Abstract
A reversible covalent bond exchange of thiols, β-sulfido-α,β-unsaturated carbonyls, and dithianes, has been studied in DMSO and D2O/DMSO mixtures. The equilibrium between thiols and β-sulfido-α,β-unsaturated carbonyls is obtained within a few hours, while the equilibration starting with the β-dithiane carbonyls and thiols takes few days. This time scale makes the system ideal for utilization in dynamic combinatorial chemistry.
The field of Dynamic Combinatorial Chemistry (DCC) has expanded dramatically since it was first established over a decade ago.1 The popularity of DCC is due to the simplicity with which hosts and ligands,2 or enzyme inhibitors,3 can be discovered with minimal synthetic effort as compared to traditional approaches. There are, however, only a handful of dynamic covalent motifs that exchange components within a few hours or days, the most common being disulfide4, transimination,5 as well as hydrazone6 and thioester exchange.7 Thus, there is interest in expanding the ‘tool kit’ available to the DCC community by developing new dynamic covalent chemistry.
A lesser used reaction for DCC is the thia-Michael addition, which takes approximately a week to achieve equilibria in slightly basic conditions. For example, Greaney used this reaction for the discovery of inhibitors for glutathione-S-transferase.3d Recently, Taunton used α-cyano acrylamides as the conjugate acceptor to target noncatalytic cysteines, thereby creating kinase inhibitors.8 In a slightly different approach, Che has used thia-Michael additions to β-sulfido-α,β-unsaturated carbonyls (Vinyl Sulfides Carbonyls, VSCs) to modify cysteines,9 and as sensors for cysteine.10 He found the reaction to be dynamic, but he did not explore the mechanistic details of the exchange.
Herein, we present experiments aimed at uncovering the timescale and mechanism of thia-Michael additions to VSCs, thereby creating new VSCs, and we report the formation of β-dithiane carbonyls. We find that the thia-conjugate addition is kinetically fast to exchange thiols and VSCs, typically complete in less than an hour, while equilibration with the β-dithiane carbonyls (BdTCs) can take as long as a few days. Importantly, the reactions proceed in DMSO and DMSO water mixtures at physiological pH.
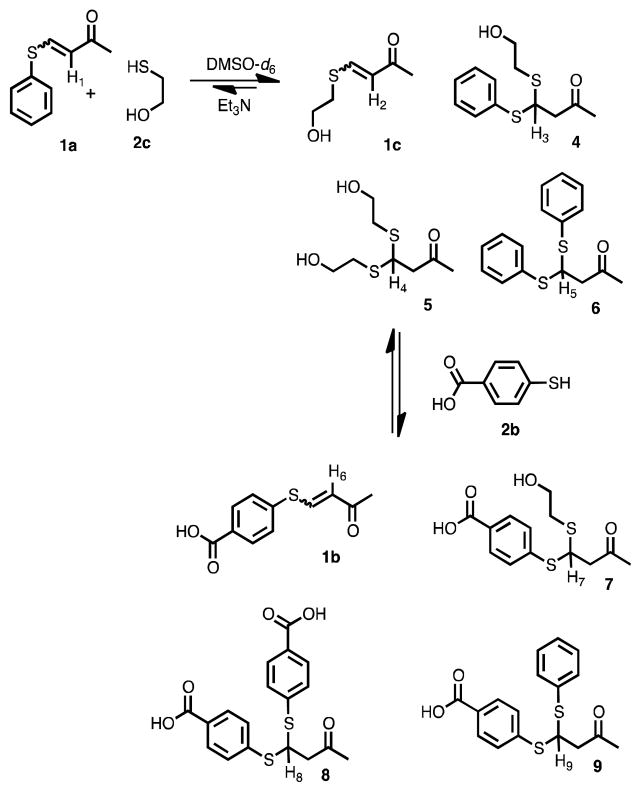
To examine the reversible covalent bond exchange, a series of β-sulfido-α,β-unsaturated ketones (i.e. VSCs) were required. The syntheses of 1a-c were achieved by treating the butynone 3 with one equivalent of thiols 2a-c in good yields (Scheme 1). The conjugate addition yields both E and Z isomers, but the Z isomer dominates, as has been reported earlier.11
Scheme 1.
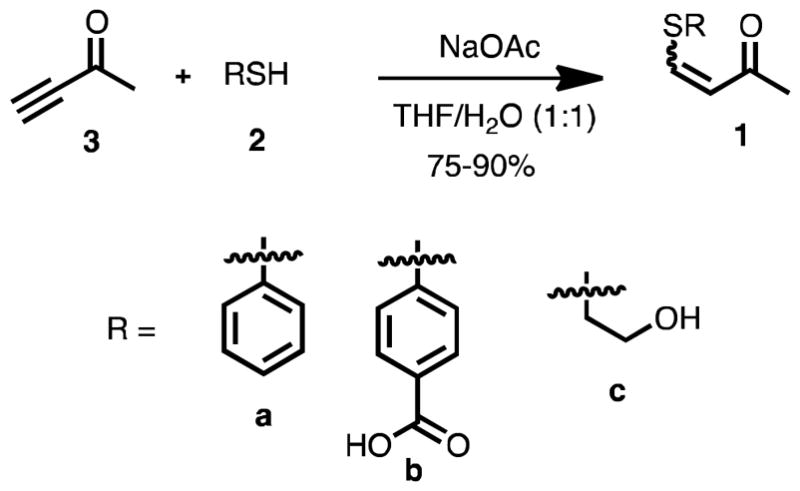
Synthesis of 1a-c.
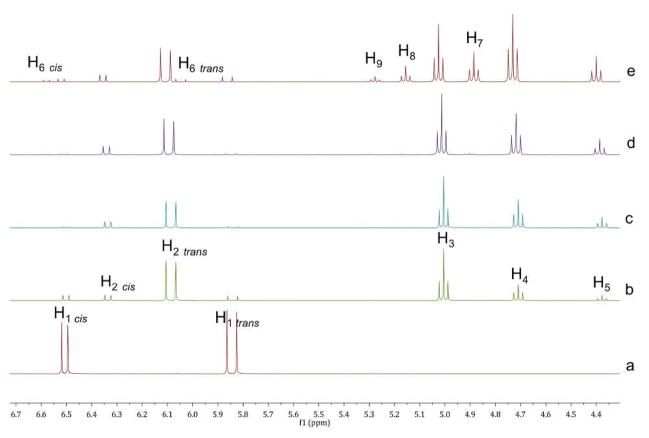
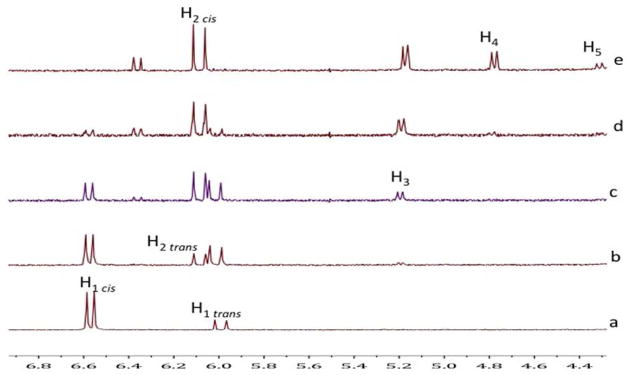
Thiol exchange with the VSCs was first carried out in DMSO-d6 and monitored by 1H NMR spectroscopy. The addition of 1 equivalent of 2c with 1a in the presence of Et3N (Scheme 2) quickly showed an equilibrating mixture of VSCs 1a and 1c (doublets between 5.8 and 6.8 ppm), while within an hour the BdTCs 4, 5, 6 (triplets at 4.7, 4.4 and 5.1 ppm respectively)12 were present, with all five species in a ratio of 3:33:19:8:37, respectively, as determined by 1H NMR integration (for the VSCs, the ratios are inclusive of both E and Z isomers). The spectra did not further change with time, indicative that the mixture had reached equilibrium (Figure 1). Although, thiols are known to oxidize in DMSO under basic conditions, we found that the equibrium between the VSC’s and the thiols is attained faster (hours) than the disulfide-thiol formation (days).13
Scheme 2.
Exchange studies with vinyl-sulfide 1a and thiols 2c and 2b.
Figure 1.
1H NMR monitoring. (a) Partial spectra of 1a in DMSO-d6 and Et3N. (b) 5 minutes after the the addition of 2c. (c) 50 minutes after the addition of 2c. (d) 5 minutes after the addition of 2b. (e) 14 hours after the addition of 2b. The labels correspond to the H’s in Scheme 2.
The subsequent addition of 4-mercaptobenzoic acid (2b) to the equilibrated mixture further generated 1b, 7, 8 and 9 as depicted in Scheme 2, while the first products decreased but remained. This confirmed that all the species are interconverting. Interestingly, we find an equilibrating mixture of VSCs and BdTCs, even though excess thiols are present in the solution. Thus, the thermodynamic stability of vinyl sulfide carbonyls and β-dithiane carbonyls are comparable under the experimental conditions.
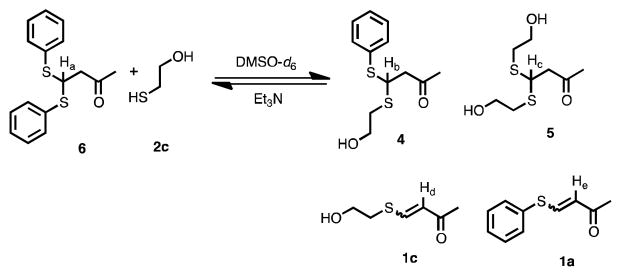
We sought to confirm the dynamic nature of the BdTCs by treating pure 6 with several equivalents of 2c in DMSO-d6 and Et3N, monitoring the reaction by 1H NMR spectroscopy (Scheme 3). Within an hour, the formation of 1a, 1c, 4 and 5 was confirmed by the appearance of proton resonances at δ = 6.0, 4.6, 4.3 ppm, respectively.14 While acid catalyzed dithianes exchange was previously reported by Sutton and co-workers,15 to our knowledge this is the first report of a base catalyzed dithiane exchange, which is undoubtedly due to the β-keto group.
Scheme 3.
Base catalyzed thioacetal exchange.
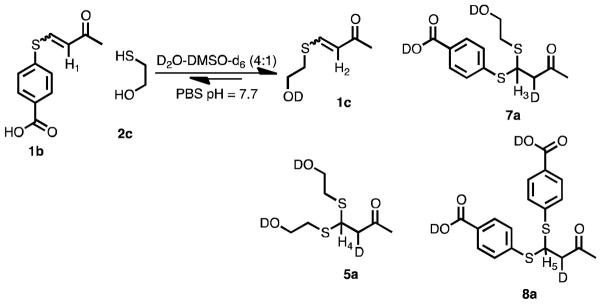
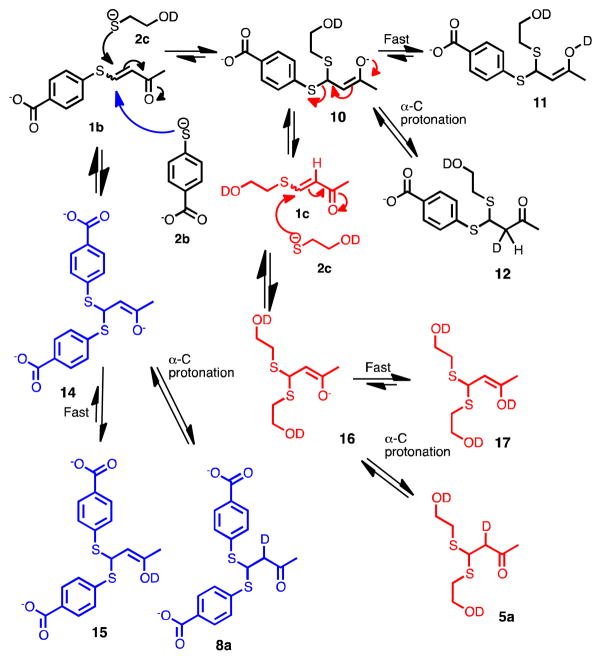
To elucidate the mechanism of the dynamic exchange of thiols, VSCs, and BdTCs, we carried out studies using D2O:DMSO-d6 (4:1) at pD 7.7 in phosphate buffer (Scheme 4), and the reaction was monitored by 1H NMR spectroscopy and LCMS.16 This solvent system allows one to follow the formation of any enol or enolate intermediates because they will necessarily be deuterated. When 1 equivalent of 2c was added to a solution of 1b, a proton resonance at δ = 6.1 ppm appeared in less than 2 minutes, which is indicative of formation of the product 1c (Figure 2). A resonance at δ = 5.2 ppm appeared a few minutes later, assigned to the methine proton of product 8a (α-deuterio-8), and exists as a doublet rather a triplet due to mono-deutration of the α–carbon. After 2 hours, products 7a and 5a were observed (see Scheme 4), the corresponding β-proton resonances were at δ = 4.8 and 4.3 ppm, respectively.
Scheme 4.
Thiol scrambling in D2O:DMSO-d6 (4:1) in PBS and deuteration of the α-carbon.
Figure 2.
1H NMR monitoring of thiol scrambling in D2O:DMSO-d6 (4:1) in phosphate buffer solution (a) 1b. (b) 2 mins. after the addition of 2c. (c) 10 mins after the addition of 2c. (d) 1 hour after the addition of 2c. (e) 2 hours after the addition of 2c. The labels correspond to H’s in Scheme 4.
The results of Figures 1 and 2 show that the scrambling of thiols and VSCs occurs faster than the formation, as well as the scrambling, of BdTCs. We expected the BdTCs to be the intermediates through which the thiols and VSCs would exchange. Hence, we wondered -How can VSCs exchange thiols faster than BdTCs if BdTCs are the intermediates? Therefore, the results show that these species must not be the correct intermediates.
Instead, the results force us to the following conclusions (Scheme 4). The attack of thiolate 2c at the β-position of 1b is a slow step. The attack generates an enolate 10, which is ultimately deuterated at the α-carbon to create 12. However, it is well known that protonation of enolates, such as 10, occurs faster on oxygen.17 Hence, enol 11 would be the first formed neutral intermediate. The enol can regenerate the enolate leading to a thiolate leaving group departure, which can rapidly scramble thiols and the stereochemistry of the alkene as observed. Any other VSCs would undergo the same fate, leading to enols 15 and 17. However, ultimately protonation at the α-carbon gives 12, 5a, and 8a, which must revert slowly to the enolates because BdTC scrambling is the slowest process.
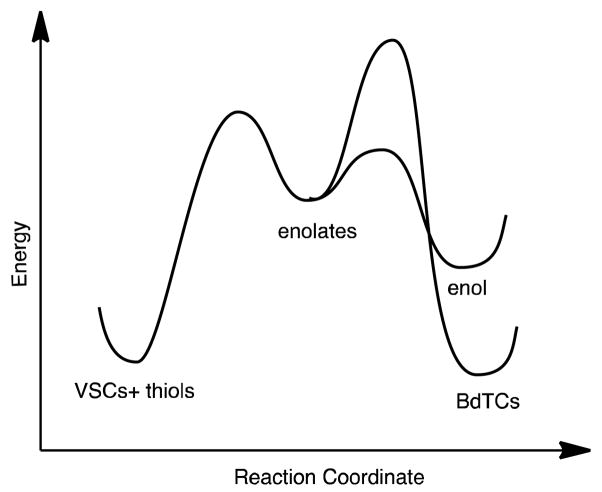
From the conclusions above, a qualitative energy profile diagram can be derived as shown in the Figure 3. In order for the BdTCs to be the slowest species to exchange thiols, one must conclude that the deprotonation of the α-carbon is the slowest step in the entire sequence, including the original conjugate addition of the thiols. The relative energy levels of the enolates and enols fall into place as shown. Future computational studies can put quantitative numbers on this qualitative diagram.
Figure 3.
Reaction coordinate diagram showing relative energies of reactions, intermediates, products, and barriers, that are consistent with the experimental observations for rates of conjugate additions, thiol scrambling, and dithiane exchange.
In summary, we report that β-sulfido-α,β-unsaturated carbonyls will reversibly and rapidly exchange thiols in organic and aqueous media, ultimately with an equilibrated mixture of thiols, vinyl sulfide carbonyls, and β-dithiane carbonyls. These later species equilibrate the slowest, leading to the conclusion that enols are the neutral intermediates through which vinyl sulfide carbonyls exchange thiols rather than dithane carbonyls.
Supplementary Material
Scheme 5.
Mechanism for the thiol exchange with 1b.
Acknowledgments
Support for this work from National Institute of Health (R01GM065515) and the Welch Foundation (F-1151) is gratefully acknowledged. Karin Keller and Zhong Ye are thanked for the assistance with LCMS data. Unai Cossio is acknowledged for reproducing the experiments.
Footnotes
Supporting Information Available. General experimental procedures and spectroscopic data for all the compounds, experimental protocol for thiol exchange and LCMS traces are mentioned in the Supporting Information. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Reviews: Moulin E, Cormosw G, Giuseppone N. Chem Soc Rev. 2012;41:1031. doi: 10.1039/c1cs15185a.Cougnon FBL, Sanders JKM. Acc Chem Res. 2012 doi: 10.1021/ar200240m. ASAP.Corbett PT, Vial L, West KR, Wietor J-L, Sanders JKM, Otto S. Chem Rev. 2006;106:3652. doi: 10.1021/cr020452p.Ladame S. Org Biomol Chem. 2008;6:219. doi: 10.1039/b714599c.Lehn JM. Chem Soc Rev. 2007;36:151. doi: 10.1039/b616752g.Rowan SJ, Cantrill SJ, Cousins GRL, Sanders JKM, Stoddart JF. Angew Chem Int Ed. 2002;41:898. doi: 10.1002/1521-3773(20020315)41:6<898::aid-anie898>3.0.co;2-e.
- 2.(a) Reek JNH, Otto S. Dynamic combinatorial chemistry. Wiley-VCH; Weinheim: 2010. [Google Scholar]; (b) Bugaut A, Jantos K, Wietor JL, Rodrigues R, Sanders JKM, Balasubramanian S. Angew Chem Int Ed. 2008;47:2677. doi: 10.1002/anie.200705589. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Ludlow RF, Otto S. J Am Chem Soc. 2008;130:12218. doi: 10.1021/ja803317k. [DOI] [PubMed] [Google Scholar]; (d) Milanesi L, Hunter CA, Sedelnikova SE, Waltho JP. Chem Eur J. 2008;12:1081. doi: 10.1002/chem.200500357. [DOI] [PubMed] [Google Scholar]
- 3.(a) Nguyen R, Huc I. Angew Chem, Int Ed. 2001;40:1774. [PubMed] [Google Scholar]; (b) Erlanson DA, Braisted AC, Raphael DR, Randal M, Stroud RM, Gordon EM, Wells JA. Proc Natl Acad Sci USA. 2000;97:9367. doi: 10.1073/pnas.97.17.9367. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Krasinski A, Radic AZ, Manetsch R, Raushel J, Taylor P, Sharpless KB, Kolb HC. J Am Chem Soc. 2005;127:6686. doi: 10.1021/ja043031t. [DOI] [PubMed] [Google Scholar]; (d) Shi B, Stevenson R, Campopiano DJ, Greaney MF. J Am Chem Soc. 2006;128:8459. doi: 10.1021/ja058049y. [DOI] [PubMed] [Google Scholar]
- 4.(a) Otto S, Furlan RLE, Sanders JKM. J Am Chem Soc. 2000;122:12063. [Google Scholar]; (b) Krishnan-Ghosh Y, Balasubramanian S. Angew Chem, Int Ed. 2003;42:2171. doi: 10.1002/anie.200250551. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Otto S, Kubik S. J Am Chem Soc. 2003;125:780. doi: 10.1021/ja0351589. [DOI] [PubMed] [Google Scholar]; (d) West KR, Bake KD, Otto S. Org Lett. 2005;7:2615. doi: 10.1021/ol0507524. [DOI] [PubMed] [Google Scholar]
- 5.(a) Huc I, Lehn JM. Proc Nat Acad Sci USA. 1997;94:2106. doi: 10.1073/pnas.94.6.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hochgurtel M, Kroth H, Piecha D, Hofmann MW, Nicolau C, Krause S, Schaaf O, Sonnenmoser G, Eliseev AV. Proc Nat Acad Sci USA. 2002;99:3382. doi: 10.1073/pnas.052703799. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Gerber-Lemaire S, Popowycz F, Rodriguez-Garcia E, Asenjo ATC, Robina I, Vogel P. Chem Bio Chem. 2002;3:466. doi: 10.1002/1439-7633(20020503)3:5<466::AID-CBIC466>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]; (d) Ramstrom O, Lohmann S, Bunyapaiboonsri T, Lehn JM. Chem Eur J. 2004;10:1711. doi: 10.1002/chem.200305551. [DOI] [PubMed] [Google Scholar]; (e) Bugaut A, Toulme AJ-J, Rayner B. Angew Chem, Int Ed. 2004;43:3144. doi: 10.1002/anie.200454041. [DOI] [PubMed] [Google Scholar]; (f) Zameo S, Vauzeilles B, Beau JM. Angew Chem, Int Ed. 2005;44:965. doi: 10.1002/anie.200462150. [DOI] [PubMed] [Google Scholar]
- 6.(a) Beeren SR, Sanders JKM. J Am Chem Soc. 2011;133:3804. doi: 10.1021/ja200130h. [DOI] [PubMed] [Google Scholar]; (b) Simpson MG, Pittelkow M, Watson SP, Sanders JKM. Org Biomol Chem. 2010;8:1181. doi: 10.1039/b917146k. [DOI] [PubMed] [Google Scholar]
- 7.(a) Ghosh S, Ingerman LA, Frye AG, Lee SJ, Gagne MJ, Waters ML. Org Lett. 2010;12:1860. doi: 10.1021/ol1004752. [DOI] [PubMed] [Google Scholar]; (b) Larsson R, Pei Z, Ramstrom O. Angew Chem Int Ed. 2004;43:3716. doi: 10.1002/anie.200454165. [DOI] [PubMed] [Google Scholar]
- 8.Serafimova IM, Pufall MA, Krishnan S, Duda K, Cohen MS, Maglathlin RL, McFarland JM, Miller RM, Frodin M, Taunton T. Nat Chem Biol. 2012;8:471. doi: 10.1038/nchembio.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shiu HY, Chan TY, Ho CM, Liu Y, Wong MK, Che CM. Chem Eur J. 2009;15:3839. doi: 10.1002/chem.200800669. [DOI] [PubMed] [Google Scholar]
- 10.Shiu HY, Chong HC, Leung YC, Wong MK, Che CM. Chem Eur J. 2010;16:3308. doi: 10.1002/chem.200903121. [DOI] [PubMed] [Google Scholar]
- 11.(a) Truce WE, Tichenor GJW. J Org Chem. 1972;37:2391. [Google Scholar]; (b) Halphen PD, Owen TC. J Org Chem. 1973;38:3507. [Google Scholar]
- 12.The assignments were made based on the 1H NMR of 5 and 6. The dithiane 6 was synthesized and purified, while the dithiane 5 was synthesized in NMR tube from 1c and 2c in DMSO-d6 and Et3N. See supporting information for the 1H NMR spectra.
- 13.Singh R, Whitesides GM. J Am Chem Soc. 1990;112:1191. [Google Scholar]
- 14.See supporting information, Figure S7.
- 15.Sutton LR, Donaubauer WA, Hampel F, Hirsch A. Chem Commun. 2004:1758. doi: 10.1039/b401021c. [DOI] [PubMed] [Google Scholar]
- 16.The reaction for LCMS was carried out in H2O/DMSO (4:1) and diluted with methanol. The LCMS traces are included in the supporting information.
- 17.Bernasconi CF, Ni JX. J Org Chem. 1994;59:4910. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.