Abstract
The efficient sequestration of nutrients is vital for the growth and survival of microorganisms. Some nutrients, such as CO2 and NH3, are readily diffusible across the cell membrane. The large membrane permeability of these nutrients obviates the need of transporters when the ambient level is high. When the ambient level is low, however, maintaining a high intracellular nutrient level against passive back diffusion is both challenging and costly. Here, we study the delicate management of ammonium (NH4+/NH3) sequestration by E. coli cells using microfluidic chemostats. We find that as the ambient ammonium concentration is reduced, E. coli cells first maximize their ability to assimilate the gaseous NH3 diffusing into the cytoplasm and then abruptly activate ammonium transport. The onset of transport varies under different growth conditions, but always occurring just as needed to maintain growth. Quantitative modeling of known interactions reveals an integral feedback mechanism by which this need-based uptake strategy is implemented. This novel strategy ensures that the expensive cost of upholding the internal ammonium concentration against back diffusion is kept at a minimum.
Keywords: active transport, futile cycle, integral feedback, metabolic coordination, microfluidics
Introduction
Microorganisms must acquire nutrients from the external environment. It is critical for their proliferation to take in these nutrients rapidly across cell membranes and maintain sufficient nutrient levels in the cytoplasm. Some essential nutrients are readily diffusible across the cell membrane. For example, NH3 or CO2 can diffuse across the membrane ∼30 times faster than water (Simon and Gutknecht, 1980; Walter and Gutknecht, 1986). The large membrane permeability of these molecules is both a blessing and a curse. When the ambient nutrient level is high, passive diffusion alone can supply enough nutrient needed for rapid cell growth. When the ambient level is low, however, maintaining high internal levels by active transport is challenging and costly because the transported molecules can diffuse out rapidly, resulting in a futile cycle. Thus, transporting these molecules has a steep price. However, it is not known what strategies the organisms adopt to deal with this problem.
Here, we study the management of ammonium (NH4+/NH3) sequestration by E. coli as a model system. Nitrogen is essential for cell growth, and ammonium is the preferred nitrogen source for many microorganisms including E. coli (Reitzer, 2003). These microorganisms often live in environments with limited ammonium supplies, for example, μM range in fresh and seawater (Rees et al, 2006). Under such conditions, it is critical for their growth and survival to transport ammonium efficiently while minimizing the obligatory futile cycle.
To study how these cells manage ammonium sequestration in such ammonium-limited conditions, it is desirable to maintain low steady ammonium concentrations in the growth medium through multiple rounds of cell doubling, a very challenging task in both batch and continuous cultures (Atkinson et al, 2002; Soupene et al, 2002). Instead, we cultured cells in microfluidic chambers that can maintain nutrients including ammonium to the desired concentrations (Groisman et al, 2005). By monitoring the growth and gene expression of exponentially growing cells using time-lapse microscopy, and analyzing the data quantitatively using flux analysis, we reveal a delicate control of ammonium sequestration strategy: as the ambient ammonium concentration is reduced, E. coli cells first increase their ability to assimilate ammonium that diffuses freely across the membrane. Upon maximizing ammonium assimilation, ammonium transport by the transporter AmtB is activated abruptly, but only to the minimal level needed to sustain cell growth. The onset of transport changes under different growth conditions that support different growth rates (and hence different cellular demands for nitrogen) when ammonium is replete, but it always occurs just at the concentration where an isogenic strain unable to transport ammonium begins to show a growth defect. Mathematical modeling of known interactions reveals an integral feedback strategy by which two distinct signals of the cellular nitrogen status are used to provide seamless coordination between ammonium assimilation and transport, such that the degree of ammonium transport is the minimum necessary to sustain cell growth.
Results
AmtB is necessary to maintain rapid cell growth at low ambient ammonium concentrations
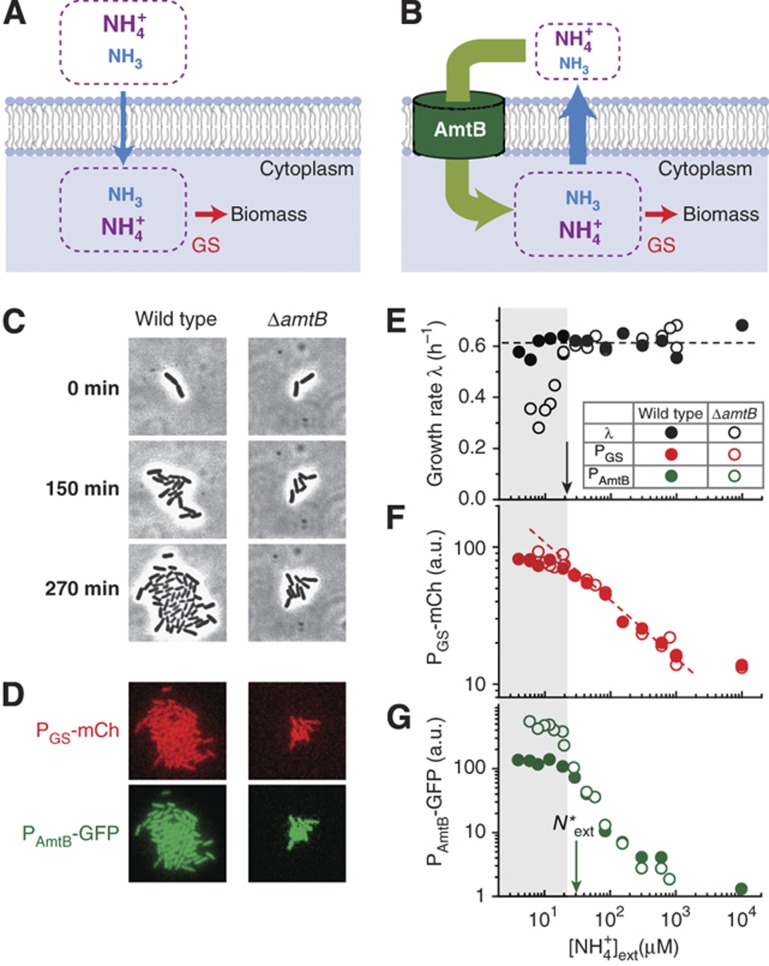
Ammonium exists predominantly in the ionic form (NH4+) at neutral pH, and the minor gaseous species (NH3) can diffuse rapidly through the cell membrane (Walter and Gutknecht, 1986). At high ambient ammonium concentrations, the passive diffusion of NH3 can provide enough nitrogen for optimal cell growth (blue arrow, Figure 1A; Soupene et al, 1998; Andrade and Einsle, 2007; Fong et al, 2007). As the ambient ammonium concentration is reduced and active ammonium transport is needed to sustain cell growth, a wide range of organisms expresses the Amt family proteins (Boussiba et al, 1984; Loque and von Wiren, 2004; Andrade and Einsle, 2007) which concentrate ammonium inside cells (green arrow, Figure 1B) (Boussiba et al, 1984; Andrade and Einsle, 2007; Fong et al, 2007). However, the higher internal concentration of NH4+/NH3 imposes a dangerous futile cycle on the organism (Kleiner, 1985), as NH3 unavoidably diffuses outward down the gradient (blue arrow), forcing a much larger ammonium uptake than the nitrogen flux needed for biosynthesis (red arrow).
Figure 1.
Ammonium sequestration by AmtB. (A) NH3 is in equilibrium with NH4+ and diffuses across the cell membrane rapidly (blue arrow) (Walter and Gutknecht, 1986). The passive diffusion of NH3 alone can support rapid cell growth when the ambient ammonium concentration is high (Soupene et al, 1998; Andrade and Einsle, 2007; Fong et al, 2007). Intracellular ammonium is assimilated into biomass through glutamine synthetase (GS) (Reitzer, 2003). (B) When the ambient ammonium concentration is low, AmtB concentrates it internally (green arrow) (Boussiba et al, 1984; Andrade and Einsle, 2007; Fong et al, 2007). Higher internal concentrations of NH4+ and NH3 lead to the outward diffusion of NH3 (blue arrow), forming an energetically costly futile cycle; see Supplementary Figure 5 for the estimate of the energetic cost. (C) Time-lapse phase-contrast images of E. coli cells growing in microfluidic chambers with minimal medium containing a very low concentration (12 μM) of NH4Cl as the sole nitrogen source and saturating amounts of glycerol as the sole carbon source. The ΔamtB strain (EQ130, right) grew more slowly than the control (EQ66, left); see Supplementary Table 1 for strain details. From these images, growth rates were determined during the first three generations when the increase is clearly exponential (Supplementary Figure 1C). Here and elsewhere, the reported external NH4+ concentration includes a residual concentration of ∼4 μM estimated in the medium (Supplementary Figure 6). (D) mCherry (red) and GFP (green) intensities reflect the GS and amtB promoter activities, respectively. (E) While the wild type (solid circles) maintained its growth rate, the ΔamtB strain (open circles) grew more slowly (gray zone) below ∼20 μM of external NH4+ (black arrow), in agreement with previous findings obtained in low pH medium (Soupene et al, 1998, 2002; Fong et al, 2007); see also Supplementary Figure 7. (F, G) The promoter activities of GS (reported by mCherry, red) and AmtB (reported by GFP, green) for the wild-type (solid circle) and ΔamtB strain (open circle) increase as the ambient ammonium concentration is reduced. Below a characteristic NH4+ concentration, N*ext≈30μM (green arrow), the GFP intensities of the two strains deviate, indicating differences in the internal nitrogen status; see text. All the data plotted here are provided in Supplementary Tables 6 and 7. a.u., arbitrary units.
To study how E. coli cells control its Amt protein, AmtB, at low ammonium concentrations, we developed microfluidic chambers (Groisman et al, 2005) which can maintain ammonium at low concentrations (Supplementary Figure 1A). Fresh medium flows actively through the channel (in green) and diffuses into the growth chambers (in red). The rapid diffusive medium exchange between the growth chambers and the surrounding channels (Supplementary Figure 1B) due to the small geometry ensures that steady low nutrient levels are maintained inside the chamber (Supplementary Note 1). This allows us to monitor the exponential growth cell growth at as low as ∼4 μM NH4+ (Supplementary Figure 1C).
To examine when AmtB becomes necessary for cell growth, we compared the exponential growth of the wild-type and ΔamtB strains (see Supplementary Tables 1 and 2 for strain details) in minimal medium with various NH4+ concentrations and saturating amounts of glycerol as the sole carbon source; the effect of different carbon sources will be discussed below. Typical phase-contrast images of cells growing in microfluidic chambers in Figure 1C show ΔamtB strains growing more slowly than the wild type at 12 μM of NH4+. With similar time-lapse images of 20–30 colonies recorded twice per doubling, we quantified the growth rate for wild-type and ΔamtB strains in Figure 1E; see Materials and methods. While the wild-type strain (solid black circles) maintained its growth rate from 10 mM down to a few μM, the ΔamtB strain (open black circles) grew more slowly below ∼20 μM (black arrow). Thus, AmtB is needed to sustain the growth below ∼20 μM NH4+ (gray zone).
GS and AmtB expression is upregulated at low ammonium concentrations
Glutamine synthetase (GS) is the major enzyme assimilating ammonium needed for cell growth at low ammonium concentrations (below 1 mM of NH4+) (Reitzer, 2003). Km of GS is reported to be ∼100 μM for NH4+ (Meek and Villafranca, 1980; Alibhai and Villafranca, 1994) under physiological conditions (Supplementary Note 2). To check how the ΔamtB strain maintains the growth down to 20 μM NH4+ which is far below the Km of GS, we monitored the expression level of GS using the mCherry fluorescence protein (Figure 1D and F). As the ambient NH4+ concentration was reduced, the mCherry fluorescence levels for ΔamtB strain (open red circles) increased gradually, beginning to approach its maximum induction level at ∼20 μM (right of the gray zone). GS expression of wild type (solid red circles) behaves indistinguishably. Thus, from 100 μM down to ∼20 μM of external NH4+, cell growth is maintained by elevating the GS expression level for both the wild-type and ΔamtB strain.
We also monitored the activity of the amtB promoter using a green fluorescence protein (GFP) reporter (Figure 1D). Figure 1G shows that the GFP signals of the wild-type and ΔamtB strain (solid and open green circles) are indistinguishable down to a characteristic ambient NH4+ concentration of N*ext≈μM (green arrow). Below this point, the GFP signal of the wild type reached a plateau while that of the ΔamtB strain continued to rise before leveling off at a higher plateau. Notably, the fold change of AmtB expression (>100-fold) between high ammonium (∼mM of external NH4+) and low ammonium (∼μM of external NH4+) conditions is much higher than that of GS expression (<10-fold in Figure 1F). Similar fold changes of AmtB and GS expression levels were observed in a bulk experiment when cells initially growing in an ammonium-replete condition transitioned to ammonium starvation (Atkinson et al, 2002).
Deducing the internal ammonium concentration of ΔamtB strain
The expression and activity levels of GS and AmtB respond to the internal nitrogen status (Reitzer, 2003). To elucidate the regulation of ammonium assimilation and transport, it is crucial to quantify the internal nitrogen status. However, there is no known way to measure directly the internal ammonium concentration in vivo. Here, we describe an approach to deduce it from the data in Figure 1G. We will first describe it here for the ΔamtB strain, which is quite straightforward. This approach will be extended below to deduce the internal ammonium concentration of wild-type cells.
The only way for the ΔamtB strain to acquire ammonium is through the passive diffusion of the gaseous form NH3 (Walter and Gutknecht, 1986), with the diffusive flux Jdiffusion given by the NH3 concentration difference between the outside and inside of cells. The latter can be related to the external and internal NH4+ concentrations ([NH4+]ext and [NH4+]int, respectively) from the NH4+/NH3 equilibrium, expressed as
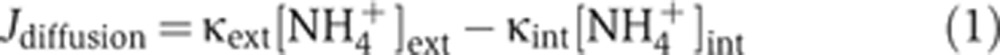 |
where κext and κint are proportionality constants given by the NH3 permeability, cell geometry, and extra- and intra-cellular pH; see Supplementary Equations S1–S4 for details of this derivation, and Supplementary Equations S5–S7 for the details of the proportionality constants. The definitions and values of the parameters in the constants are given in Supplementary Table 3.
For a given external NH4+ concentration, we can deduce the internal NH4+ concentration by equating the diffusion flux Jdiffusion with the rate of nitrogen assimilation, Jbiomass, which is given as required by the nitrogen content of biomass (n0) and the measured growth rate (λ), that is,
 |
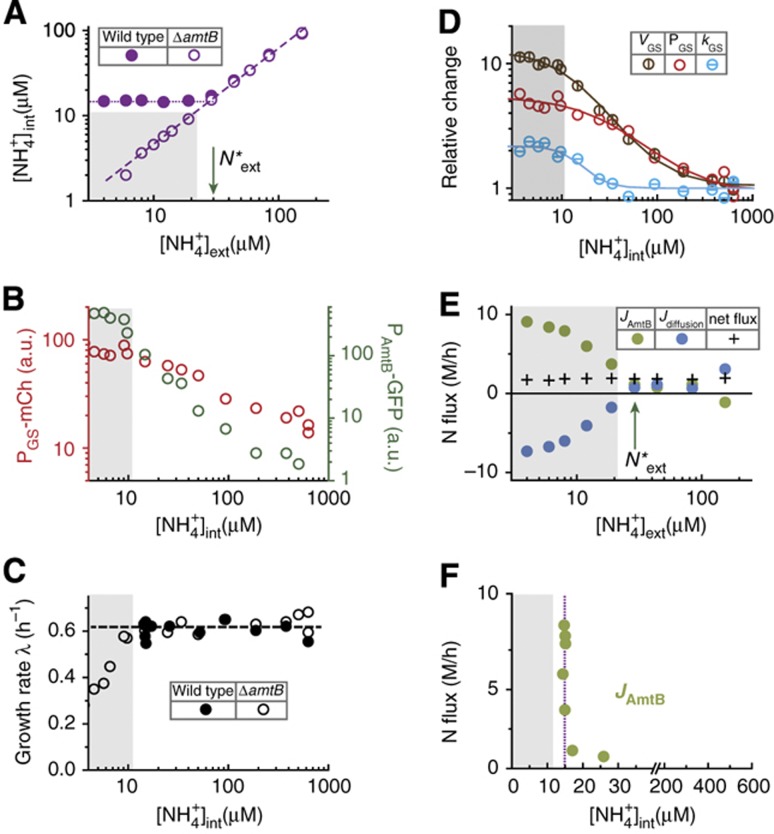
The internal NH4+ concentrations ([NH4+]ext) of ΔamtB cells, deduced from Equations (1) and (2) with Jdiffusion=Jbiomass using the measured growth rate λΔamtB for each external NH4+ concentration (open circles in Figure 1E), are plotted for various external NH4+ concentrations as shown in Figure 2A (open purple circles); see Supplementary Equations S8–S11. The linear relation is expected due to the passive diffusion of NH3 in ΔamtB cells. Here and below, the gray region, adopted from that in Figure 1E, indicates the conditions in which the ΔamtB strain exhibits reduced growth.
Figure 2.
Steady maintenance of internal ammonium and the abrupt activation of AmtB. (A) The deduced internal NH4+ concentrations of the ΔamtB strain (EQ130, open purple circles) and wild type (EQ66, solid purple circles) grown in glycerol with varying NH4+ concentrations; see Equations (1) and (2), and the text. The gray region (defined in Figure 1E) indicates the conditions in which the ΔamtB strain exhibits reduced growth. (B) The dependences of the GS (left axis) and amtB (right axis) promoter activities of the ΔamtB strain on the deduced internal NH4+ concentrations. (C) The growth rate of the ΔamtB strain and wild type on the deduced internal NH4+ concentrations. (D) The Vmax of GS (VGS, brown), the GS expression level (PGS, red) and the specific activity of GS (kGS, cyan) are plotted relative to their values in ammonium-replete conditions. Here, VGS is obtained from Equation (3), and kGS is obtained from the ratio of VGS and PGS. The lines of respective colors are fit using Hill functions (Supplementary Equation S18); see Supplementary Table 4 for the kinetic parameters of the fit. (E) The deduced ammonium transport flux through AmtB (green circles) and the NH3 diffusion flux (blue circles); see Equations (1), (2) and (4). Black crosses represent the net nitrogen influx (the sum of the two fluxes), which the cells utilize for biomass synthesis. Note that the maintenance of the net flux is accompanied by strong increases in ammonium transport by AmtB, and NH3 leakage through passive diffusion. (F) A sharp increase in the ammonium transport flux through AmtB (green circles) when plotted against the deduced internal NH4+ concentrations.
Deducing the GS activity
The scattered plot of PGS-mCherry reporter levels observed (open circles in Figure 1F) with the deduced internal NH4+ concentrations for the corresponding external NH4+ concentrations (open circles in Figure 2A) is shown as red circles in Figure 2B, revealing a simple dependence of GS promoter activity on the internal NH4+ concentration. It shows that as the internal NH4+ concentration is reduced, the ΔamtB strain increases the capacity of ammonium assimilation by increasing the GS expression level. However, GS expression does not reflect the actual capacity of ammonium assimilation by GS, because GS activity can be altered by glutamine via adenylylation (Kingdon et al, 1967; Wulff et al, 1967; Okano et al, 2010) and by the end products of glutamine metabolism via allosteric inhibition (Woolfolk and Stadtman, 1964; Woolfolk and Stadtman, 1967). With the knowledge of the internal NH4+ concentrations (open circles in Figure 2A), we can further deduce GS activity from the growth rate and GS expression (open circles in Figure 1E and F) as described below.
First, the total flux of ammonium assimilation by GS per cell volume, JGS, is given by the Michaelis–Menten kinetics (Meek and Villafranca, 1980),
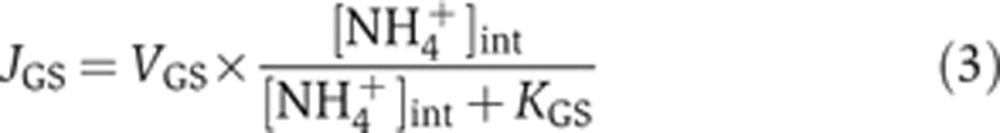 |
Here, VGS and KGS are the associated Vmax and the Michaelis constant, with KGS∼100 μM for NH4+ (Meek and Villafranca, 1980; Alibhai and Villafranca, 1994). Molecularly, VGS is determined by the levels of GS expression and its specific activity, with the latter affected by allosteric product inhibition (Woolfolk and Stadtman, 1964; Woolfolk and Stadtman, 1967) and reversible adenylylation (Kingdon et al, 1967; Wulff et al, 1967; Okano et al, 2010).
Since GS is the key enzyme assimilating internal ammonium into biomass at low ambient NH4+ concentrations (e.g., <1 mM; Reitzer, 2003), the ammonium assimilation flux JGS can be obtained from the nitrogen demand for cellular growth, Jbiomass=λΔamtB
n0 (Equation (2)), where 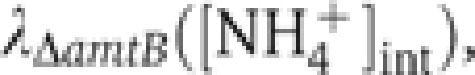 , shown as the open circles in Figure 2C, is obtained from the measured growth rates in various external NH4+ concentrations (open circles in Figure 1E) and the corresponding internal NH4+ concentrations (open circles in Figure 2A). Using this resulting form of JGS([NH4+]int) in Equation (3) yields VGS for various internal NH4+ concentrations, plotted as brown circles with vertical lines in Figure 2D. In the same figure, the relative GS expression level, PGS([NH4+]int), from Figure 2B (red circles) is re-plotted as red circles for comparison. The ratio of VGS([NH4+]int) gives the relative changes in the specific activity of GS, kGS, at the different internal NH4+ concentrations, shown as the cyan circles with horizontal lines in Figure 2D. As the internal NH4+ concentration is reduced, kGS([NH4+]int) increases by ∼2-folds, approaching its maximum level near the concentration at which PGS([NH4+]int) is maximized. Previously, it was found from batch culture studies that GS is approximately half-adenylylated in nitrogen-replete conditions and completely unadenylylated in nitrogen-limited conditions (Okano et al, 2010). Thus, the changes in kGS may arise primarily from the changes in the adenylylation state. We note that VGS, PGS and kGS can be well described by Hill functions (Supplementary Equation S18), as shown by the lines of respective colors in Figure 2D: see Supplementary Table 4 for parameters of the fit.
, shown as the open circles in Figure 2C, is obtained from the measured growth rates in various external NH4+ concentrations (open circles in Figure 1E) and the corresponding internal NH4+ concentrations (open circles in Figure 2A). Using this resulting form of JGS([NH4+]int) in Equation (3) yields VGS for various internal NH4+ concentrations, plotted as brown circles with vertical lines in Figure 2D. In the same figure, the relative GS expression level, PGS([NH4+]int), from Figure 2B (red circles) is re-plotted as red circles for comparison. The ratio of VGS([NH4+]int) gives the relative changes in the specific activity of GS, kGS, at the different internal NH4+ concentrations, shown as the cyan circles with horizontal lines in Figure 2D. As the internal NH4+ concentration is reduced, kGS([NH4+]int) increases by ∼2-folds, approaching its maximum level near the concentration at which PGS([NH4+]int) is maximized. Previously, it was found from batch culture studies that GS is approximately half-adenylylated in nitrogen-replete conditions and completely unadenylylated in nitrogen-limited conditions (Okano et al, 2010). Thus, the changes in kGS may arise primarily from the changes in the adenylylation state. We note that VGS, PGS and kGS can be well described by Hill functions (Supplementary Equation S18), as shown by the lines of respective colors in Figure 2D: see Supplementary Table 4 for parameters of the fit.
Deducing the AmtB activity
In contrast to the ΔamtB strain that relies only on passive diffusion of NH3 for ammonium uptake, wild-type cells can employ AmtB to actively transport ammonium. However, a futile cycle associated with the ammonium transport (Figure 1B) may impose a significant burden on cells. Thus, it is critical for cells to control the AmtB activity tightly. This can be seen from the comparison of the amtB promoter activities between wild-type and ΔamtB strains (Figure 1G); they are indistinguishable down to the low external NH4+ concentration marked with N*ext below which they deviate, indicating that the internal nitrogen status of the two strains differs below N*ext. This suggests that although AmtB expression is upregulated below ∼1 mM of external NH4+, its activity is turned on only below N*ext, which is slightly above when is needed to maintain rapid growth (gray region). Indeed, it is known the AmtB activity is inhibited by the regulatory protein GlnK, which is expressed in the same operon as AmtB, binds tightly to it, and inhibits its activity in ammonium-replete conditions (Coutts et al, 2002; Blauwkamp and Ninfa, 2003; Javelle et al, 2004).
We wish to determine AmtB activity quantitatively. Since this information is contained in the internal NH4+ concentration which is directly affected by AmtB activity, we determine the internal NH4+ concentration of wild type first and extract the AmtB activity from it. To deduce the internal NH4+ concentration of the wild type, we make a scattered plot (green circles in Figure 2B) of PAmtB-GFP reporter levels measured for the ΔamtB strain at various external NH4+ concentrations (open circles in Figure 1G) against the corresponding internal NH4+ concentrations (open circles in Figure 2A), which reveals a simple and sensitive dependence of the amtB promoter activity on the internal NH4+ concentration. Assuming that the dependence is the same for the wild-type cells, which amounts to the assumption that the amtB promoter activity is primarily determined by the internal NH4+ concentration only when the ambient NH4+ concentration is varied (Supplementary Note 3), the internal NH4+ concentration of the wild type can be directly read off from the measured amtB promoter activity in wild-type cells (solid circle in Figure 1G). The internal NH4+ concentration obtained in this way is shown as the solid purple circles in Figure 2A. It is seen to be maintained at ∼15 μM as the external NH4+ concentration is reduced below N*ext≈30μM (green arrow in Figure 2A).
With the knowledge of the internal NH4+ concentration of the wild-type cells, we can deduce the total activity of AmtB, JAmtB, defined as the ammonium transport flux through AmtB (Supplementary Equation S13), by balancing the supply and assimilation of nitrogen: for exponentially growing wild-type cells, the active ammonium transport by AmtB must supply for both the nitrogen needed for growth and the passive out diffusion of NH3 through the membrane (Figure 1B). Thus, we have
 |
where Jdiffusion and Jbiomass are given by Equations (1) and (2), respectively: see Supplementary Equations S12–S15 for details. The external and internal NH4+ concentrations of the wild-type cells given in Figure 2A (solid circles) yield the diffusion flux Jdiffusion, plotted against the external NH4+ concentration as the blue circles in Figure 2E, with the negative values indicating a flux out of the cell (see also the blue arrow in Figure 1B). Together with Jbiomass from the observed growth rate of wild-type cells (solid circles in Figure 1E), JAmtB is deduced from Equation (4); the results are plotted as the green circles in Figure 2E. Note that this analysis does not depend on the exact molecular species (NH4+ versus NH3) transported by AmtB, a contentious subject in the literature (Andrade and Einsle, 2007; Fong et al, 2007). The ammonium transport flux through AmtB here only refers to the rate of nitrogen atoms being transported. In Figure 2E, we also indicate the net ammonium uptake flux, given by the sum of Jdiffusion and JAmtB, as the crosses. It is clear that net uptake flux is much (∼5-fold) smaller than the flux through AmtB in the gray region where AmtB activity is needed, indicating that much of the flux through AmtB is lost back to the medium by diffusion as will be discussed below.
The activity of AmtB is delicately controlled
The internal NH4+ concentrations and AmtB activity deduced above reveal a delicate management of ammonium sequestration by wild-type cells. In Figure 2A, the internal NH4+ concentrations for wild-type and ΔamtB strain are indistinguishable for ambient NH4+ concentrations above N*ext≈30μM (green arrow), both decreasing linearly. Below that point, however, the internal NH4+ concentrations of ΔamtB strain continued to decrease linearly whereas the wild type maintained its internal NH4+ concentration at an approximately constant value (∼15 μM), referred to as the ‘maintenance concentration’ (purple dotted line). The deviation of internal NH4+ concentrations for the two strains below N*ext≈30μM (green arrow) is accompanied by a strong increase in ammonium transport by AmtB (JAmtB, green circles in Figure 2E), and in NH3 back diffusion (Jdiffusion, blue circles). Importantly, the net ammonium influx (black crosses) remained unchanged, providing a steady flow of nitrogen needed for biomass synthesis at the constant growth rate. The dependence of the ammonium transport on the availability of internal ammonium (Figure 2F) shows that the ammonium flux through AmtB increased sharply at the maintenance concentration (purple dotted lines in Figure 2A and F), which is slightly above the concentration at which the ΔamtB strain begins to grow slowly (gray zone in Figure 2C and F).
These observations highlight the sensitive and precise control of AmtB activity, which is crucial in light of the futile cycle associated with it. The ammonium transport by AmtB enables cells to maintain their optimal growth at low ambient NH4+ concentrations (solid circles in gray region in Figure 1E) by concentrating NH4+ internally (below N*ext in Figure 2A). However, the higher internal NH4+ concentrations result in large leaky flux of NH3 (blue circles in Figure 2E and blue arrow in Figure 1B), forcing cells to transport more ammonium than necessary (green circles in Figure 2E and green arrow in Figure 1B). To minimize the futile cycle, cells exert a tight control on AmtB activity. As the ambient ammonium concentration is reduced, they first increase the ability to assimilate NH3 diffusing into the cytoplasm by increasing GS expression/activity (Figures 1F and 2D), but keep AmtB inactive (green circles above N*ext in Figure 2E). Only as GS expression/activity approaches the maximum (the gray zone in Figures 1F and 2D, also the region where the lack of AmtB would slow down growth as in Figures 1E and 2C), AmtB is activated abruptly (Figure 2F) and transports just enough ammonium to maintain the internal NH4+ barely above the minimum level needed for optimal growth (purple dotted line above gray zone in Figure 2A and F), thereby minimizing the futile cycle.
The delicate control of AmtB activity is coordinated with cellular nitrogen demand
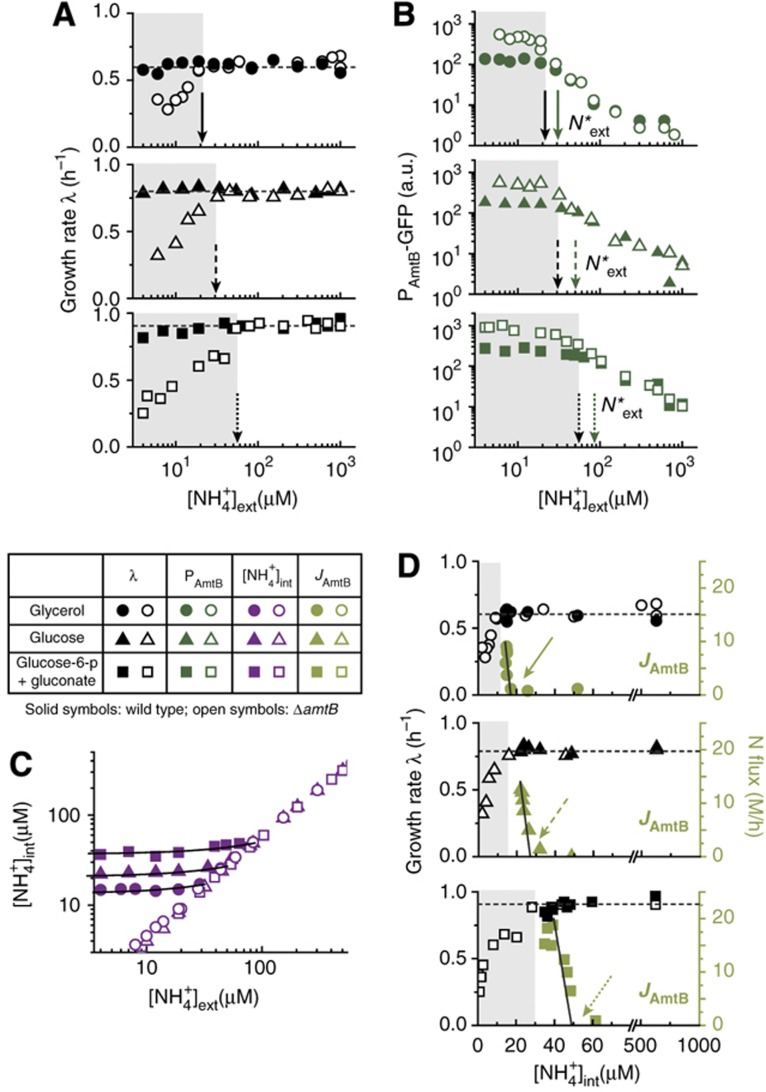
Cells coordinate nitrogen and carbon metabolism through various mechanisms (Commichau et al, 2006; Doucette et al, 2011). Since cells growing on different carbon sources exhibit different growth rates in ammonium-replete conditions and hence have different demands for nitrogen, we varied the carbon source in the growth medium to ones that provided successively faster growth than what glycerol can provide. We expect this would force the cells to activate AmtB at higher ammonium concentrations if the faster growth rates are to be maintained as the external ammonium concentration is reduced. Indeed, the data on growth rates (Figure 3A) show that the wild type (solid symbols) was able to maintain growth throughout the range of NH4+ concentrations tested. In contrast, the ΔamtB strain (open symbols) exhibited growth reduction as the ambient NH4+ concentration was reduced, and the onset of the growth defect (black arrows) occurred at successively higher ambient NH4+ concentrations for the medium supporting faster growth. Also, Figure 3B shows that the NH4+ concentrations at which the amtB promoter activity (GFP) of the wild-type and ΔamtB strain deviate (green arrows) are always just slightly above the respective onset of the growth defect for the three carbon sources tested.
Figure 3.
Coordination of AmtB activity with the cell’s growth status. The growth rate of the culture in ammonium-replete condition was changed by using different carbon sources: glucose-6-phosphate (g6p) plus gluconate (squares), and glucose (triangles). Data from Figures 1 and 2 (glycerol as the carbon source) are re-plotted for comparison (circles). Solid, dashed, and dotted arrows are associated with glycerol, glucose, and g6p plus gluconate. Solid and open symbols indicate the wild-type and ΔamtB strain (EQ66 and EQ130), respectively. (A) The growth rate of the wild-type and ΔamtB strain for all carbon sources tested. The onset of the growth defect of the ΔamtB strain (black arrows) was at successively higher ambient NH4+ concentrations for the carbon sources supporting faster growth. (B) The amtB promoter activities (reported by GFP) of the wild-type and ΔamtB strains deviated (green arrows) at slightly higher ambient NH4+ concentrations than where growth defect set in for the ΔamtB strain (gray zone), indicating that ammonium transport in the wild type occurred barely above where it would be needed to maintain the growth, in coordination with the different growth conditions. (C) The maintenance of the internal NH4+ concentration for the different carbon sources. (D) The growth rate (black symbols) and ammonium transport flux (green symbols) plotted against the internal NH4+ concentrations for the different carbon sources. The abrupt increase in the ammonium flux occurred above the respective onset of growth defect of the ΔamtB strain (right of the gray zone). The black lines in Figures 3C and D show the linear fits of our model (Supplementary Equations S36–S38), from which the onset of AmtB activation N*int is determined (green arrows); see Supplementary Table 5 for the parameters. All the data plotted here are given in Supplementary Tables 8–11. a.u., arbitrary units.
The internal NH4+ concentration and the NH4+ flux through AmtB were subsequently deduced from the data in Figure 3A and B as described above, for wild-type cells grown in the two additional types of medium. The internal NH4+ concentrations were again maintained at constant values at low ambient NH4+ concentrations (solid purple symbols in Figure 3C), with successively higher maintenance concentrations for the faster growing cells, consistent with the higher internal NH4+ concentrations needed for assimilation by GS at the higher rates. The growth rate and the ammonium flux through AmtB are plotted against the internal NH4+ concentrations for the three medium studied in Figure 3D (black and green symbols, respectively). We see that for each medium studied, the abrupt increase in ammonium flux occurred at a value of N*int (green arrows in Figure 3D) which is slightly above the respective onset of growth defect of the ΔamtB strain (the right of gray region); see Supplementary Table 5 for the values of N*ext, N*int and the onset of growth defect of the ΔamtB strain. These results show that the onset of ammonium transport and the maintenance level of the internal NH4+ is not preset to a fixed value, but is instead determined dynamically, such that ammonium transport by AmtB is only employed as necessary to maintain cell growth.
Discussion
Glutamine is unlikely to be a signal controlling AmtB activity
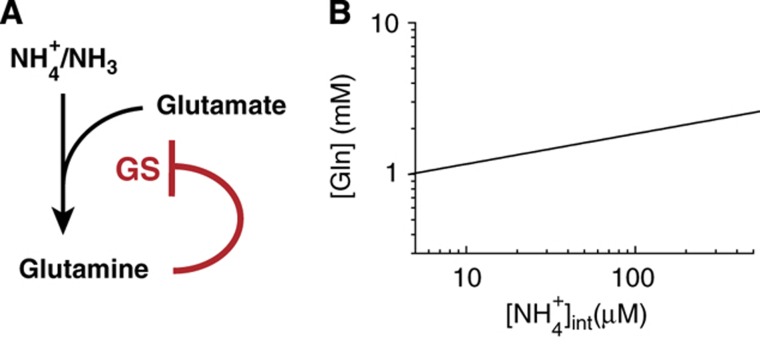
The delicate control of AmtB activity and its coordination with cellular nitrogen demand require intricate signaling system(s). Glutamine has long been established as a signal of the internal nitrogen status (Reitzer, 2003), serving as the major effector of GS expression (Hirschman et al, 1985; Reitzer and Magasanik, 1986; Jiang et al, 1998a, b) and activity (Kingdon et al, 1967; Wulff et al, 1967; Okano et al, 2010) (Figure 4A). It is therefore a possible candidate for controlling AmtB activity, via for example, the known effect of glutamine on GlnK that inhibits AmtB (Coutts et al, 2002; Javelle et al, 2004). The intracellular glutamine concentration unfortunately cannot be directly measured for cells grown under the very low ammonium conditions provided by the microfluidic chambers, due to the small number of cells in the chambers. However, because GS is the sole assimilator of ammonium at low ambient NH4+ concentrations (Reitzer, 2003), the negative feedback regulation of glutamine on GS imposes an obligatory relation between the internal NH4+ concentration and the glutamine pool which can be deduced from two relations: (i) the empirically obtained relation between the relative PGS-mCherry promoter activity and the internal NH4+ concentration, PGS([NH4+]int) (red circles and line in Figure 2D) and (ii) the mechanism regulating GS expression, controlled primarily by the glutamine concentration [Gln] under nitrogen limitation (Hirschman et al, 1985; Reitzer and Magasanik, 1986; Jiang et al, 1998a, b; Reitzer, 2003).
Figure 4.
GS regulation by glutamine and the relation between glutamine pool and internal ammonium concentration. (A) At low ambient ammonium concentrations, GS is the major ammonium assimilation enzyme, catalyzing the formation of glutamine from ammonium and glutamate (Reitzer, 2003). GS expression is repressed by glutamine (red line) through the NtrBC two-component signaling system (Hirschman et al, 1985; Reitzer and Magasanik, 1986; Jiang et al, 1998a, b) while the specific activity of GS is inhibited by adenylylation by GlnE whose activity is stimulated by glutamine (Kingdon et al, 1967; Wulff et al, 1967; Okano et al, 2010). (B) This negative feedback regulation of glutamine on GS imposes an obligatory relation between the internal NH4+ concentration and the glutamine pool, described by Equation (5): see text and Supplementary Equations S18–S20 for details. As shown in the plot, this obligatory relation features a very weak dependence of the glutamine pool on the internal NH4+ concentration, reflecting the homeostatic nature of negative feedback control by glutamine (Reitzer, 2003).
Writing the relative GS expression as EGS([Gln]), the relation between [Gln] and [NH4+]int follows readily by equating PGS([NH4+]int). We model EGS([Gln]) by a Hill function with the Michaelis parameter KGlne, maximum fold-change fGlne, and a Hill coefficient HGlne (see Supplementary Equation S19), whose values are highly constrained by existing data on GS expression and glutamine concentration (Ikeda et al, 1996; Atkinson et al, 2002; Okano et al, 2010); as shown in Supplementary Figure 2, the existing data are best described by 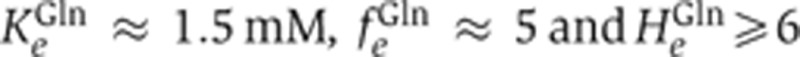 . Comparing EGS([Gln]) with the Hill function for PGS([NH4+]int) described by the red line in Figure 2D, we obtain
. Comparing EGS([Gln]) with the Hill function for PGS([NH4+]int) described by the red line in Figure 2D, we obtain
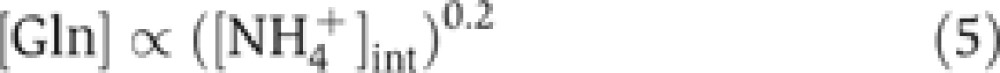 |
which is plotted in Figure 4B (see Supplementary Equations S18–S20 for details). It shows an extremely weak dependence of the glutamine pool on the internal NH4+ concentration, reflecting the homeostatic nature of negative feedback control by glutamine (Reitzer, 2003).
The weak dependence of the glutamine pool on the internal NH4+ concentration, together with the steady maintenance of the internal NH4+ concentration when AmtB activity abruptly increases (Figure 2F), makes it unlikely for glutamine to be the major effector of AmtB activity. This is further supported by the results of Figure 3D, which show that AmtB transport is activated at different internal NH4+ concentrations (and hence different glutamine concentrations) under the different growth conditions.
AmtB is regulated by α-ketoglutarate via an integral feedback control
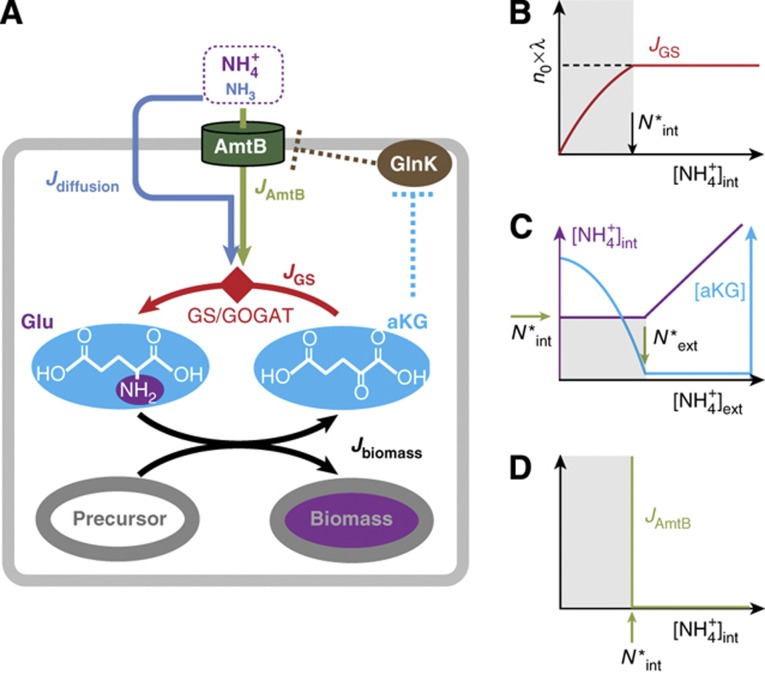
The known molecular interactions actually suggest an alternative signaling scheme as depicted in Figure 5A. It is known that GlnK binds tightly to AmtB and inhibits its activity in ammonium-replete conditions (brown dashed line) (Coutts et al, 2002; Blauwkamp and Ninfa, 2003; Javelle et al, 2004). Also, GlnK dissociates from AmtB at elevated α-ketoglutarate (aKG) concentrations, as predicted by Gruswitz et al (2007) and later confirmed by Radchenko et al (2010) and Truan et al (2010), thereby setting AmtB free to transport ammonium (cyan dashed line). What ties these two pieces of biochemical interactions together is that the aKG pool can be dramatically affected by the ammonium influx which it controls, as established in the literature (Yuan et al, 2009; Radchenko et al, 2010; Doucette et al, 2011; Yan et al, 2011). At low internal ammonium concentrations, ammonium is assimilated by the GS/GOGAT cycle (Supplementary Figure 3; Reitzer, 2003), producing glutamate (Glu) from aKG (red arrows in Figure 5A). The nitrogen group in Glu is passed on to various precursors for biomass synthesis, turning Glu back to aKG (black arrows). If the internal ammonium level drops, then the rate of ammonium assimilation will drop immediately. This slows aKG drainage (red arrow), resulting in aKG accumulation: see Supplementary Figure 3 for details. Indeed, the rapid accumulation of aKG upon ammonium downshift (>5-folds within 15 s) was reported recently (Yan et al, 2011).
Figure 5.
The integral feedback model of AmtB activation control. (A) Intracellular ammonium is assimilated into the biomass in two steps: first, it is captured in the form of glutamate (Glu) using the carbon skeleton α-ketoglutarate (aKG) via the GS/GOGAT pathway; see Supplementary Figure 3 for details. Then, the N-group in Glu is transferred to various carbon precursors to synthesize amino acids and incorporated into the biomass, while recycling the carbon skeleton back to aKG. Importantly, the aKG pool, which integrates imbalances between the ammonium assimilation flux (JGS) and the biomass incorporation flux (Jbiomass) (Equation (6)), is known to activate AmtB strongly (Gruswitz et al, 2007; Radchenko et al, 2010; Truan et al, 2010) via the regulatory GlnK (Coutts et al, 2002; Blauwkamp and Ninfa, 2003; Javelle et al, 2004), as indicated by the dashed cyan and brown lines. These interactions form the integral feedback loop (Equations (6), (7) and (8)). (B) The flux of ammonium assimilation by GS, JGS, plotted against the internal ammonium concentration, [NH4+]int. JGS is obtained from λ × n0, based on the form of 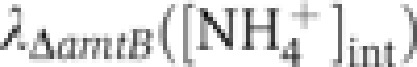 shown in Figure 2C. JGS decreases when [NH4+]int decreases below a certain level, N*int (gray region). (C, D) The steady-state aKG concentration ([aKG], cyan), internal ammonium concentration (purple), and AmtB activity (JAmtB, green) are deduced from Equation (8); see Supplementary Figure 4 for detailed explanation. N*ext is the external ammonium concentration ([NH4+]ext) below which JGS decreases without AmtB. For [NH4+]ext > N*ext, [aKG] remains at its basal level, and [NH4+]ext changes linearly with [NH4+]ext. For [NH4+]ext<N*ext, [aKG] increases and activates AmtB to the level needed to uphold [NH4+]int at N*int.
shown in Figure 2C. JGS decreases when [NH4+]int decreases below a certain level, N*int (gray region). (C, D) The steady-state aKG concentration ([aKG], cyan), internal ammonium concentration (purple), and AmtB activity (JAmtB, green) are deduced from Equation (8); see Supplementary Figure 4 for detailed explanation. N*ext is the external ammonium concentration ([NH4+]ext) below which JGS decreases without AmtB. For [NH4+]ext > N*ext, [aKG] remains at its basal level, and [NH4+]ext changes linearly with [NH4+]ext. For [NH4+]ext<N*ext, [aKG] increases and activates AmtB to the level needed to uphold [NH4+]int at N*int.
Quantitative description of these processes elucidates an intriguing integral feedback scheme. Integral feedback is an effective control scheme to stabilize a complex system to a desired set point (Leigh, 2004). Importantly, when the system deviates from the desired set point, a control variable integrates the deviation and activates a controller to reduce it, maintaining the system at the desired set point robustly. In the regulation of ammonium transport, aKG and AmtB serve as the control variable and controller, respectively. The aKG pool ([aKG]) is balanced primarily by the rate of nitrogen incorporation into biomass (Jbiomass, black arrow in Figure 5A), and ammonium assimilation rate (JGS, red arrow), that is,
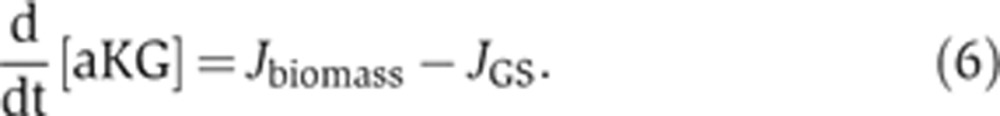 |
JGS is provided by the sum of the NH3 influx via diffusion (Jdiffusion, blue arrow) and the AmtB transport flux (JAmtB, green arrow), that is,
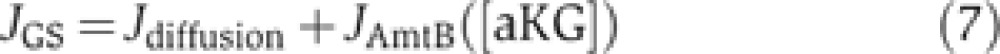 |
with JAmtB being an increasing function of [aKG] (Radchenko et al, 2010).
In a steady state, Equations (6) and (7) yield
As described above, JGS([NH4+]int) is obtained from 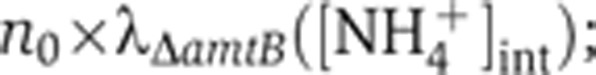 ; its form is sketched in Figure 5B based on the form of
; its form is sketched in Figure 5B based on the form of 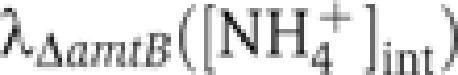 in Figure 2C. Under ammonium-replete conditions,
in Figure 2C. Under ammonium-replete conditions, 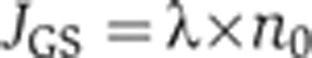 with the growth rate λ determined by other metabolic constraints (such as the carbon source). However, as the internal NH4+ concentration [NH4+]int decreases, JGS eventually decreases when [NH4+]int decreases below a certain level, N*int (the gray zone). Thus for the cell to maintain growth at the rate λ, it is necessary to keep
with the growth rate λ determined by other metabolic constraints (such as the carbon source). However, as the internal NH4+ concentration [NH4+]int decreases, JGS eventually decreases when [NH4+]int decreases below a certain level, N*int (the gray zone). Thus for the cell to maintain growth at the rate λ, it is necessary to keep 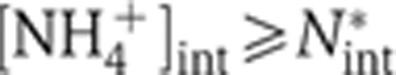 (above the gray zone in Figure 5B). This can be accomplished by passive diffusion alone (i.e., JAmtB=0) for sufficiently large external NH4+ concentration, [NH4+]ext, or by turning on the AmtB flux when the external NH4+ concentration is so low that [NH4+]int would drop below N*int by passive diffusion alone. The latter scenario can be mediated by aKG according to Equation (6) since any decrease in JGS due to [NH4+]int < N*int (Figure 5B) would result in aKG accumulation (control variable), which then activates AmtB (Gruswitz et al, 2007; Radchenko et al, 2010; Truan et al, 2010) (controller) until the steady-state condition defined by Equation (8) is restored.
(above the gray zone in Figure 5B). This can be accomplished by passive diffusion alone (i.e., JAmtB=0) for sufficiently large external NH4+ concentration, [NH4+]ext, or by turning on the AmtB flux when the external NH4+ concentration is so low that [NH4+]int would drop below N*int by passive diffusion alone. The latter scenario can be mediated by aKG according to Equation (6) since any decrease in JGS due to [NH4+]int < N*int (Figure 5B) would result in aKG accumulation (control variable), which then activates AmtB (Gruswitz et al, 2007; Radchenko et al, 2010; Truan et al, 2010) (controller) until the steady-state condition defined by Equation (8) is restored.
The robust maintenance of internal ammonium concentration and the abrupt onset of ammonium transport through AmtB
The above mode of regulation ensures that AmtB is activated only when [NH4+]int decreases below N*int. The steady-state concentrations of aKG and internal ammonium that determine the AmtB activity can be readily deduced from Equation (8) assuming a generic Hill form for the activation of JAmtB by aKG. For [NH4+]ext > N*ext, the passive diffusion alone can supply for enough nitrogen needed for cell growth and AmtB is inactive (JAmtB≈0). There, [aKG] remains at its basal level (cyan line in Figure 5C), and [NH4+]int changes linearly (purple line) with [NH4+]ext as described by Equation (8), together with Jdiffusion given by Equation (1). For [NH4+]ext < N*ext, AmtB is activated only to uphold [NH4+]int at N*int with the activity determined by Equation (8). [aKG] is raised to the level needed, while [NH4+]int remains at N*int; see Supplementary Figure 4 for details.
The feedback scheme described here ensures that [NH4+]int ≈ N*int while JAmtB increases for [NH4+]ext < N*ext, giving rise to three prominent features: (i) the robust maintenance of internal ammonium at the minimum level (N*int in Figure 5C) needed to sustain the growth is achieved autonomously without the need of fine-tuning parameters (the characteristics of an integral feedback control). (ii) A plot of JAmtB versus [NH4+]int must inevitably have an abrupt increase of JAmtB at N*int as shown in Figure 5D. Notably, neither of these features requires any cooperative molecular interactions. (iii) The abrupt activation of AmtB is not preset, but is determined by the onset of the decrease in JGS (N*int in Figure 5B and D); see Equation (6). As a result, AmtB activation is adjusted automatically to maintain rapid cell growth in different ammonium-limiting conditions.
The perfect maintenance of the internal NH4+ concentration for [NH4+]int<N*ext (horizontal purple line in Figure 5C) and infinitely sharp activation of AmtB (vertical line in Figure 5D) in the simple feedback model described here resemble qualitatively the data shown in Figure 3C and D. However, the actual maintenance of the internal NH4+ is not perfect but has minor declines (Figure 3C); also the activation of AmtB is not infinitely sharp but occurs over a narrow range of [NH4+]int (Figure 3D). These modest blurring effects can be readily accounted for by the known (weak) effect of the stimulation of JGS by aKG (Jiang et al, 1998b, c; Reitzer, 2003). Although we described that JGS depends only on [NH4+]int with a well-defined N*int (Figure 5B) in our simple model, it is known that aKG has a marginal effect on JGS via changes in GS expression and specific activity (Jiang et al, 1998b, c; Reitzer, 2003). Thus, when the aKG concentration increases for [NH4+]ext < N*ext (cyan curve in Figure 5C), the resulting weak increase in JGS allows minor declines in [NH4+]int below N*int without slowing down growth. We included this effect in the more elaborate analysis described in Supplementary information (Supplementary Equations S24–S39). The extended model adequately explains the data in Figure 3C and D (black lines). It showed that the slopes of the minor decline in the maintenance of internal NH4+ and in the activation of AmtB (Figure 3C and D) were determined by one parameter, reflecting the response of GS expression and activity relative to that of AmtB activity as aKG concentration changes.
Tight coordination of GS and AmtB activities
For [NH4+]ext>N*ext, [NH4+]int (purple curve in Figure 5C) changes with [NH4+]ext while [aKG] (cyan curve) remains constant. For [NH4+]ext<N*ext, [aKG] changes while [NH4+]int remains constant. The interlaced dependence of [NH4+]int and [aKG] on the external NH4+ concentration highlights the tight coordination of the two lines of defense against ammonium shortage. For the external NH4+ concentration above N*ext, glutamine (via the obligatory relation with [NH4+]int described by Equation (5)) can be an effective controller of the nitrogen status, increasing the capacity to assimilate the gaseous NH3 diffusing into the cytoplasm by upregulating increasing GS expression and activity (Figures 1F and 2D; Reitzer, 2003). However, for the external NH4+ concentration below N*ext, [NH4+]int is kept frozen at its maintenance level N*int (purple line in Figure 5C). There, it is aKG which senses the nitrogen status and activates transport by AmtB (cyan line in Figure 5C and green line in Figure 5D).
Glutamine has long been established as a signal of the internal nitrogen status (Ikeda et al, 1996; Reitzer, 2003). aKG is a well-known signal of internal nitrogen status for other organisms, such as cyanobacteria (Muro-Pastor et al, 2001). Here, we established that aKG is also a signal of the internal nitrogen status at very low ammonium concentrations. The key point here is that the two distinct signaling pathways are needed to provide the seamless shift of the need-based ammonium acquisition strategy, from the upregulation of assimilation by GS to the upregulation of transport by AmtB.
Perspective
As the ambient ammonium level is reduced, cells step up the transport of ammonium and keep growing as fast as permitted by other (e.g., carbon) conditions, in spite of clues of a possible nitrogen crisis (i.e., the depletion of ambient ammonium). Despite this seemingly lavish strategy, however, the integral feedback scheme ensures that NH4+ is not transported more than necessary. This tight control may be necessary in light of a very high cost of ammonium transport, estimated to be a substantial part of the cellular energy budget (see Supplementary Figure 5 for the estimate of the associated energy cost), due to the obligatory futile NH3 cycle (Figure 1B). This result is directly applicable to microorganisms in the marine environment in which ammonium concentration is very low (Rees et al, 2006). Furthermore, similar strategies may underlie how organisms deal with other essential but highly permeable nutrients.
Materials and methods
Strain and media
All strains were derived from Escherichia coli K12 strain NCM3722 (Soupene et al, 2003; Lyons et al, 2011) as listed in Supplementary Table 1 and detailed in Supplementary Methods. MOPS-buffered minimal media (pH 7.4) were used for cell growth as described by Neidhardt et al (1974). For carbon sources, glycerol (0.4% w/v), glucose (0.4% w/v), or 10 mM each of glucose-6 phosphate and gluconate were used. For nitrogen sources, different concentrations of NH4Cl were used as specified. All the media were filtered through 0.45 μm filters.
Microfluidic device fabrication
Microfluidic devices were fabricated by molding silicone elastomer (PDMS Slygard 184, Dow Corning) to the master molds (Groisman et al, 2005), consisted of two layers of cross-linked epoxy (Su-8s, MicroChem) patterned by negative phototransparency masks (FineLine Imaging, CO) on silicon wafers (Supplementary Figure 1A). The layer for growth chambers (∼1.5 μm) was deposited first using SU-8 2002 and processed according to manufacturer’s manual. On top of the first layer, the second layer for channels (∼25 μm) was deposited using SU-8 2025 (side view).
PDMS was mixed 1:15 ratio of catalyst and resin, poured to the master mold, degassed for 30 min and cured in an 80°C oven for 90 min. After the elastomer was peeled off the mold, inlet and outlet holes were punched. Then, the device was boiled in 1% HCl solution for 1 h, attached to No. 1 microscope cover glass (pre-cleaned with ethanol) and baked in the 80°C oven overnight for maximum binding.
Cell growth in microfluidic chamber
All cultures were grown at 37°C. The cells were first cultured in LB broth in 20 mm test tubes with shaking (250 r.p.m.) in a water bath (New Brunkswick Scientific). After 5–6 h of growth, they were transferred to a MOPS medium containing the selected carbon source with 20 mM of NH4Cl and grew overnight in the same condition (pre-culture). The pre-culture was inoculated with fewer than 105 cells/ml so that cells were in an exponential phase at the time of experiment. The next morning, the pre-culture was diluted to a fresh growth medium containing the same concentration of NH4Cl as in the experimental growth medium, and 0.1% BSA (bovine serum albumin, Sigma; BSA prevents cells from binding to surfaces of microfluidic devices) to an optical density (OD600) of 0.005–0.01 as measured on a Genesys20 spectrophotometer (Thermo-Fisher) with the standard sample holder (16.100-Q-10/Z8.5, Starna Cells Incl; ∼200 μl per measurement). To load cells into the microfluidic device, the diluted pre-culture was pressured at 1–2 psi to the outlet of the device (Supplementary Figure 1A). After the channel and growth chambers were completely filled with the pre-culture, the culture was removed and a fresh experimental growth medium was introduced from the inlet of the device. Pressured at ∼0.5 psi, the growth medium flowed actively through a channel at the flow rate of 20–50 μm/s. The microfluidic device was fixed onto a motorized microscope stage equipped with autofocus (Proscan II, Prior) in a fluorescent microscope (Nikon TI-U) that were housed in a microscope incubator (InVivo Scientific). After 3–4 generations of unperturbed growth at 37°C in the growth chambers, the pressure in the inlet was increased to 3–4 psi to introduce an active flow in the chambers and wash most of the cells out of the chambers. When only a few cells were left in each chamber, the pressure was removed. Then, −0.5 to −1.5 of vacuum was applied from the outlet to bring down the ceiling of the growth chambers and loosely sandwich the trapped cells (side view). Since the vacuum induces the fresh medium flow in a channel (flow rate of 50–100 μm/s), no additional pressure was applied from the inlet. The 20–40 positions that contain a single cell in the view (∼100 μm × ∼100 μm) of charge-coupled device (CCD) camera (Clara, Andor) with a 60 × phase-contrast objective were saved in the motorized stage. Phase-contrast images of the growing cells for each position were recorded two times per doubling. Fluorescence images were taken once per doubling, immediately after phase-contrast images for each position with a Xenon excitation lamp (Sutter Inst.). The images were analyzed with a custom-built Matlab program. First, the program identified pixel positions occupied by cells with phase-contrast images, obtained a size of a growing colony in time series for each position and calculated the growth rate of the colony. In order to get fluorescence levels, fluorescence intensities over the cell-occupying area identified by phase-contrast images were averaged.
Supplementary Material
Acknowledgments
We are grateful to Sydney Kustu for extensive advice and encouragement throughout this research, and to Jason Hall, Peter Lenz, Jilong Wang and Mike Merrick for discussions. MK also acknowledges Mark Polinkovsky and Edgar Gutierrez for their help with the fabrication of microfluidic chambers. This work was supported by the NIH through grant (R01-GM038361) to Sydney Kustu, the Human Frontiers in Science Program (RGP0022), and by the NSF through grant PHY-1058793 to TH.
Author contributions: MK, DY, AG and TH designed research; MK and HO performed the experiments; MK and TH developed the models and analyzed the data; ZZ constructed strains; MK and TH wrote the paper with contribution from all.
Footnotes
The authors declare that they have no conflict of interest.
References
- Alibhai M, Villafranca JJ (1994) Kinetic and mutagenic studies of the role of the active site residues Asp-50 and Glu-327 of Escherichia coli glutamine synthetase. Biochemistry 33: 682–686 [DOI] [PubMed] [Google Scholar]
- Andrade SLA, Einsle O (2007) The Amt/Mep/Rh family of ammonium transport proteins. Mol Membr Biol 24: 357–365 [DOI] [PubMed] [Google Scholar]
- Atkinson MR, Blauwkamp TA, Bondarenko V, Studitsky V, Ninfa AJ (2002) Activation of the glnA, glnK, and nac promoters as Escherichia coli undergoes the transition from nitrogen excess growth to nitrogen starvation. J Bacteriol 184: 5358–5363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blauwkamp TA, Ninfa AJ (2003) Antagonism of PII signalling by the AmtB protein of Escherichia coli. Mol Microbiol 48: 1017–1028 [DOI] [PubMed] [Google Scholar]
- Boussiba S, Dilling W, Gibson J (1984) Methylammonium transport in Anacystis nidulans R-2. J Bacteriol 160: 204–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commichau FM, Forchhammer K, Stulke J (2006) Regulatory links between carbon and nitrogen metabolism. Curr Opin Microbiol 9: 167–172 [DOI] [PubMed] [Google Scholar]
- Coutts G, Thomas G, Blakey D, Merrick M (2002) Membrane sequestration of the signal transduction protein GlnK by the ammonium transporter AmtB. EMBO J 21: 536–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucette CD, Schwab DJ, Wingreen NS, Rabinowitz JD (2011) Alpha-ketoglutarate coordinates carbon and nitrogen utilization via enzyme I inhibition. Nat Chem Biol 7: 894–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong RN, Kim KS, Yoshihara C, Inwood WB, Kustu S (2007) The W148L substitution in the Escherichia coli ammonium channel AmtB increases flux and indicates that the substrate is an ion. Proc Natl Acad Sci USA 104: 18706–18711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groisman A, Lobo C, Cho HJ, Campbell JK, Dufour YS, Stevens AM, Levchenko A (2005) A microfluidic chemostat for experiments with bacterial and yeast cells. Nat Methods 2: 685–689 [DOI] [PubMed] [Google Scholar]
- Gruswitz F, O’Connell J, Stroud RM (2007) Inhibitory complex of the transmembrane ammonia channel, AmtB, and the cytosolic regulatory protein, GlnK, at 1.96 A. Proc Natl Acad Sci USA 104: 42–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschman J, Wong PK, Sei K, Keener J, Kustu S (1985) Products of nitrogen regulatory genes ntrA and ntrC of enteric bacteria activate glnA transcription in vitro: evidence that the ntrA product is a sigma factor. Proc Natl Acad Sci USA 82: 7525–7529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda TP, Shauger AE, Kustu S (1996) Salmonella typhimurium apparently perceives external nitrogen limitation as internal glutamine limitation. J Mol Biol 259: 589–607 [DOI] [PubMed] [Google Scholar]
- Javelle A, Severi E, Thornton J, Merrick M (2004) Ammonium sensing in Escherichia coli. Role of the ammonium transporter AmtB and AmtB-GlnK complex formation. J Biol Chem 279: 8530–8538 [DOI] [PubMed] [Google Scholar]
- Jiang P, Peliska JA, Ninfa AJ (1998a) Enzymological characterization of the signal-transducing uridylyltransferase/uridylyl-removing enzyme (EC 2.7.7.59) of Escherichia coli and its interaction with the PII protein. Biochemistry 37: 12782–12794 [DOI] [PubMed] [Google Scholar]
- Jiang P, Peliska JA, Ninfa AJ (1998b) Reconstitution of the signal-transduction bicyclic cascade responsible for the regulation of Ntr gene transcription in Escherichia coli. Biochemistry 37: 12795–12801 [DOI] [PubMed] [Google Scholar]
- Jiang P, Peliska JA, Ninfa AJ (1998c) The regulation of Escherichia coli glutamine synthetase revisited: Role of 2-ketoglutarate in the regulation of glutamine synthetase adenylylation state. Biochemistry 37: 12802–12810 [DOI] [PubMed] [Google Scholar]
- Kingdon HS, Shapiro BM, Stadtman ER (1967) Regulation of glutamine synthetase. 8. ATP: glutamine synthetase adenylyltransferase, an enzyme that catalyzes alterations in regulatory properties of glutamine synthetase. Proc Natl Acad Sci USA 58: 1703–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner D (1985) Bacterial ammonium transport. FEMS Microbiol Rev 32: 87–100 [Google Scholar]
- Leigh JR (2004) Control Theory Institution of Electrical Engineers, London, UK [Google Scholar]
- Loque D, von Wiren N (2004) Regulatory levels for the transport of ammonium in plant roots. J Exp Bot 55: 1293–1305 [DOI] [PubMed] [Google Scholar]
- Lyons E, Freeling M, Kustu S, Inwood W (2011) Using genomic sequencing for classical genetics in E. coli K12. PLoS ONE 6: e16717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek TD, Villafranca JJ (1980) Kinetic mechanism of Escherichia coli glutamine synthetase. Biochemistry 19: 5513–5519 [DOI] [PubMed] [Google Scholar]
- Muro-Pastor MI, Reyes JC, Florencio FJ (2001) Cyanobacteria perceive nitrogen status by sensing intracellular 2-oxoglutarate levels. J Biol Chem 276: 38320–38328 [DOI] [PubMed] [Google Scholar]
- Neidhardt FC, Bloch PL, Smith DF (1974) Culture medium for enterobacteria. J Bacteriol 119: 736–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano H, Hwa T, Lenz P, Yan D (2010) Reversible adenylylation of glutamine synthetase is dynamically counterbalanced during steady state growth of Escherichia coli. J Mol Biol 404: 522–536 [DOI] [PubMed] [Google Scholar]
- Radchenko MV, Thornton J, Merrick M (2010) Control of AmtB-GlnK complex formation by intracellular levels of ATP, ADP, and 2-oxoglutarate. J Biol Chem 285: 31037–31045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees AP, Woodward EMS, Joint I (2006) Concentrations and uptake of nitrate and ammonium in the Atlantic ocean between 60 degrees N and 50 degrees S. Deep Sea Res Part II Top Stud Oceanogr 53: 1649–1665 [Google Scholar]
- Reitzer L (2003) Nitrogen assimilation and global regulation in Escherichia coli. Annu Rev Microbiol 57: 155–176 [DOI] [PubMed] [Google Scholar]
- Reitzer LJ, Magasanik B (1986) Transcription of glnA in Escherichia coli is stimulated by activator bound to sites far from the promoter. Cell 45: 785–792 [DOI] [PubMed] [Google Scholar]
- Simon SA, Gutknecht J (1980) Solubility of carbon dioxide in lipid bilayer membranes and organic solvents. Biochim Biophys Acta 596: 352–358 [DOI] [PubMed] [Google Scholar]
- Soupene E, He LH, Yan DL, Kustu S (1998) Ammonia acquisition in enteric bacteria: Physiological role of the ammonium/methylammonium transport B (AmtB) protein. Proc Natl Acad Sci USA 95: 7030–7034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soupene E, Lee H, Kustu S (2002) Ammonium/methylammonium transport (Amt) proteins facilitate diffusion of NH3 bidirectionally. Proc Natl Acad Sci USA 99: 3926–3931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soupene E, van Heeswijk WC, Plumbridge J, Stewart V, Bertenthal D, Lee H, Prasad G, Paliy O, Charernnoppakul P, Kustu S (2003) Physiological studies of Escherichia coli strain MG1655: growth defects and apparent cross regulation of gene expression. J Bacteriol 185: 5611–5626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truan D, Huergo LF, Chubatsu LS, Merrick M, Li XD, Winkler FK (2010) A new P(II) protein structure identifies the 2-oxoglutarate binding site. J Mol Biol 400: 531–539 [DOI] [PubMed] [Google Scholar]
- Walter A, Gutknecht J (1986) Permeability of small nonelectrolytes through lipid bilayer membranes. J Membr Biol 90: 207–217 [DOI] [PubMed] [Google Scholar]
- Woolfolk CA, Stadtman ER (1964) Cumulative feedback inhibition in multiple end product regulation of glutamine synthetase activity in Escherichia coli. Biochem Biophys Res Commun 17: 313–319 [Google Scholar]
- Woolfolk CA, Stadtman ER (1967) Regulation of glutamine synthetase 3. Cumulative feedback inhibition of glutamine synthetase from Escherichia coli. Arch Biochem Biophys 118: 736–755 [DOI] [PubMed] [Google Scholar]
- Wulff K, Mecke D, Holzer H (1967) Mechanism of enzymatic inactivation of glutamine synthetase from E. coli. Biochem Biophys Res Commun 28: 740–745 [DOI] [PubMed] [Google Scholar]
- Yan D, Lenz P, Hwa T (2011) Overcoming fluctuation and leakage problems in the quantification of intracellular 2-oxoglutarate levels in Escherichia coli. Appl Environ Microbiol 77: 6763–6771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Doucette CD, Fowler WU, Feng XJ, Piazza M, Rabitz HA, Wingreen NS, Rabinowitz JD (2009) Metabolomics-driven quantitative analysis of ammonia assimilation in E. coli. Mol Syst Biol 5: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.