeno-associated viral vectors. Nat. Protoc. 2006;1:1412–1428. doi: 10.1038/nprot.2006.207. [DOI] [PubMed] [Google Scholar]
- Handa A. Muramatsu S. Qiu J., et al. Adeno-associated virus (AAV)-3-based vectors transduce haematopoietic cells not susceptible to transduction with AAV-2-based vectors. J. Gen. Virol. 2000;81:2077–2084. doi: 10.1099/0022-1317-81-8-2077. [DOI] [PubMed] [Google Scholar]
- Harmer N.J. Insights into the role of heparan sulphate in fibroblast growth factor signalling. Biochem. Soc. Trans. 2006;34:442–445. doi: 10.1042/BST0340442. [DOI] [PubMed] [Google Scholar]
- Harmer N.J. Robinson C.J. Adam L.E., et al. Multimers of the fibroblast growth factor (FGF)–FGF receptor–saccharide complex are formed on long oligomers of heparin. Biochem. J. 2006;393:741–748. doi: 10.1042/BJ20050985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgard P. Stockert R. Heparan sulfate proteoglycans initiate dengue virus infection of hepatocytes. Hepatology. 2000;32:1069–1077. doi: 10.1053/jhep.2000.18713. [DOI] [PubMed] [Google Scholar]
- Hoggan M.D. Blacklow N.R. Rowe W.P. Studies of small DNA viruses found in various adenovirus preparations: Physical, biological, and immunological characteristics. Proc. Natl. Acad. Sci. U.S.A. 1966;55:1467–1474. doi: 10.1073/pnas.55.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter D.J. Kraft P. Jacobs K.B., et al. A genome-wide association study identifies alleles in FGFR2 associated with risk of sporadic postmenopausal breast cancer. Nat. Genet. 2007;39:870–874. doi: 10.1038/ng2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinno-Oue A. Oue M. Ruscetti S.K. A unique heparin-binding domain in the envelope protein of the neuropathogenic PVC-211 murine leukemia virus may contribute to its brain capillary endothelial cell tropism. J. Virol. 2001;75:12439–12445. doi: 10.1128/JVI.75.24.12439-12445.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaludov N. Brown K.E. Walters R.W., et al. Adeno-associated virus serotype 4 (AAV4) and AAV5 both require sialic acid binding for hemagglutination and efficient transduction but differ in sialic acid linkage specificity. J. Virol. 2001;75:6884–6893. doi: 10.1128/JVI.75.15.6884-6893.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwakura Y. Tamayose K. Iwabuchi K., et al. Hepatocyte growth factor receptor is a coreceptor for adeno-associated virus type 2 infection. J. Virol. 2005;79:609–614. doi: 10.1128/JVI.79.1.609-614.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiesz R. S. Buszman P. Martin J. L., et al. Local delivery of enoxaparin to decrease restenosis after stenting: results of initial multicenter trial: Polish-American Local Lovenox NIR Assessment study (The POLONIA study) Circulation. 2001;103:26–31. doi: 10.1161/01.cir.103.1.26. [DOI] [PubMed] [Google Scholar]
- Lerch T.F. Chapman M.S. Identification of the heparin binding site on adeno-associated virus serotype 3B (AAV-3B) Virology. 2012;423:6–13. doi: 10.1016/j.virol.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling C. Lu Y. Kalsi J.K., et al. Human hepatocyte growth factor receptor is a cellular coreceptor for adeno-associated virus serotype 3. Hum. Gene Ther. 2010;21:1741–1747. doi: 10.1089/hum.2010.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastakov M.Y. Baer K. Symes C.W., et al. Immunological aspects of recombinant adeno-associated virus delivery to the mammalian brain. J. Virol. 2002;76:8446–8454. doi: 10.1128/JVI.76.16.8446-8454.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffa A.B. Ethier S.P. Differential signal transduction of alternatively spliced FGFR2 variants expressed in human mammary epithelial cells. J. Cell. Physiol. 2007;210:720–731. doi: 10.1002/jcp.20880. [DOI] [PubMed] [Google Scholar]
- Moffa A.B. Tannheimer S.L. Ethier S.P. Transforming potential of alternatively spliced variants of fibroblast growth factor receptor 2 in human mammary epithelial cells. Mol. Cancer Res. 2004;2:643–652. [PubMed] [Google Scholar]
- Mohammadi M. McMahon G. Sun L., et al. Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science. 1997;276:955–960. doi: 10.1126/science.276.5314.955. [DOI] [PubMed] [Google Scholar]
- Mohammadi M. Olsen S.K. Ibrahimi O.A. Structural basis for fibroblast growth factor receptor activation. Cytokine Growth Factor Rev. 2005;16:107–137. doi: 10.1016/j.cytogfr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Moscatelli D. Quarto N. Transformation of NIH 3T3 cells with basic fibroblast growth factor or the hst/K-fgf oncogene causes downregulation of the fibroblast growth factor receptor: Reversal of morphological transformation and restoration of receptor number by suramin. J. Cell Biol. 1989;109:2519–2527. doi: 10.1083/jcb.109.5.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu S. Mizukami H. Young N.S. Brown K.E. Nucleotide sequencing and generation of an infectious clone of adeno-associated virus 3. Virology. 1996;221:208–217. doi: 10.1006/viro.1996.0367. [DOI] [PubMed] [Google Scholar]
- Nguyen J.B. Sanchez-Pernaute R. Cunningham J. Bankiewicz K.S. Convection-enhanced delivery of AAV-2 combined with heparin increases TK gene transfer in the rat brain. Neuroreport. 2001;12:1961–1964. doi: 10.1097/00001756-200107030-00037. [DOI] [PubMed] [Google Scholar]
- Opie S.R. Warrington K.H., Jr. Agbandje-McKenna M., et al. Identification of amino acid residues in the capsid proteins of adeno-associated virus type 2 that contribute to heparan sulfate proteoglycan binding. J. Virol. 2003;77:6995–7006. doi: 10.1128/JVI.77.12.6995-7006.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz D.M. FGFs, heparan sulfate and FGFRs: Complex interactions essential for development. Bioessays. 2000;22:108–112. doi: 10.1002/(SICI)1521-1878(200002)22:2<108::AID-BIES2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Ornitz D.M. Itoh N. Fibroblast growth factors. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-3-reviews3005. REVIEWS3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz D.M. Xu J. Colvin J.S., et al. Receptor specificity of the fibroblast growth factor family. J. Biol. Chem. 1996;271:15292–15297. doi: 10.1074/jbc.271.25.15292. [DOI] [PubMed] [Google Scholar]
- Piacentino V. Milano C.A. Bolanos M., et al. XIAP mediated attenuation of apoptosis using a novel cardiac enhanced adeno-associated viral vector. Hum. Gene Ther. 2012;23:635–646. doi: 10.1089/hum.2011.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing K. Mah C. Hansen J., et al. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat. Med. 1999;5:71–77. doi: 10.1038/4758. [DOI] [PubMed] [Google Scholar]
- Rabinowitz J.E. Rolling F. Li C., et al. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J. Virol. 2002;76:791–801. doi: 10.1128/JVI.76.2.791-801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz J.E. Bowles D.E. Faust S.M., et al. Cross-dressing the virion: The transcapsidation of adeno-associated virus serotypes functionally defines subgroups. J. Virol. 2004;78:4421–4432. doi: 10.1128/JVI.78.9.4421-4432.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge E.A. Halbert C.L. Russell D.W. Infectious clones and vectors derived from adeno-associated virus (AAV) serotypes other than AAV type 2. J. Virol. 1998;72:309–319. doi: 10.1128/jvi.72.1.309-319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuettrumpf J. Zou J. Zhang Y., et al. The inhibitory effects of anticoagulation on in vivo gene transfer by adeno-associated viral or adenoviral vectors. Mol. Ther. 2006;13:88–97. doi: 10.1016/j.ymthe.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Sleeman M. Fraser J. McDonald M., et al. Identification of a new fibroblast growth factor receptor, FGFR5. Gene. 2001;271:171–182. doi: 10.1016/s0378-1119(01)00518-2. [DOI] [PubMed] [Google Scholar]
- Summerford C. Samulski R.J. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J. Virol. 1998;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerford C. Bartlett J.S. Samulski R.J. αvβ5 integrin: A co-receptor for adeno-associated virus type 2 infection. Nat. Med. 1999;5:78–82. doi: 10.1038/4768. [DOI] [PubMed] [Google Scholar]
- Tannheimer S.L. Rehemtulla A. Ethier S.P. Characterization of fibroblast growth factor receptor 2 overexpression in the human breast cancer cell line SUM-52PE. Breast Cancer Res. 2000;2:311–320. doi: 10.1186/bcr73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia M. Fragale A. Battaglia P.A. A competitive PCR-based method to measure human fibroblast growth factor receptor 1–4 (FGFR1–4) gene expression. DNA Cell Biol. 2001;20:367–379. doi: 10.1089/10445490152122488. [DOI] [PubMed] [Google Scholar]
- Walters R.W. Yi S.M. Keshavjee S., et al. Binding of adeno-associated virus type 5 to 2,3-linked sialic acid is required for gene transfer. J. Biol. Chem. 2001;276:20610–20616. doi: 10.1074/jbc.M101559200. [DOI] [PubMed] [Google Scholar]
- Wilensky R. L. Tanguay J. F. Ito S., et al. Heparin infusion prior to stenting (HIPS) trial: final results of a prospective, randomized, controlled trial evaluating the effects of local vascular delivery on intimal hyperplasia. Am Heart J. 2000;139:1061–1070. doi: 10.1067/mhj.2000.106614. [DOI] [PubMed] [Google Scholar]
- Wu Z. Miller E. Agbandje-McKenna M. Samulski R.J. α2,3 and α2,6 N-linked sialic acids facilitate efficient binding and transduction by adeno-associated virus types 1 and 6. J. Virol. 2006;80:9093–9103. doi: 10.1128/JVI.00895-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. Ibrahimi O.A. Olsen S.K., et al. Receptor specificity of the fibroblast growth factor family: The complete mammalian FGF family. J. Biol. Chem. 2006;281:15694–15700. doi: 10.1074/jbc.M601252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.