Abstract
The dentate gyrus of the hippocampus continues generating new neurons throughout life. These neurons originate from radial astrocytes within the subgranular zone (SGZ). Here, we find that Sox1, a member of the SoxB1 family of transcription factors, is expressed in a subset of radial astrocytes. Lineage tracing using Sox1-tTA;tetO-Cre;Rosa26 reporter mice shows that the Sox1-expressing cells represent an activated neural stem/progenitor population that gives rise to most if not all newly born granular neurons, as well as a small number of mature hilar astrocytes. Furthermore, a subpopulation of Sox1-marked cells have long-term neurogenic potential, producing new neurons 3 months after inactivation of tetracycline transactivator. Remarkably, after 8 weeks of labeling and a 12-week chase, as much as 44% of all granular neurons in the dentate gyrus were derived from Sox1 lineage-traced adult neural stem/progenitor cells. The fraction of Sox1-positive cells within the radial astrocyte population decreases with age, correlating with a decrease in neurogenesis. However, expression profiling shows that these cells are transcriptionally stable throughout the lifespan of the mouse. These results demonstrate that Sox1 is expressed in an activated stem/progenitor population whose numbers decrease with age while maintaining a stable molecular program.
Keywords: Sox1, Neural stem cells, Hippocampus, Subgranular zone
INTRODUCTION
Neurogenesis continues in the adult brains of mammals in two regions: the subgranular zone (SGZ) of the dentate gyrus in the hippocampus and along the lateral walls of the lateral ventricles in the ventricular-subventricular zone (V-SVZ) (Altman and Das, 1965a; Altman and Das, 1965b; Kaplan and Bell, 1984; Cameron et al., 1993; Lois and Alvarez-Buylla, 1994; Kempermann et al., 1997; Eriksson et al., 1998; Gage et al., 1998; Doetsch et al., 1999; Ming and Song, 2005). Putative neural stem cells (NSCs) in the SGZ give rise to mature neurons of the granular layer, which have been suggested to be involved in learning and memory (Shors et al., 2001; Schmidt-Hieber et al., 2004; Tashiro et al., 2006). NSCs in the V-SVZ contribute to migrating neuroblasts along the rostral migratory stream that go on to become interneurons within the olfactory bulb (Luskin, 1993; Lois and Alvarez-Buylla, 1994; Petreanu and Alvarez-Buylla, 2002; Carleton et al., 2003). Although much evidence exists to support the presence of a population of cells with stem/progenitor properties in the adult brain, there is not a consistent understanding regarding the full potential and self-renewal properties of this cell population. Furthermore, additional markers are needed to identify stem/progenitor cells actively involved in neurogenesis. The Sox transcription factors are central regulators of neurodevelopment (Pevny and Placzek, 2005; Wegner and Stolt, 2005; Favaro et al., 2009; Scott et al., 2010). They share homology with the testis determining factor SRY through their HMG-box DNA-binding domain (Sinclair et al., 1990; Gubbay et al., 1990; Denny et al., 1992; Coriat et al., 1993; Wright et al., 1993). Members of the SoxB1 subfamily, comprising of Sox1, Sox2 and Sox3, show distinct and overlapping expression patterns during embryogenesis (Uwanogho et al., 1995; Collignon et al., 1996; Pevny and Lovell-Badge, 1997; Rex et al., 1997; Streit et al., 1997; Pevny et al., 1998; Wood and Episkopou, 1999). Sox1 is the earliest known specific marker of the neuroectoderm lineage, being activated during gastrulation. Sox2 and Sox3 show broader expression patterns turning on at the pre-implantation and epiblast stages, respectively (Collignon et al., 1996; Pevny et al., 1998; Wood and Episkopou, 1999). The only non-neural role described for SOX1 is in the developing eye lens, where SOX1 has been shown to regulate the γ-crystallin genes (Kamachi et al., 1998; Nishiguchi et al., 1998). Contradictory reports claim a role for SOX1 in neural lineage commitment and differentiation (Pevny et al., 1998; Kan et al., 2004; Ekonomou et al., 2005) versus maintenance of the progenitor pool (Bylund et al., 2003; Zhao et al., 2004; Suter et al., 2009; Elkouris et al., 2011). A model reconciling both scenarios has been proposed whereby SOX1 plays an initial role in maintenance of division in the progenitor pool, but, upon continued expression, leads to neuronal differentiation (Kan et al., 2007). Loss of SOX1 is largely compensated by SOX2 and SOX3 during development, but leads to epilepsy and eventual lethality (Wegner, 1999; Malas et al., 2003; Graham et al., 2003; Kan et al., 2007).
In the adult, there have been reports that SOX1 is localized to the germinal regions as well as a putative stem cell pool in the postnatal cerebellum (Aubert et al., 2003; Pevny and Rao, 2003; Barraud et al., 2005; Sottile et al., 2006; Kan et al., 2007). These studies, however, did not fully characterize the cell type(s) positive for SOX1 within these adult germinal regions. By contrast, SOX2 expression has been well characterized in adult neurogenic zones (Ellis et al., 2004; Ferri et al., 2004; Komitova and Eriksson, 2004; Brazel et al., 2005; Episkopou, 2005; Sottile et al., 2006; Suh et al., 2007). SOX2 marks both radial astrocytes (also known as type 1 cells) and early progenitor cells (also known as type 2a cells) within the adult dentate gyrus (Steiner et al., 2006). Radial astrocytes are positive for the astrocyte marker GFAP and are morphologically identified by having a cell body residing in the SGZ with a radial process extending into the granular layer. They are well established as the relatively quiescent NSCs of the SGZ. Early progenitors represent a nonradial, intermediate proliferating cell population within the subgranular zone (Yamaguchi et al., 2000; Seri et al., 2001; Kempermann et al., 2003; Fukuda et al., 2003; Seri et al., 2004; Encinas et al., 2011; Bonaguidi et al., 2011). In this study, we investigate the nature of Sox1-positive cells in SGZ of the adult hippocampus using real-time reporters and lineage tracing. We find that Sox1 marks a subset of the radial astrocytes and early progenitors that have been activated to produce new neurons and astrocytes.
MATERIALS AND METHODS
Gene targeting
The targeting vector to generate Sox1-tTA ES cells was prepared by replacing the eGFP gene used to generate Sox1-GFP mice with the gene expressing the tetracycline transactivator (tTA) as well as replacing the loxP flanked hygromycinR-thymidine kinase dual selection cassette with a neomycin resistance cassette also loxP flanked (Sox1-GFP construct kindly provided by Austin Smith, University of Cambridge, Cambridge, UK). Sox1 knockout ES cells were generated by electroporation of SfiI linearized vector into V6.5 ES cells. Targeted clones were selected in neomycin (0.25 mg/ml) and verified by Southern analysis with flanking 5′ and 3′ probes. The expected band size with the 5′ probe is 10 kb for the wild-type locus and 6.5 kb for the targeted locus using XbaI for genomic DNA digestion. The expected band size with the 3′ probe is 12 kb for the wild-type locus and 6.5 kb for the targeted vector using EcoRI for genomic DNA digestion. The selection cassette was removed by transiently transfecting a Cre recombinase expression vector. Clones that had undergone excision were verified by Southern as well as negative selection on G418. The expected band size with the 3′ probe is 8.8 kb for targeted locus with the selection cassette and 7.1 kb for targeted locus with the selection cassette removed by Cre using NotI for genomic DNA digestion. One clone was injected into blastocysts and germline chimeras were identified. Sox1-tTA mice were outcrossed into a C57BL/6 background and maintained in a heterozygous state.
Animals and tissue processing
All animals were maintained according to institutional regulations. LC-1, R26R, R26eYFP and Z/EG mice were previously published (Soriano, 1999; Novak et al., 2000; Srinivas et al., 2001; Schönig et al., 2002). All crosses involving Sox1-tTA and LC-1 mice were fed doxycycline (20 mg/l) in their drinking water and experimental mice were maintained on doxycycline until 6 weeks of age. Long-term BrdU labeling was achieved by introducing BrdU in the drinking water (0.8 mg/ml) for 1 week prior to sacrificing the mice. BrdU pulse labeling was accomplished with a single IP injection of BrdU (0.05 mg/g) 1 hour before sacrificing the animal. Genotypes were confirmed by PCR using genomic DNA prepared from tail clippings. For tissue collection, mice were deeply anesthetized and perfused first with phosphate-buffered saline (PBS) followed by 4% paraformaldehyde (PFA). Brains were postfixed overnight in 4% PFA at 4°C, washed in PBS, cryoprotected in 30% sucrose, then embedded in optimal cutting temperature compound (OCT) and stored at −80°C. Coronal sections were cut using a Leica cryostat at 12 μm directly onto slides and stored at −80°C or as 50 μm floating sections into PBS and stored at 4°C.
Immunocytochemistry and imaging
Sections were postfixed for 15 minutes at room temperature in 4% PFA followed by PBS washes then incubated in blocking solution (5% goat serum, 0.1% Triton X-100 in PBS). Primary antibodies were diluted in blocking solution and used as follows overnight at 4°C: GFP (chicken, 1:500, Aves Labs, Tigard, OR, USA), SOX1 (kind gift from Dr Yusuke Kamachi and Dr Hisato Kondoh, Osaka University, Japan), SOX2 (rabbit, 1:500, Abcam, Cambridge, MA, USA; mouse, 1:100, R&D Systems, Minneapolis, MN, USA), GFAP (rabbit, 1:300, Dako, Carpinteria, CA, USA; mouse, 1:200, Millipore, Billerica, MA, USA), DCX (rabbit, 1:500, Cell Signaling, Danvers, MA, USA), PSA-NCAM (mouse, 1:400, Chemicon), TUJ1 (mouse, Covance, 1:500), NeuN (mouse, 1:500, Millipore) and BrdU (rat, Abcam, 1:200). All secondary antibodies were Alexa Fluor (1:500, Invitrogen, Grand Island, NY, USA) labeled with 488, 546 or 633, mixed in blocking solution and incubated for 2 hours at room temperature. DNA was stained using 4′,6-diamidino-2-phenylindole (DAPI, 10 μg/ml, Sigma, St Louis, MO, USA) or TO-PRO-3 iodide (Invitrogen) diluted in PBS for 5 minutes at room temperature. For detection of BrdU, sequential IHC was performed. Following initial primary antibody staining, antibody antigen complexes were stabilized by fixation using 4% PFA for 15 minutes at room temperature. The BrdU antigen was retrieved by treatment of the tissue with 2 N HCl for 30 minutes at 37°C followed by PBS washes. Primary antibody staining was then performed for BrdU followed by secondary detection for all antibodies used. All images from stained brain sections were captured using a Zeiss or Leica confocal microscope and stacked images were produced using the corresponding system software then resized and false colored in Photoshop (Adobe, San Jose, CA, USA).
X-gal staining
Sections were postfixed with 4% PFA for 15 minutes at room temperature. They were then washed twice in 100 mM phosphate buffer (pH 7.4), 2 mM MgCl2 and 5 mM EGTA, followed by a wash in 100 mM phosphate buffer (pH 7.4), 2 mM MgCl2, 0.01% sodium desoxycholate and 0.02% NP40. Sections were then stained in X-gal staining solution [5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6, 1 mg/ml X-gal] at 37°C overnight. Sections were then mounted and imaged using an Olympus upright microscope.
Cell counting/quantification
At least three coronal sections representing more ventral, medial or dorsal areas of the dentate gyrus were stained with the appropriate antibodies and confocal z-stacks were taken of the dentate gyrus from each hemisphere using the 63× objective. Cells positive for each antibody were counted first in individual channels followed by counting colocalization in the merged image. Colocalization was then verified in the serial scans comprising the 3D projection of the z-stack. P-values were calculated using a two-tailed unpaired t-test.
Dissociation, FACS, RNA preparation and profiling
Hippocampi were isolated from Sox1-GFP mice at 5-7 months (n=4) or 1 year 9 months (n=4) using a Leica dissecting scope. Cells were immediately dissociated using papain (Worthington Biochemical Corporation. Lakewood, NJ, USA) and sorted for the GFP high population using a FACSAria Flow Cytometer (BD Biosciences, San Diego, CA, USA). RNA was harvested using the Arcturus PicoPure RNA isolation kit (Life Technologies, Carlsbad, CA, USA) and the quality and quantity of each sample was checked using Agilent BioAnalyzer (Santa Clara, CA, USA) and Nanodrop spectrophotometer (Wilmington, DE, USA). Samples were amplified using the NuGEN FFPE kit (San Carlos, CA, USA) and cDNA was prepared using an oligo(dT)-T7 RNA polymerase primer before biotinylation by in vitro transcription, partial hydrolysis and final hybridization to the Affymetrix (Santa Clara, CA, USA) GeneChip Mouse Gene 1.0 ST Array (Gladstone Genomics Core). Arrays were normalized using Robust Multichip Average (RMA) and the latest probe map to MM9 RefSeq genes (Irizarry et al., 2003; Dai et al., 2005). To detect potential outlier arrays, the distributions of fold changes between all possible pairs within each group were analyzed. One sample from a young mouse was found to have a skewed distribution when compared with other biological replicate samples and was removed from further analysis. The statistical package R was used to perform average linkage hierarchical clustering of the remaining samples. Significance analysis of microarrays (SAM) was performed to determine significant differentially expressed genes between the old and young mice with a false discovery rate cutoff of 5% (Tusher et al., 2001).
RESULTS
SOX1 is expressed in a subset of radial astrocytes and early progenitor cells in the SGZ of the hippocampus
SOX1 is found in the neurogenic zones of the adult mouse brain (Aubert et al., 2003; Barraud et al., 2005; Kan et al., 2007). To determine the specific cell types expressing SOX1 within these zones, we performed co-staining with markers for radial astrocytes (GFAP), early progenitor cells (SOX2 positive non-radial cells), late progenitor/neuroblasts (PSA-NCAM, DCX) and neurons (TUJ1, NeuN). For SOX1, we used both an antibody to endogenous SOX1 and a Sox1-GFP reporter mouse (Aubert et al., 2003). SOX1 and Sox1-GFP staining overlapped both in the dentate gyrus and V-SVZ (Fig. 1A,B; supplementary material Fig. S1A,B). Specificity of the SOX1 antibody was confirmed by the lack of staining in Sox1-null mice (Sox1-tTA homozygous) (supplementary material Fig. S2A,B). As Sox1-GFP served as a more robust cellular marker, we used this reporter in most experiments. In the V-SVZ, Sox1-GFP only colocalized with a marker for late progenitors (PSA-NCAM), although we cannot rule out rare GFAP-positive cells (supplementary material Fig. S1C). By contrast, in the dentate gyrus, Sox1-GFP did not co-stain with markers of late progenitors/neuroblasts (PSA-NCAM or DCX) or neurons (Tuj1 or NeuN), with the exception of rare dim GFP-positive late progenitors (data not shown), which probably reflects GFP perdurance, as similar cells were not seen with the SOX1 antibody. Co-staining with GFAP and SOX2 identified two independent SOX1 populations in the dentate gyrus: (1) GFAP-positive, Sox2-positive radial astrocytes (Fig. 1C,D; supplementary material Fig. S2C); and (2) SOX2 positive non-radial cells (Fig. 1C). For simplicity, we call the former GFAP-positive radial astrocytes and the latter SOX2-positive non-radial cells throughout the remainder of the text. From an average of 200 GFP-positive cells counted per Sox1-GFP mouse, 35.30% (±3.62%) were GFAP-positive radial astrocytes and 30.20% (±1.99%) were SOX2-positive non-radial cells (Fig. 1E). From an average of 300 SOX1 antibody-positive cells per Sox1-tTA heterozygous mouse, 23.20% (±0.56%) were GFAP-positive radial astrocytes and 37.00% (±1.61%) were SOX2-positive non-radial cells (Fig. 1E). Notably, unlike SOX1, SOX2 antibody also stained astrocytes outside the SGZ zone (Fig. 1C). The remaining Sox1-GFP and SOX1-positive cells did not colocalize with any of the markers used in these studies and may represent SOX1 positivity during a transition between cell types or a distinct, unidentified, population of cells within the SGZ. Evaluation of the number of SOX1-positive cells within the radial astrocytes and non-radial cells revealed that SOX1 was expressed in only a subset of each of the two cell populations. Sox1-GFP and SOX1 antibody marked 39.29% (±7.06%) and 34.62% (±4.28%), respectively, of the GFAP positive radial astrocytes (Fig. 1F). Sox1-GFP and SOX1 marked 42.36% (±3.72%) and 38.85% (±2.10%), respectively, of the SOX2-positive non-radial cells (Fig. 1G). BrdU incorporation studies supported the more proliferative nature of the Sox1-GFP, SOX2-positive non-radial cells (supplementary material Fig. S2D). Therefore, SOX1 specifically marks two populations of cells that have anatomical, immunohistochemical and morphological patterns consistent with previously described putative stem cells and early progenitors.
Fig. 1.
Sox1 is expressed in a subset of putative NSCs of the SGZ. Sox1-GFP mice were sacrificed at 4 months of age and brains were harvested and sectioned into 50 μm slices for antibody staining and imaging by confocal microscopy. (A) GFP (green), Sox1 (red) and DNA (ToPro3, blue) staining of the dentate gyrus. (B) GFP (green), Sox1 (red) and DNA (ToPro3, blue) staining of a representative radial astrocyte in the SGZ. (C) GFP (green), Sox2 (red) and GFAP (blue) staining of the dentate gyrus. Arrowheads mark cells where all three antibodies colocalize. Arrows mark cells where Sox2 and GFP colocalize. (D) GFP (green), GFAP (red) and DNA (ToPro3, blue) staining of the dentate gyrus. Arrowheads mark radial astrocytes positive for GFP and GFAP. Arrow marks radial process that are only GFAP positive. (E) Quantification of Sox1-GFP cells or anti-SOX1 cells that are GFAP positive radial (blue bar), Sox2-positive non-radial (red bar) or uncharacterized (gray bar) in the dentate gyrus (n=3). (F) Quantification of the percentage of all GFAP-positive radial astrocytes that are also GFP (n=3, each circle represents an individual Sox1-GFP mouse) or Sox1 (n=5, each triangle represents an individual Sox1-tTA mouse) positive in the dentate gyrus. (G) Quantification of the percentage of Sox2-positive non-radial cells that are also GFP positive (n=3, each circle represents an individual Sox1-GFP mouse) or anti-SOX1 positive (n=3, each triangle represents an individual Sox1-tTA mouse) in the dentate gyrus. Scale bars: 10 μm. NS, no statistical significance. Averages of 200 cells per Sox1-GFP mouse and 300 cells per Sox1-tTA mouse were counted. Black bars indicate average; error bars indicate s.d.
SOX1-positive cells of the SGZ produce granular neurons and hilar astrocytes
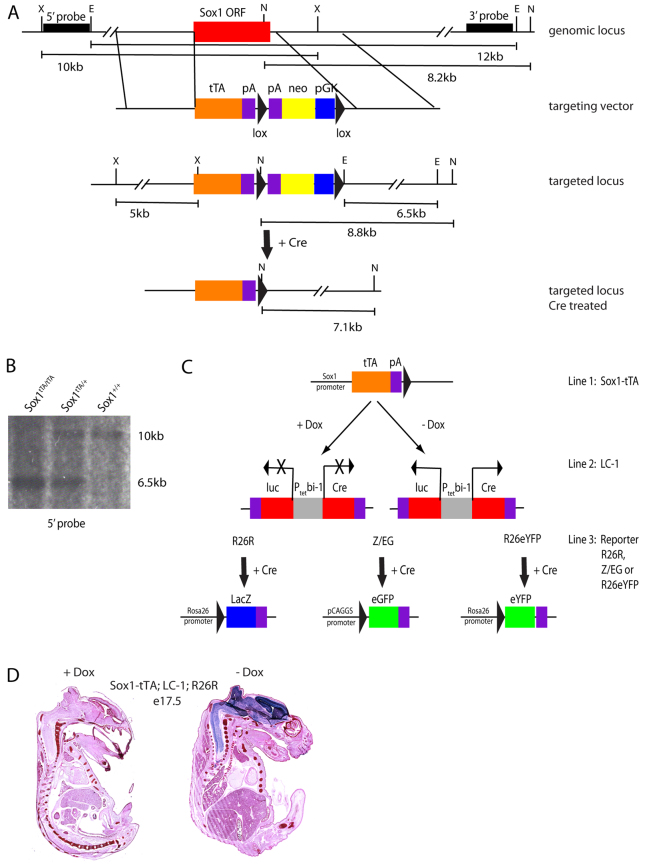
To determine the fate of SOX1-positive cells in the adult SGZ, we performed lineage-tracing experiments. Specifically, we developed an inducible system based on the tetracycline transactivator (tTA) (Fig. 2A). The inducible system was crucial as Sox1 is expressed throughout the neuroectoderm during embryogenesis; therefore, in order to trace Sox1-derived cells in the adult mouse, it was essential to keep the system off during this earlier developmental time period. Using the Sox1-GFP targeting construct, a tTA cDNA was exchanged for GFP (Fig. 2A) so that tTA expression would recapitulate Sox1-GFP expression. Targeted embryonic stem cell clones were confirmed by Southern blotting (Fig. 2B) and used to produce Sox1-tTA transgenic mice. To initially test effectiveness of the Sox1-tTA system, the mice were crossed to mice carrying a tetO promoter driving Cre (LC-1) and a Rosa26;lox-stop-lox;lacZ reporter (R26R) (Soriano, 1999; Schönig et al., 2002). In the triple transgenic mice (Sox1-tTA; LC-1; R26R), the tTA drives expression of Cre, resulting in removal of the lox-stop-lox cassette hence permanently activating the lacZ gene (Fig. 2C). Therefore, all Sox1-expressing cells along with downstream progeny should be marked. Indeed, in the absence of doxycycline, the entire developing nervous system of E17.5 embryos stained positively for X-gal as expected (Fig. 2D, right). The system appeared to be specific to the neural tissue, although expression in small subsets of cells in other tissues could not be ruled out (Fig. 2D, right and data not shown). The introduction of doxycycline into the drinking water of mothers effectively silenced the tTA system, as shown by the lack of reporter expression in similarly aged embryos (Fig. 2D, left).
Fig. 2.
Production of a Sox1 driven inducible mouse model. (A) The genomic locus, targeting vector, targeted locus and targeted locus after Cre treatment to remove the selection cassette are shown. The Sox1 open reading frame (ORF) is shown as a red box. The 5′ and 3′ probes for Southern blotting are shown as black bars. Restriction sites are represented by X (XbaI), E (EcoRI) and N (NotI). Expected sizes of fragments resulting from the digest of the genomic locus are represented below (X to X=10 kb, E to E=12 kb, N to N= 8.2 kb). The arms for homologous recombination are represented by the regions between the vertical lines. The knock-in construct consists of: the tetracycline trasactivator gene (tTA, orange box), polyA sequence (pA, purple boxes), lox sequence (black triangles) and pGK-neo gene (blue and yellow boxes). The targeted locus is shown with the resulting restriction fragment size changes represented below (X to X=5 kb, E to E=6.5 kb, N to N=8.8 kb). The targeted locus following Cre treatment is shown with the resulting restriction fragment size change for NotI shown below (N to N=7.1 kb). (B) Representative Southern blot of genomic DNA from a Sox1 wild-type, a Sox1-tTA heterozygous and a Sox1-tTA homozygous mouse digested with XbaI and probed with the 5′ probe. (C) Diagram of the alleles used for lineage tracing of Sox1 cells. Sox1-tTA; LC-1; reporter mice are either kept in the presence of doxycycline to silence the system or removed from doxycycline to allow for system activation. In the absence of doxycycline, tTA can act on the bi-directional tet-responsive promoter at the LC-1 transgenic locus (Ptetbi-1). This allows for expression of Cre as well as luciferase (luc) (red boxes). Cre can then act on loxP sites within the reporter alleles. The R26R reporter will then express β-gal (blue box), the Z/EG reporter will express eGFP (green box) and the R26eYFP reporter will express eYFP (green box). The promoter for each of the reporters is shown. (D) E17.5 mice were harvested from mothers on dox (left embryo) or not on dox (right embryo) and stained with Eosin (pink) and X-gal (blue).
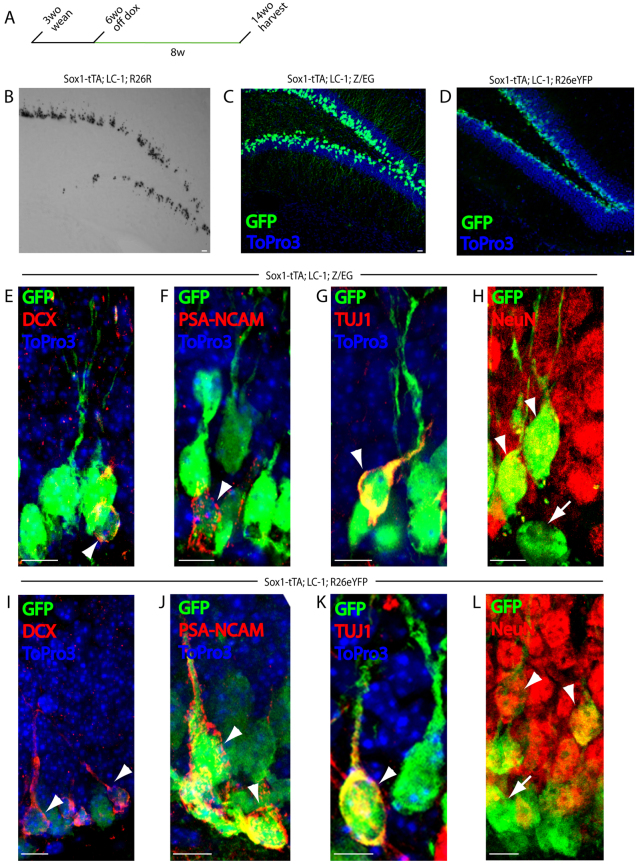
To trace the lineage of Sox1-expressing cells in the hippocampus of adult mice, triple transgenic mice were kept on doxycycline throughout embryonic development and postnatally until 6 weeks of age at which time doxycycline was removed to activate tTA and remove the lox-stop-lox cassette of the reporters. Mice were kept off doxycycline for 8 weeks before being sacrificed for analysis (Fig. 3A). X-gal staining of sections from the hippocampus of mice kept off doxycycline for 8 weeks showed a distinct pattern of clusters of cells extending from the SGZ into the granular layer of the dentate gyrus consistent with the Sox1-expressing cells giving rise to neuroblasts and granular neurons (Fig. 3B). Two additional reporter systems were crossed to the Sox1-tTA; LC-1 mice: a CAGGS;lox-βgal-stop-lox;GFP (Z/EG) and a Rosa26;lox-stop-lox;eYFP (R26R-eYFP) reporter (Fig. 2C) (Novak et al., 2000; Srinivas et al., 2001). Both systems produced a similar pattern of labeled cells into the granular layer as seen with the R26R system (Fig. 3C,D). Importantly, no reporter activity was seen in control mice kept on doxycycline for the 8-week labeling period (supplementary material Fig. S3). As the GFP and YFP reporters provided better resolution at the cellular level, they were used in co-staining experiments.
Fig. 3.
Sox1-expressing cells give rise to new neurons. (A) Schematic of the timeline for lineage-tracing experiments. Sox1-tTA; LC-1; reporter mice were taken off doxycycline at 6 weeks of age (system on) for 8 weeks then sacrificed at 14 weeks of age, except for R26R mice which were kept off doxycyline for 26 weeks. (B) X-gal staining of a 50 μm section of the dentate gyrus from an 8-month-old Sox1-tTA; LC-1; R26R mouse. (C) GFP (green) and DNA (ToPro3, blue) staining of a 50 μm section of the dentate gyrus from a 3.5-month-old Sox1-tTA; LC-1; Z/EG mouse. (D) GFP (green) and DNA (ToPro3, blue) staining of a 50 μm section of the dentate gyrus from a 3.5-month-old Sox1-tTA; LC-1; R26eYFP mouse. (E-H) GFP (green), DCX (red) and DNA (Topro3, blue) staining (E); GFP (green), PSA-NCAM (red) and DNA (Topro3, blue) staining (F); GFP (green), Tuj1 (red) and DNA (Topro3, blue) staining (G); and GFP (green) and NeuN (red) staining (H) of a 50 μm section of the dentate gyrus from a 3.5-month-old Sox1-tTA; LC-1; Z/EG mouse. (I-L) GFP (green), DCX (red) and DNA (Topro3, blue) staining (I); GFP (green), PSA-NCAM (red) and DNA (Topro3, blue) staining (J); GFP (green), Tuj1 (red) and DNA (Topro3, blue) staining (K); and GFP (green) and NeuN (red) (L) staining of a 50 μm section of the dentate gyrus from a 3.5-month-old Sox1-tTA; LC-1; R26eYFP mouse. All mice were taken off of doxycycline at 1.5 months. Arrowheads in E-L indicate cells that are double positive for GFP and described marker. Arrows in H and L indicate cells marked only by GFP. Scale bars: 10 μm.
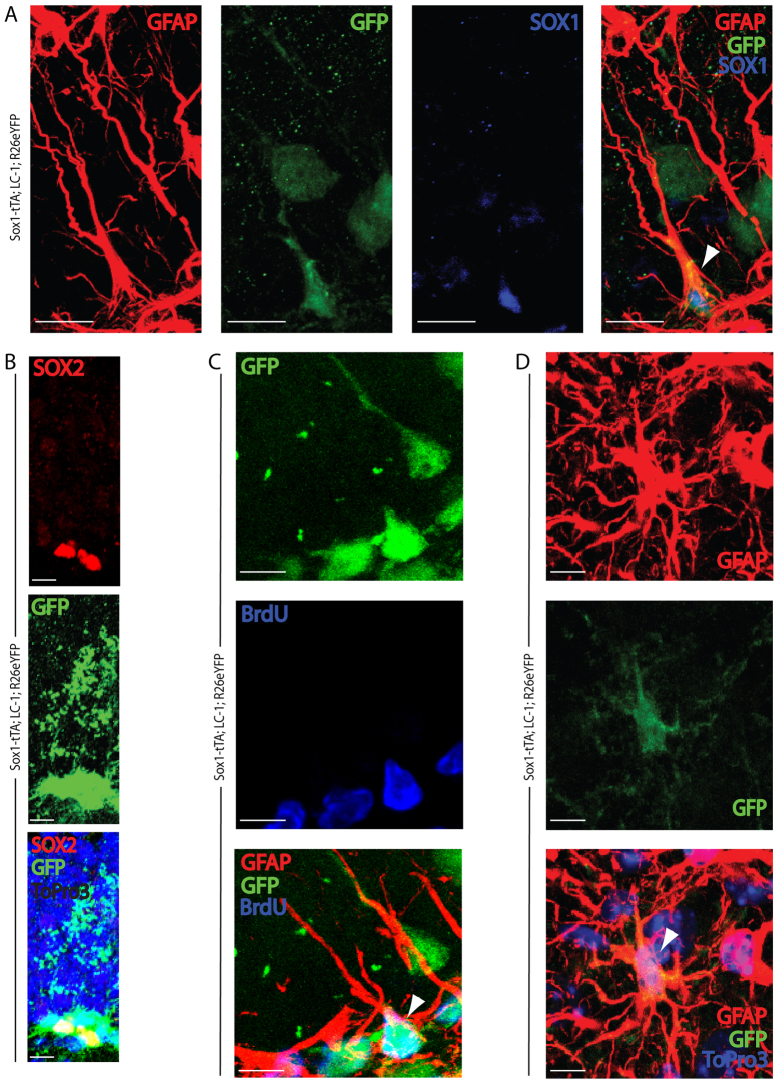
To determine which cell types were being marked in the lineage tracing experiments, we co-stained sections from the hippocampus of triple transgenic mice with anti-GFP and markers for late progenitors/neuroblasts, neurons and astrocytes. The mice carrying the Z/EG reporter showed GFP stained PSA-NCAM late progenitors, DCX neuroblasts, TUJ1 immature neurons and NeuN mature granular neurons of the dentate gyrus (Fig. 3E-H; supplementary material Fig. S4). However, the Z/EG reporter did not show GFP staining in the radial astrocytes (data not shown), even though Sox1-GFP was expressed in a subset of these cells (Fig. 1). We considered two possibilities: (1) Sox1 is expressed in a subset of radial astrocytes that are already committed to the neuroblast lineage and have transformed into this lineage by the time we analyzed the mice; and/or (2) the Z/EG reporter is poorly expressed in the Sox1-positive radial astrocytes. To test the later possibility, we examined mice carrying the R26R-eYFP reporter instead of Z/EG. Similar to Z/EG mice, the R26R-eYFP was activated and expressed throughout neuronal differentiation (Fig. 3I-L; supplementary material Fig. S5). In contrast to the Z/EG reporter, the R26R-eYFP reporter was also expressed in a subset of GFAP-positive radial astrocytes and SOX2-positive non-radial SGZ cells (Fig. 4A,B). A 1-week pulse of BrdU showed that these cells as well as PSA-NCAM-positive late progenitors were actively cycling (Fig. 4C; supplementary material Fig. S6). Non-radial GFAP-positive astrocytes within the hilar region of the dentate gyrus also expressed the reporter (Fig. 4D). These cells, which had morphology consistent with mature parenchymal astrocytes, did not express Sox1-GFP or stain positive with the SOX1 antibody. Therefore, we infer they were derived from the Sox1-expressing progenitors. Together, these lineage-tracing experiments show that Sox1-expressing cells, including radial astrocytes and non-radial early progenitors, give rise to both granular neurons and mature astrocytes.
Fig. 4.
Sox1 lineage tracing marks a subset of putative stem cells, as well as newly born neuroblasts, granule neurons and astrocytes. Sox1-tTA; LC-1; R26eYFP mice were sacrificed at 3.5 months of age and brains were harvested and sectioned into 50 μm slices for antibody staining and imaging by confocal microscopy. (A) GFAP (red), GFP (green) and Sox1 (blue) staining of the dentate gyrus. (B) Sox2 (red), GFP (green) and DNA (ToPro3, blue) staining of the dentate gyrus. Note that grainy GFP staining above Sox2-positive cells is background. (C) GFAP (red), GFP (green) and BrdU (blue) staining of the dentate gyrus. (D) GFAP (red), GFP (green) and DNA (ToPro3, blue) staining of an astrocyte within the hilus. Arrowheads mark cells that are positive for all three antibodies. All mice were taken off of doxycycline at 1.5 months. Scale bars: 10 μm.
SOX1 marks cells with long-term capacity to produce new neurons
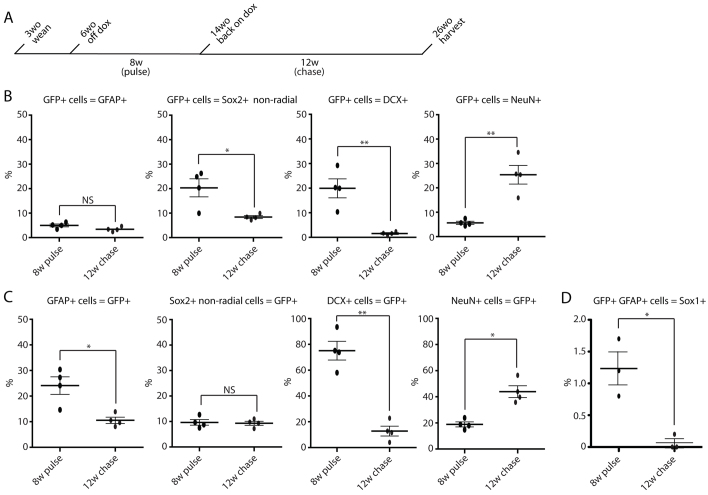
Although activation of Sox1-tTA for 8 weeks showed that Sox1-expressing cells could give rise to granular neurons and mature astrocytes, it was unclear whether the lineage marked radial astrocytes were a stable population within the stem cell compartment that could give rise to new neurons at a later time point. To test this possibility, we took mice that had been off doxycycline for 8 weeks (pulse; tTA system on) and put them back on doxycycline for 12 weeks (chase; tTA system off) (Fig. 5A). This enabled the initial marking of Sox1-expressing cells followed by a 12-week chase. Marked cells at the end of the 12-week chase represent cells or the progeny of cells that had expressed Sox1 at some point during the 8-week pulse of tTA activity. If Sox1 marks a stable population of stem/progenitor cells, there should be ongoing reporter expression in these cells as well as downstream neuroblasts at the end of the chase. Quantification of the distribution of GFP positive, lineage-traced cells at the 8-week pulse versus 12-week chase timepoints showed: GFAP-positive radial astrocytes [4.96% (±0.83%) versus 3.41% (±0.68%)], SOX2-positive non-radial cells [20.33% (±5.22%) versus 8.40% (±0.81%)], DCX-positive cells [19.93% (±5.45%) versus 1.56% (±0.51%)] and NeuN positive granular neurons [5.65% (±0.91%) versus 25.38% (±5.39%)] (Fig. 5B). Overall, this distribution of Sox1-traced cells showed a shift from the progenitor to the mature neuron pool during the chase period, although a population of labeled radial astrocytes and neuroblasts remained, consistent with ongoing neurogenesis from cells marked 12 weeks earlier.
Fig. 5.
Sox1-marked cells within the stem cell compartment are long lived and continue to give rise to new neurons. (A) Schematic of the timeline for back-on-doxycycline experiments. Sox1-tTA; LC-1; R26eYFP mice were taken off doxycycline at 6 weeks of age for 8 weeks (pulse; system on). They were then put back on doxycycline at 14 weeks of age for 12 weeks (chase; system silenced) and sacrificed at 26 weeks of age. (B) Quantification of GFP-positive lineage-traced cells that are radial GFAP positive, Sox2 non-radial positive, DCX positive or NeuN positive in mice kept off doxycycline (pulse) for 8 weeks (n=4, each black circle represents an individual mouse) or put back on doxycycline (chase) for 12 weeks (n=4, each red circle represents an individual mouse). Black bar represents the average. (C) Quantification of the percentage of all GFAP-positive radial astrocytes, Sox2-positive non-radial cells, Dcx-positive neuroblasts or NeuN-positive neurons that are also GFP positive in mice kept off doxycycline (pulse) for 8 weeks (n=4, each black circle represents an individual mouse) or put back on doxycycline (chase) for 12 weeks (n=4, each red circle represents an individual mouse). Black bar represents the average. (D) Quantification of the percentage of GFAP positive, GFP-positive radial astrocytes that are also Sox1 positive in mice kept off doxycycline (pulse) for 8 weeks (n=3, each black circle represents an individual mouse) or put back on doxycycline (chase) for 12 weeks (n=3, each red circle represents an individual mouse). Black bars represent the average; error bars indicate s.d. An average of 200 cells were counted per mouse. NS, no statistical significance; *P<0.05, **P<0.01.
Next, we compared the fraction of GFAP-positive radial astrocytes, SOX2-positive non-radial cells, DCX neuroblasts and NeuN neurons marked by GFP at the end of the 8-week pulse versus after the 12-week chase (Fig. 5C). The percentage of GFAP-positive radial astrocytes decreased [24.10% (±4.81%) versus 10.54% (±1.74%)] whereas the Sox2-positive non-radial cells remained stable [9.65% (±1.56%) versus 9.33% (±1.12)]. By contrast, the percentage of DCX-positive cells dropped dramatically [75.12% (±10.25%) versus 12.80% (±5.46%)], while the percentage of NeuN-positive granular neurons increased [18.87% (±2.67%) versus 43.96% (±6.33%)]. BrdU or PCNA staining confirmed proliferation of the Sox1-marked cells following the 12-week chase (supplementary material Fig. S7). These pulse-chase experiments give rise to several conclusions. First, the high percentage of marked neuroblasts at the end of the 8-week pulse shows that most newly born neurons arise from Sox1-expressing cells. Second, the decrease in marked radial astrocytes after the 12-week chase suggests that some of these presumptive stem cells were actually committed progenitors or went on to die. Third, the continuing production of neural progenitors after the 12-week chase is consistent with a number of the marked radial astrocytes having long-term neurogenic potential.
Following the 12-week chase, most neuroblasts were not marked by lineage tracing and hence were not arising from the Sox1-labeled radial astrocytes (Fig. 5C). This finding may reflect a cyclic expression of Sox1. That is, most of the radial astrocytes that were marked by Sox1 expression during the 8-week pulse have since silenced the gene and entered a quiescent state. Meanwhile, a new population of Sox1-expressing cells gives rise to most of the newly born neuroblasts. To test this hypothesis, we evaluated SOX1 protein expression, using the anti-SOX1 antibody, within the lineage-traced (GFP-positive) GFAP-positive radial astroyctes after the 8-week pulse and the 12-week chase (Fig. 5D). Following the 8-week pulse, only 1.23% (±0.26%) of the marked (GFP-positive) radial astrocytes were anti-SOX1 positive. This finding suggests that Sox1 expression is highly transitory where most of the radial astrocytes that were labeled during the 8 weeks had already silenced Sox1 by the end of the pulse. Consistent with this conclusion, following the 12-week chase, only rare Sox1-traced cells [0.07% (±0.07%)] were still expressing Sox1. The vast majority of anti-SOX1-positive cells at this time point were not expressing GFP and, therefore, had activated Sox1 in previously Sox1-negative cells.
SOX1-positive cells of the SGZ decrease in number but are molecularly stable during aging
It has been proposed that the molecular constitution of NSCs changes with aging resulting in a decreased regenerative capacity (for review, see Galvan and Jin, 2007). However, considering the heterogeneity that we uncovered within the stem cell pool, the change with aging could be due to changes in cellular composition of the germinal niche and/or cell-autonomous changes in gene expression within progenitors. To test these alternatives, we asked whether the fraction of radial astrocytes that is SOX1 positive changes with age. Indeed, within the radial astrocyte pool, the fraction of SOX1-positive cells decreased from 26.23% (±2.76%) at 2.5 months to 21.48% (±2.39%) at 6 months to 10.75% (±5.59%) at 12 months (Fig. 6A). Next, we asked whether the molecular constitution of the Sox1 population itself changed with age. To test this proposition, Sox1-GFP cells were sorted from dissociated hippocampi from young (4-7 month) and old (21 month) mice. Purified mRNA from these cells was analyzed on microarrays. Very few genes were altered as defined by a false discovery rate of less than 5% (Fig. 6B,C). Only eight probe sets representing five genes were significantly up and 7 probe sets representing 5 genes were significantly down (Fig. 6C). Cluster analysis was unable to separate the young from old mice showing that their molecular constitutions were almost identical (Fig. 6D). P16 and HMGA2, which have previously been shown to increase and decrease, respectively, with age in the V-SVZ (Molofsky et al., 2006), did not show significant changes at the transcript level in the SOX1-positive cells of the SGZ (Fig. 6E). Therefore, although the fraction of SOX1-positive cells within the stem cell pool declines with aging, their molecular constitution remains remarkably stable throughout adult life.
Fig. 6.
The fraction of Sox1-positive cells within the stem cell compartment decreases with age, but is molecularly stable. (A) C57BL/6 mice were sacrificed at 2.5 months, 6 months or 12 months of age and brains were harvested and sectioned into 50 μm slices for antibody staining with GFAP and Sox1. Confocal microscopy was used for quantification of the percentage of all GFAP-positive radial astrocytes that are also Sox1 positive (n=3 for each time point; each circle represents an individual mouse). Black bar represents the average. (B) SAM analysis comparing three younger mice (4-7 month old versus 21 month old). Blue dots represent downregulated genes and red dots represent upregulated genes with FDR<5. (C) Fold difference (log2) of mean expression values of individual genes, old versus young. Red and blue dots as in A. List of differential genes with FDR<5 listed. (D) Tree diagram of clusteral analysis showing relatedness of individual mice. Note that old and young mice cannot be differentiated, consistent with the very similar expression patterns. (E) Expression levels of p16 and HMGA2.
DISCUSSION
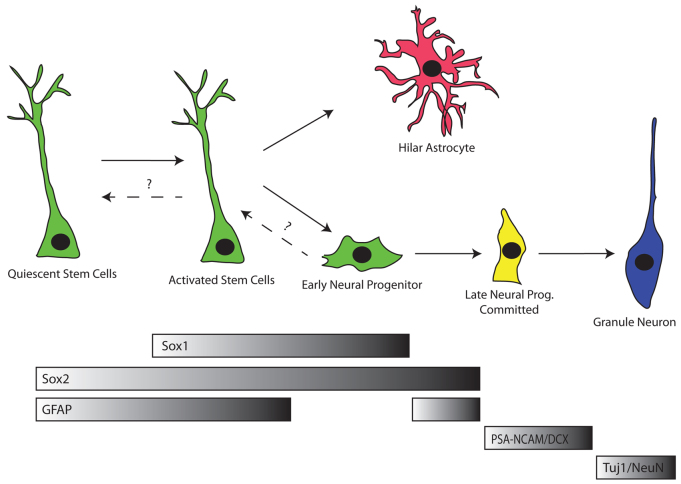
Neurogenesis occurs within the hippocampus of adult mice throughout their lifespan. However, the self-renewal properties and nature of the stem cells has remained controversial (Suh et al., 2007; Bonaguidi et al., 2011; Encinas et al., 2011). Part of this controversy stems from a lack of markers. Here, we show that SOX1 is expressed in a subset of GFAP-positive radial astrocytes and SOX2-positive non-radial cells within the SGZ. SOX1 shows a much more restricted pattern than other previously described markers of putative SGZ stem/progenitor cells (Fig. 7). That is SOX1 is expressed in a fraction of GFAP radial astrocytes and SOX2 positive non-radial cells. By contrast, SOX2 is expressed broadly across radial astrocytes, non-radial progenitors and mature astrocytes in the dentate gyrus (Steiner et al., 2006; Suh et al., 2007). Similarly, other common markers (i.e. nestin or GFAP) are expressed in a broader range of SGZ cells, endothelial cells and/or mature astrocytes (Brenner et al., 1994; Wiese et al., 2004).
Fig. 7.
Model of SGZ lineages in the adult dentate gyrus based on SOX1. SOX1 is expressed in activated stem/progenitor cells. These activated SOX1-marked stem cells can give rise to hilar astrocytes as well as to committed early neural progenitors, which then give rise to downstream lineages. Alternatively, activated SOX1 stem cells may return to a quiescent state, whereby SOX1 is silenced, with the potential to be reactivated, and re-express SOX1, at a later time.
Even though Sox1 is expressed in only a subset of stem/early progenitor cells, lineage tracing shows that these are the cells responsible for producing a majority of the neuroblasts. Considering the likely incompleteness of removal of the lox-stop-lox cassette in Cre-expressing cells, it is possible that Sox1 marks all the stem/early progenitors giving rise to neuroblasts and hence newly born neurons. However, the subset of radial astrocytes and non-radial cells that express SOX1 do not all differentiate into neurons. Lineage tracing shows a substantial fraction of Sox1 marked cells remain within the two stem/progenitor populations even 12 weeks after lineage tracing has been shut off. Interestingly, most of the Sox1 marked radial astrocytes no longer actively express Sox1. Even at the end of the 8-week pulse many traced cells are no longer expressing Sox1, suggestive of a highly transitory expression of this transcription factor. After the 12-week chase, a small fraction of late progenitors/neuroblasts continue to be marked, presumably arising from lineage traced radial astrocytes. Based on these findings, we propose a model where radial astrocytes that activate Sox1 can either commit to differentiate or to remain as stem cells. The latter cells silence Sox1 and then reactivate it at a later time point to give rise to future generations of neurons (Fig. 7). Although it is possible that the marked late progenitors/neuroblasts after the 12-week chase were somehow delayed in their differentiation rather than arising anew from traced radial astrocytes, this interpretation seems unlikely considering that the chase is three times longer than the reported 4-week time span from a committed progenitor to mature neuron (Zhao et al., 2008; Encinas et al., 2006). Furthermore, the marked late progenitors were proliferating, inconsistent with a dormant state.
Lineage-tracing experiments using inducible nestin-CreER transgenic mice have led to contradictory interpretations regarding the lifespan of adult NSCs. In one report, the authors use low dose tamoxifen to mark rare cells in the stem cell pool and follow clones over time (Bonaguidi et al., 2011). Based on this approach, they find evidence that radial astrocytes can self-renew long term and can produce both neurons and astrocytes. Consistent with this conclusion, another report using a Hes5-CreER transgenic to lineage trace NSCs, also found evidence for long-term production of neurons (Lugert et al., 2012). In a third contrasting report, the authors use higher doses of tamoxifen in the nestin-creER model along with real-time expression of nestin (nestin-GFP transgenic mice) and sequential CIdU/IdU staining to arrive at the conclusion that radial astrocytes have a limited lifespan (‘disposable stem cell model’) (Encinas et al., 2011). In particular, they conclude that once radial astrocytes are activated, they undergo several rounds of division to produce neuronally committed progenitors and then differentiate into terminal astrocytes. Our results support aspects of both models. The ongoing production of progenitors/neuroblasts from Sox1-marked cells even after 3 months of having the system off is consistent with a population of stem cells with long-term neurogenic potential that cycle between an activated and quiescent state. However, the reduction of Sox1-traced radial astrocytes over the 12-week chase is consistent with some of these cells permanently exiting the stem cell pool.
Our results also identify crucial variables when considering the NSC population of the hippocampus. First, SOX1 expression clearly identifies heterogeneity within the GFAP-positive radial astrocyte and SOX2 positive non-radial cell populations. Second, our results show the influence that reporter expression can have on interpretation of data. In particular, we find that the Z/EG reporter does not mark Sox1-expressing cells. The Bonaguidi et al. and Ecinas et al. papers both use the Z/EG reporter in most of their lineage-tracing experiments and, therefore, may be visualizing a different subset of progenitor cells. By contrast, we find the R26R-eYFP reporter is expressed in the Sox1 radial astrocytes, but not all of the Sox1-expressing cells when compared with either Sox1-GFP or anti-SOX1 staining (Fig. 5B versus Fig. 1E). This finding may reflect that either many of the Sox1 radial astrocytes have transitioned into late progenitors prior to complete activation of the reporter or the failure of the reporter to be expressed in all cells. Another important consideration is comparisons between transgenic lines. We use a Sox1 knock-in approach, while the nestin-GFP and nestin-creER are transgenics inserted at different loci. Therefore, comparisons may be complicated by local epigenetic influences on gene expression. Resolution of these confounding factors and others will be essential to resolve the very important question of the nature of hippocampal stem cells and their long-term self-renewal capacity.
SOX1 appears to play multiple roles in the adult brain. Its expression is limited to stem/progenitor cells in the dentate gyrus whereas it is expressed in mature neurons in other parts of the brain. For example, Sox1 is highly expressed in the CA1 region of the hippocampus (Kan et al., 2007). SOX1 is also expressed in other neuron subtypes in the brain and has been shown to play an important role in the migration of the ventral striatal neurons from the VZ during embryonic development (Ekonomou et al., 2005; Kan et al., 2007). SOX1 is also expressed in the V-SVZ of the adult (Kan et al., 2007). However, unlike in the SGZ, real-time expression (Sox1-GFP) and pulse-chase lineage tracing (Sox1-tTA system) suggests that the Sox1-expressing cells of the V-SVZ are predominantly, if not solely, committed intermediate progenitors (supplementary material Figs S1, S8). Why Sox1 is expressed in such distinct populations is unclear. The downstream molecular pathways will need to be dissected to better understand how SOX1 is used in the different contexts. In vitro studies have suggested that SOX1 regulates Notch signaling (Kan et al., 2004). Interestingly, Notch signaling is used iteratively in stem/progenitor cells and then again later in differentiated neurons (for review, see Ables et al., 2011). Therefore, although SOX1 may be used at different stages leading to different outcomes, it could be doing so through common molecular pathways.
A major question in the adult neurogenesis field is how aging influences the intrinsic molecular nature of the stem cells. In the V-SVZ, it has been shown that levels of the cell cycle inhibitor p16 increase with aging, resulting in a decline in proliferation and hence neurogenesis, consistent with intrinsic changes within these cells (Molofsky et al., 2006). Therefore, it appeared likely that mRNA profiles of Sox1-expressing cells from young and old mice would differ. However, our profiling data suggest that these cells are intrinsically stable. An interesting alternative to intrinsic modifications of gene expression profiles is that changes in the composition of the germinal niche cell populations occur. Therefore, with an increasing purification of stem cells, the more stable the cells seem. To test this possibility, it will be essential to develop new markers that can subdivide the presumptive stem cells to increasing homogeneous populations. Indeed, our data supports an unexpected heterogeneity among the GFAP-positive radial astrocytes and Sox2-positive non-radial cells of the SGZ (Yamaguchi et al., 2000; Seri et al., 2001; Kempermann et al., 2003; Fukuda et al., 2003; Seri et al., 2004; Encinas et al., 2011; Bonaguidi et al., 2011). We propose that this heterogeneity along with limitations in the tools used to trace the presumptive stem cells underlie the often-contradictory results within the adult NSC field. The further characterization of new markers such as Sox1 will enable the refinement and hence better understanding of the true stem cell pool within the SGZ.
Supplementary Material
Acknowledgements
We appreciate critical manuscript review by J. Rich, H. Suh, J. Lathia, D. Schonberg and D. Lim. We thank Austin Smith for the Sox1-GFP targeting construct and Yusuke Kamachi and Hisato Kondoh for the Sox1 antibody.
Footnotes
Funding
R.B. was supported by the National Institutes of Health (NIH) [K08 NS48118 and R01 NS057221], California Institute of Regenerative Medicine (CIRM) [Seed Grant RS1-00161, New Faculty Award RN2-00906] and the Pew Charitable Trust. M.V. was supported by an American Brain Tumor Association Basic Research Fellowship and was supported by a National Service Research Award [NINDS F32 NS058042]. Y.G.H. and A.A.B. were supported by NIH [R01 NS28478]. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.081133/-/DC1
References
- Ables J. L., Breunig J. J., Eisch A. J., Rakic P. (2011). Not(ch) just development: Notch signalling in the adult brain. Nat. Rev. Neurosci. 12, 269-283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J., Das G. D. (1965a). Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 124, 319-335 [DOI] [PubMed] [Google Scholar]
- Altman J., Das G. D. (1965b). Post-natal origin of microneurones in the rat brain. Nature 207, 953-956 [DOI] [PubMed] [Google Scholar]
- Aubert J., Stavridis M. P., Tweedie S., O'Reilly M., Vierlinger K., Li M., Ghazal P., Pratt T., Mason J. O., Roy D., et al. (2003). Screening for mammalian neural genes via fluorescence-activated cell sorter purification of neural precursors from Sox1-gfp knock-in mice. Proc. Natl. Acad. Sci. USA 100 Suppl. 1, 11836-11841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraud P., Thompson L., Kirik D., Björklund A., Parmar M. (2005). Isolation and characterization of neural precursor cells from the Sox1-GFP reporter mouse. Eur. J. Neurosci. 22, 1555-1569 [DOI] [PubMed] [Google Scholar]
- Bonaguidi M. A., Wheeler M. A., Shapiro J. S., Stadel R. P., Sun G. J., Ming G. L., Song H. (2011). In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell 145, 1142-1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazel C. Y., Limke T. L., Osborne J. K., Miura T., Cai J., Pevny L., Rao M. S. (2005). Sox2 expression defines a heterogeneous population of neurosphere-forming cells in the adult murine brain. Aging Cell 4, 197-207 [DOI] [PubMed] [Google Scholar]
- Brenner M., Kisseberth W. C., Su Y., Besnard F., Messing A. (1994). GFAP promoter directs astrocyte-specific expression in transgenic mice. J. Neurosci. 14, 1030-1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylund M., Andersson E., Novitch B. G., Muhr J. (2003). Vertebrate neurogenesis is counteracted by Sox1-3 activity. Nat. Neurosci. 6, 1162-1168 [DOI] [PubMed] [Google Scholar]
- Cameron H. A., Woolley C. S., McEwen B. S., Gould E. (1993). Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience 56, 337-344 [DOI] [PubMed] [Google Scholar]
- Carleton A., Petreanu L. T., Lansford R., Alvarez-Buylla A., Lledo P. M. (2003). Becoming a new neuron in the adult olfactory bulb. Nat. Neurosci. 6, 507-518 [DOI] [PubMed] [Google Scholar]
- Collignon J., Sockanathan S., Hacker A., Cohen-Tannoudji M., Norris D., Rastan S., Stevanovic M., Goodfellow P. N., Lovell-Badge R. (1996). A comparison of the properties of Sox-3 with Sry and two related genes, Sox-1 and Sox-2. Development 122, 509-520 [DOI] [PubMed] [Google Scholar]
- Coriat A. M., Müller U., Harry J. L., Uwanogho D., Sharpe P. T. (1993). PCR amplification of SRY-related gene sequences reveals evolutionary conservation of the SRY-box motif. PCR Methods Appl. 2, 218-222 [DOI] [PubMed] [Google Scholar]
- Dai M. H., Wang P. L., Boyd A. D., Kostov G., Athey B., Jones E. G., Bunney W. E., Myers R. M., Speed T. P., Akil H., et al. (2005). Evolving gene/transcript definitions significantly alter the interpretation of GeneChip data. Nucleic Acids Res. 33, e175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny P., Swift S., Connor F., Ashworth A. (1992). An SRY-related gene expressed during spermatogenesis in the mouse encodes a sequence-specific DNA-binding protein. EMBO J. 11, 3705-3712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F., Caillé I., Lim D. A., García-Verdugo J. M., Alvarez-Buylla A. (1999). Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97, 703-716 [DOI] [PubMed] [Google Scholar]
- Ekonomou A., Kazanis I., Malas S., Wood H., Alifragis P., Denaxa M., Karagogeos D., Constanti A., Lovell-Badge R., Episkopou V. (2005). Neuronal migration and ventral subtype identity in the telencephalon depend on SOX1. PLoS Biol. 3, e186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkouris M., Balaskas N., Poulou M., Politis P. K., Panayiotou E., Malas S., Thomaidou D., Remboutsika E. (2011). Sox1 maintains the undifferentiated state of cortical neural progenitor cells via the suppression of Prox1-mediated cell cycle exit and neurogenesis. Stem Cells 29, 89-98 [DOI] [PubMed] [Google Scholar]
- Ellis P., Fagan B. M., Magness S. T., Hutton S., Taranova O., Hayashi S., McMahon A., Rao M., Pevny L. (2004). Sox2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Dev. Neurosci. 26, 148-165 [DOI] [PubMed] [Google Scholar]
- Encinas J. M., Vaahtokari A., Enikolopov G. (2006). Fluoxetine targets early progenitor cells in the adult brain. Proc. Natl. Acad. Sci. USA 103, 8233-8238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas J. M., Michurina T. V., Peunova N., Park J. H., Tordo J., Peterson D. A., Fishell G., Koulakov A., Enikolopov G. (2011). Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell 8, 566-579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Episkopou V. (2005). Sox2 functions in adult neural stem cells. Trends Neurosci. 28, 219-221 [DOI] [PubMed] [Google Scholar]
- Eriksson P. S., Perfilieva E., Björk-Eriksson T., Alborn A. M., Nordborg C., Peterson D. A., Gage F. H. (1998). Neurogenesis in the adult human hippocampus. Nat. Med. 4, 1313-1317 [DOI] [PubMed] [Google Scholar]
- Favaro R., Valotta M., Ferri A. L., Latorre E., Mariani J., Giachino C., Lancini C., Tosetti V., Ottolenghi S., Taylor V., et al. (2009). Hippocampal development and neural stem cell maintenance require Sox2-dependent regulation of Shh. Nat. Neurosci. 12, 1248-1256 [DOI] [PubMed] [Google Scholar]
- Ferri A. L., Cavallaro M., Braida D., Di Cristofano A., Canta A., Vezzani A., Ottolenghi S., Pandolfi P. P., Sala M., DeBiasi S., et al. (2004). Sox2 deficiency causes neurodegeneration and impaired neurogenesis in the adult mouse brain. Development 131, 3805-3819 [DOI] [PubMed] [Google Scholar]
- Fukuda S., Kato F., Tozuka Y., Yamaguchi M., Miyamoto Y., Hisatsune T. (2003). Two distinct subpopulations of nestin-positive cells in adult mouse dentate gyrus. J. Neurosci. 23, 9357-9366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage F. H., Kempermann G., Palmer T. D., Peterson D. A., Ray J. (1998). Multipotent progenitor cells in the adult dentate gyrus. J. Neurobiol. 36, 249-266 [DOI] [PubMed] [Google Scholar]
- Galvan V., Jin K. (2007). Neurogenesis in the aging brain. Clin. Interv. Aging 2, 605-610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham V., Khudyakov J., Ellis P., Pevny L. (2003). Sox2 functions to maintain neural progenitor identity. Neuron 39, 749-765 [DOI] [PubMed] [Google Scholar]
- Gubbay J., Collignon J., Koopman P., Capel B., Economou A., Münsterberg A., Vivian N., Goodfellow P., Lovell-Badge R. (1990). A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature 346, 245-250 [DOI] [PubMed] [Google Scholar]
- Irizarry R. A., Hobbs B., Collin F., Beazer-Barclay Y. D., Antonellis K. J., Scherf U., Speed T. P. (2003). Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4, 249-264 [DOI] [PubMed] [Google Scholar]
- Kamachi Y., Uchikawa M., Collignon J., Lovell-Badge R., Kondoh H. (1998). Involvement of Sox1, 2 and 3 in the early and subsequent molecular events of lens induction. Development 125, 2521-2532 [DOI] [PubMed] [Google Scholar]
- Kan L., Israsena N., Zhang Z., Hu M., Zhao L. R., Jalali A., Sahni V., Kessler J. A. (2004). Sox1 acts through multiple independent pathways to promote neurogenesis. Dev. Biol. 269, 580-594 [DOI] [PubMed] [Google Scholar]
- Kan L., Jalali A., Zhao L. R., Zhou X., McGuire T., Kazanis I., Episkopou V., Bassuk A. G., Kessler J. A. (2007). Dual function of Sox1 in telencephalic progenitor cells. Dev. Biol. 310, 85-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M. S., Bell D. H. (1984). Mitotic neuroblasts in the 9-day-old and 11-month-old rodent hippocampus. J. Neurosci. 4, 1429-1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G., Kuhn H. G., Gage F. H. (1997). More hippocampal neurons in adult mice living in an enriched environment. Nature 386, 493-495 [DOI] [PubMed] [Google Scholar]
- Kempermann G., Gast D., Kronenberg G., Yamaguchi M., Gage F. H. (2003). Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development 130, 391-399 [DOI] [PubMed] [Google Scholar]
- Komitova M., Eriksson P. S. (2004). Sox-2 is expressed by neural progenitors and astroglia in the adult rat brain. Neurosci. Lett. 369, 24-27 [DOI] [PubMed] [Google Scholar]
- Lois C., Alvarez-Buylla A. (1994). Long-distance neuronal migration in the adult mammalian brain. Science 264, 1145-1148 [DOI] [PubMed] [Google Scholar]
- Lugert S., Vogt M., Tchorz J. S., Müller M., Giachino C., Taylor V. (2012). Homeostatic neurogenesis in the adult hippocampus does not involve amplification of Ascl1(high) intermediate progenitors. Nat. Commun. 3, 670 [DOI] [PubMed] [Google Scholar]
- Luskin M. B. (1993). Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron 11, 173-189 [DOI] [PubMed] [Google Scholar]
- Malas S., Postlethwaite M., Ekonomou A., Whalley B., Nishiguchi S., Wood H., Meldrum B., Constanti A., Episkopou V. (2003). Sox1-deficient mice suffer from epilepsy associated with abnormal ventral forebrain development and olfactory cortex hyperexcitability. Neuroscience 119, 421-432 [DOI] [PubMed] [Google Scholar]
- Ming G. L., Song H. (2005). Adult neurogenesis in the mammalian central nervous system. Annu. Rev. Neurosci. 28, 223-250 [DOI] [PubMed] [Google Scholar]
- Molofsky A. V., Slutsky S. G., Joseph N. M., He S., Pardal R., Krishnamurthy J., Sharpless N. E., Morrison S. J. (2006). Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature 443, 448-452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiguchi S., Wood H., Kondoh H., Lovell-Badge R., Episkopou V. (1998). Sox1 directly regulates the gamma-crystallin genes and is essential for lens development in mice. Genes Dev. 12, 776-781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak A., Guo C., Yang W., Nagy A., Lobe C. G. (2000). Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis 28, 147-155 [PubMed] [Google Scholar]
- Petreanu L., Alvarez-Buylla A. (2002). Maturation and death of adult-born olfactory bulb granule neurons: role of olfaction. J. Neurosci. 22, 6106-6113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevny L., Rao M. S. (2003). The stem-cell menagerie. Trends Neurosci. 26, 351-359 [DOI] [PubMed] [Google Scholar]
- Pevny L., Placzek M. (2005). Sox genes and neural progenitor identity. Curr. Opin. Neurobiol. 15, 7-13 [DOI] [PubMed] [Google Scholar]
- Pevny L. H., Lovell-Badge R. (1997). Sox genes find their feet. Curr. Opin. Genet. Dev. 7, 338-344 [DOI] [PubMed] [Google Scholar]
- Pevny L. H., Sockanathan S., Placzek M., Lovell-Badge R. (1998). A role for Sox1 in neural determination. Development 125, 1967-1978 [DOI] [PubMed] [Google Scholar]
- Rex M., Orme A., Uwanogho D., Tointon K., Wigmore P. M., Sharpe P. T., Scotting P. J. (1997). Dynamic expression of chicken Sox2 and Sox3 genes in ectoderm induced to form neural tissue. Dev. Dyn. 209, 323-332 [DOI] [PubMed] [Google Scholar]
- Schmidt-Hieber C., Jonas P., Bischofberger J. (2004). Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature 429, 184-187 [DOI] [PubMed] [Google Scholar]
- Schönig K., Schwenk F., Rajewsky K., Bujard H. (2002). Stringent doxycycline dependent control of CRE recombinase in vivo. Nucleic Acids Res. 30, e134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott C. E., Wynn S. L., Sesay A., Cruz C., Cheung M., Gomez Gaviro M. V., Booth S., Gao B., Cheah K. S., Lovell-Badge R., et al. (2010). SOX9 induces and maintains neural stem cells. Nat. Neurosci. 13, 1181-1189 [DOI] [PubMed] [Google Scholar]
- Seri B., García-Verdugo J. M., McEwen B. S., Alvarez-Buylla A. (2001). Astrocytes give rise to new neurons in the adult mammalian hippocampus. J. Neurosci. 21, 7153-7160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seri B., García-Verdugo J. M., Collado-Morente L., McEwen B. S., Alvarez-Buylla A. (2004). Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J. Comp. Neurol. 478, 359-378 [DOI] [PubMed] [Google Scholar]
- Shors T. J., Miesegaes G., Beylin A., Zhao M., Rydel T., Gould E. (2001). Neurogenesis in the adult is involved in the formation of trace memories. Nature 410, 372-376 [DOI] [PubMed] [Google Scholar]
- Sinclair A. H., Berta P., Palmer M. S., Hawkins J. R., Griffiths B. L., Smith M. J., Foster J. W., Frischauf A. M., Lovell-Badge R., Goodfellow P. N. (1990). A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature 346, 240-244 [DOI] [PubMed] [Google Scholar]
- Soriano P. (1999). Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21, 70-71 [DOI] [PubMed] [Google Scholar]
- Sottile V., Li M., Scotting P. J. (2006). Stem cell marker expression in the Bergmann glia population of the adult mouse brain. Brain Res. 1099, 8-17 [DOI] [PubMed] [Google Scholar]
- Srinivas S., Watanabe T., Lin C. S., William C. M., Tanabe Y., Jessell T. M., Costantini F. (2001). Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1, 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner B., Klempin F., Wang L., Kott M., Kettenmann H., Kempermann G. (2006). Type-2 cells as link between glial and neuronal lineage in adult hippocampal neurogenesis. Glia 54, 805-814 [DOI] [PubMed] [Google Scholar]
- Streit A., Sockanathan S., Pérez L., Rex M., Scotting P. J., Sharpe P. T., Lovell-Badge R., Stern C. D. (1997). Preventing the loss of competence for neural induction: HGF/SF, L5 and Sox-2. Development 124, 1191-1202 [DOI] [PubMed] [Google Scholar]
- Suh H., Consiglio A., Ray J., Sawai T., D'Amour K. A., Gage F. H. (2007). In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell 1, 515-528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter D. M., Tirefort D., Julien S., Krause K. H. (2009). A Sox1 to Pax6 switch drives neuroectoderm to radial glia progression during differentiation of mouse embryonic stem cells. Stem Cells 27, 49-58 [DOI] [PubMed] [Google Scholar]
- Tashiro A., Sandler V. M., Toni N., Zhao C., Gage F. H. (2006). NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature 442, 929-933 [DOI] [PubMed] [Google Scholar]
- Tusher V. G., Tibshirani R., Chu G. (2001). Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. USA 98, 5116-5121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uwanogho D., Rex M., Cartwright E. J., Pearl G., Healy C., Scotting P. J., Sharpe P. T. (1995). Embryonic expression of the chicken Sox2, Sox3 and Sox11 genes suggests an interactive role in neuronal development. Mech. Dev. 49, 23-36 [DOI] [PubMed] [Google Scholar]
- Wegner M. (1999). From head to toes: the multiple facets of Sox proteins. Nucleic Acids Res. 27, 1409-1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner M., Stolt C. C. (2005). From stem cells to neurons and glia: a Soxist's view of neural development. Trends Neurosci. 28, 583-588 [DOI] [PubMed] [Google Scholar]
- Wiese C., Rolletschek A., Kania G., Blyszczuk P., Tarasov K. V., Tarasova Y., Wersto R. P., Boheler K. R., Wobus A. M. (2004). Nestin expression – a property of multi-lineage progenitor cells? Cell. Mol. Life Sci. 61, 2510-2522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood H. B., Episkopou V. (1999). Comparative expression of the mouse Sox1, Sox2 and Sox3 genes from pre-gastrulation to early somite stages. Mech. Dev. 86, 197-201 [DOI] [PubMed] [Google Scholar]
- Wright E. M., Snopek B., Koopman P. (1993). Seven new members of the Sox gene family expressed during mouse development. Nucleic Acids Res. 21, 744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M., Saito H., Suzuki M., Mori K. (2000). Visualization of neurogenesis in the central nervous system using nestin promoter-GFP transgenic mice. Neuroreport 11, 1991-1996 [DOI] [PubMed] [Google Scholar]
- Zhao C., Deng W., Gage F. H. (2008). Mechanisms and functional implications of adult neurogenesis. Cell 132, 645-660 [DOI] [PubMed] [Google Scholar]
- Zhao S., Nichols J., Smith A. G., Li M. (2004). SoxB transcription factors specify neuroectodermal lineage choice in ES cells. Mol. Cell. Neurosci. 27, 332-342 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.