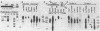Abstract
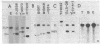
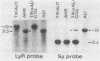
A DNA region not associated with conventional immunoglobulin gene rearrangement is rearranged in many lymphoid tumors. This region, designated here as lymphoid rearranging (LyR) DNA, was cloned from plasmacytoma J558 in which it had recombined 5' to a constant (C) region of the alpha heavy (H) chain gene, C alpha, within a switch (S) region, S alpha, involved in the switching of CH genes. Sequence determination established that LyR DNA had recombined within a S alpha recombination unit. LyR DNA does not originate from the H chain locus, and discordance between LyR DNA and CH copy number in certain lines suggests that LyR DNA probably derives from another chromosome. LyR DNA rearrangement is a characteristic of tumors of mature B cells; it was detected in 24 of 28 plasmacytomas and B-cell lymphomas, usually as LyR-S alpha, but not in 11 Abelson retrovirus-induced lymphomas of B-cell precursors nor detectably in normal B cells. In contrast, rearrangement was observed in only 3 of 18 T-cell lymphomas, and none of seven nonlymphoid lines. Most tumor lines (49 of 52), whether lymphoid or not, contained a low level of polyadenylylated LyR transcript(s), but several new RNA species with differences in their 5' regions appeared in B-cell lines in which LyR DNA was rearranged, suggesting that rearrangement may activate a new promoter or mode of splicing. The results suggest that the LyR-S alpha rearrangement represents a translocation to chromosome 12 that alters expression of LyR-encoded genes; hence, it may have participated in lymphoid tumor oncogenesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Webb E., Gerondakis S., Cory S. Cloned embryonic DNA sequences flanking the mouse immunoglobulin C gamma 3 and C gamma 1 genes. Nucleic Acids Res. 1980 Dec 20;8(24):6019–6032. doi: 10.1093/nar/8.24.6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt F., Rosenberg N., Lewis S., Thomas E., Baltimore D. Organization and reorganization of immunoglobulin genes in A-MULV-transformed cells: rearrangement of heavy but not light chain genes. Cell. 1981 Dec;27(2 Pt 1):381–390. doi: 10.1016/0092-8674(81)90421-9. [DOI] [PubMed] [Google Scholar]
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Coleclough C., Cooper D., Perry R. P. Rearrangement of immunoglobulin heavy chain genes during B-lymphocyte development as revealed by studies of mouse plasmacytoma cells. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1422–1426. doi: 10.1073/pnas.77.3.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook W. D. Rapid thymomas induced by Abelson murine leukemia virus. Proc Natl Acad Sci U S A. 1982 May;79(9):2917–2921. doi: 10.1073/pnas.79.9.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory S., Adams J. M. Deletions are associated with somatic rearrangement of immunoglobulin heavy chain genes. Cell. 1980 Jan;19(1):37–51. doi: 10.1016/0092-8674(80)90386-4. [DOI] [PubMed] [Google Scholar]
- Cory S., Adams J. M., Kemp D. J. Somatic rearrangements forming active immunoglobulin mu genes in B and T lymphoid cell lines. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4943–4947. doi: 10.1073/pnas.77.8.4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. M., Kim S. K., Hood L. E. DNA sequences mediating class switching in alpha-immunoglobulins. Science. 1980 Sep 19;209(4463):1360–1365. doi: 10.1126/science.6774415. [DOI] [PubMed] [Google Scholar]
- Gutman G. A., Warner N. L., Harris A. W. Immunoglobulin production by murine B-lymphoma cells. Clin Immunol Immunopathol. 1981 Feb;18(2):230–244. doi: 10.1016/0090-1229(81)90029-5. [DOI] [PubMed] [Google Scholar]
- Harris L. J., Lang R. B., Marcu K. B. Non-immunoglobulin-associated DNA rearrangements in mouse plasmacytomas. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4175–4179. doi: 10.1073/pnas.79.13.4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B. In vitro packaging of lambda and cosmid DNA. Methods Enzymol. 1979;68:299–309. doi: 10.1016/0076-6879(79)68021-7. [DOI] [PubMed] [Google Scholar]
- Kemp D. J., Harris A. W., Cory S., Adams J. M. Expression of the immunoglobulin C mu gene in mouse T and B lymphoid and myeloid cell lines. Proc Natl Acad Sci U S A. 1980 May;77(5):2876–2880. doi: 10.1073/pnas.77.5.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch I. R., Ravetch J. V., Kwan S. P., Max E. E., Ney R. L., Leder P. Multiple immunoglobulin switch region homologies outside the heavy chain constant region locus. Nature. 1981 Oct 15;293(5833):585–587. doi: 10.1038/293585a0. [DOI] [PubMed] [Google Scholar]
- Kurosawa Y., Tonegawa S. Organization, structure, and assembly of immunoglobulin heavy chain diversity DNA segments. J Exp Med. 1982 Jan 1;155(1):201–218. doi: 10.1084/jem.155.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcu K. B. Immunoglobulin heavy-chain constant-region genes. Cell. 1982 Jul;29(3):719–721. doi: 10.1016/0092-8674(82)90431-7. [DOI] [PubMed] [Google Scholar]
- Marshak-Rothstein A., Fink P., Gridley T., Raulet D. H., Bevan M. J., Gefter M. L. Properties and applications of monoclonal antibodies directed against determinants of the Thy-1 locus. J Immunol. 1979 Jun;122(6):2491–2497. [PubMed] [Google Scholar]
- Messing J., Gronenborn B., Müller-Hill B., Hans Hopschneider P. Filamentous coliphage M13 as a cloning vehicle: insertion of a HindII fragment of the lac regulatory region in M13 replicative form in vitro. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3642–3646. doi: 10.1073/pnas.74.9.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido T., Nakai S., Honjo T. Switch region of immunoglobulin Cmu gene is composed of simple tandem repetitive sequences. Nature. 1981 Aug 27;292(5826):845–848. doi: 10.1038/292845a0. [DOI] [PubMed] [Google Scholar]
- Ohno S., Babonits M., Wiener F., Spira J., Klein G., Potter M. Nonrandom chromosome changes involving the Ig gene-carrying chromosomes 12 and 6 in pristane-induced mouse plasmacytomas. Cell. 1979 Dec;18(4):1001–1007. doi: 10.1016/0092-8674(79)90212-5. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Shimizu A., Takahashi N., Yaoita Y., Honjo T. Organization of the constant-region gene family of the mouse immunoglobulin heavy chain. Cell. 1982 Mar;28(3):499–506. doi: 10.1016/0092-8674(82)90204-5. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spira J., Wiener F., Babonits M., Gamble J., Miller J., Klein G. The role of chromosome 15 in murine leukemogenesis. I. Contrasting behavior of the tumor vs. normal parent-derived chromosomes No. 15 in somatic hybrids of varying tumorigenicity. Int J Cancer. 1981 Dec;28(6):785–798. doi: 10.1002/ijc.2910280618. [DOI] [PubMed] [Google Scholar]