Abstract
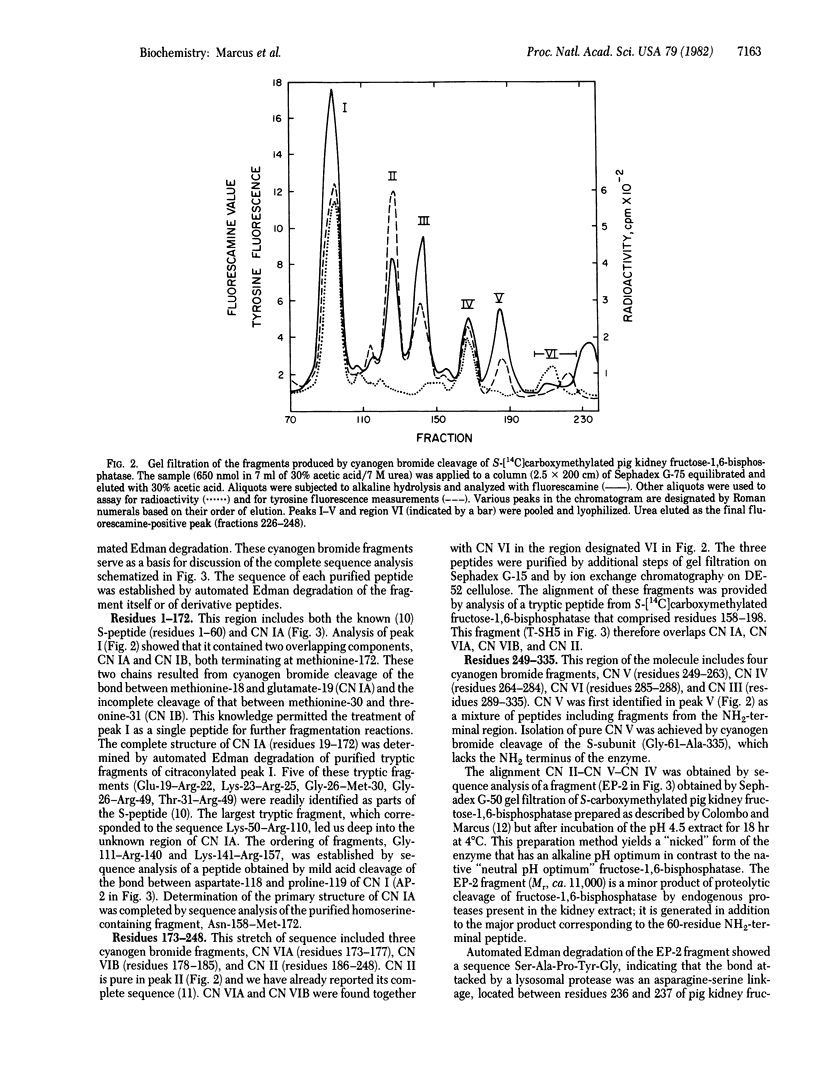
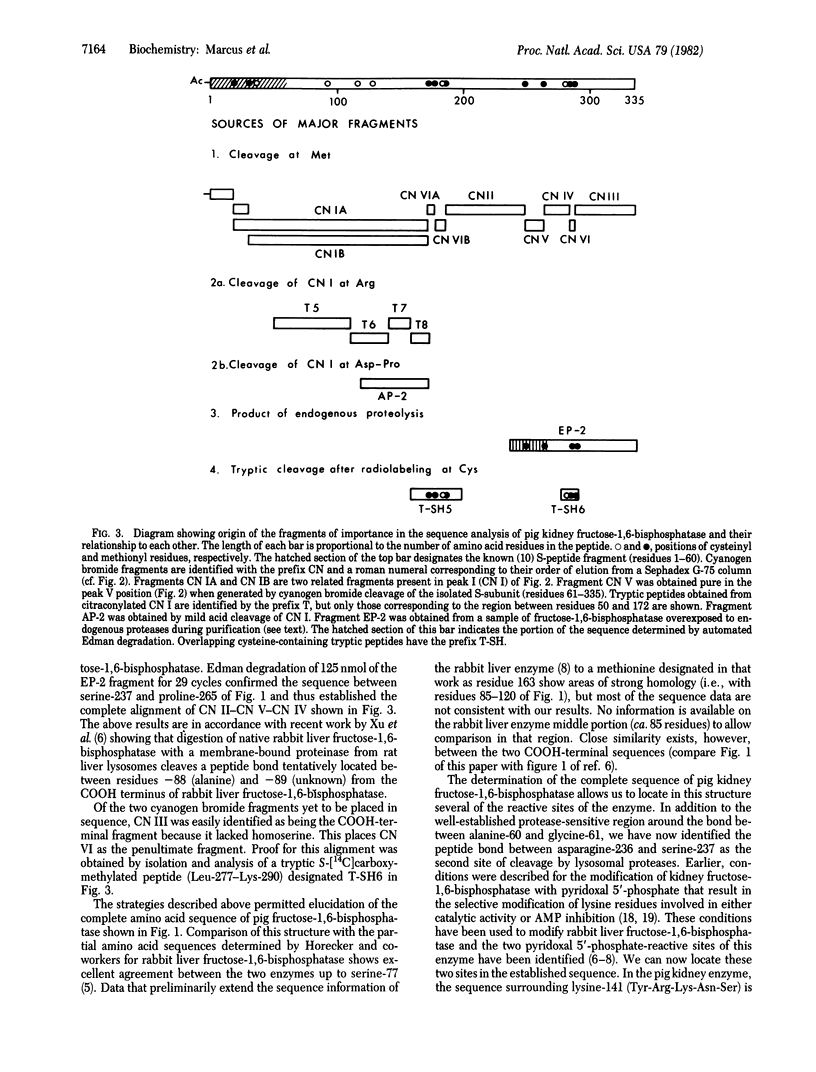
The covalent structure of the pig kidney fructose-1,6-bisphosphatase (D-fructose-1,6-bisphosphate 1-phosphohydrolase, EC 3.1.3.11) subunit has been determined. Placement of the 335 amino acid residues in the polypeptide chain was based largely on automated Edman degradation of eight purified cyanogen bromide fragments generated from the S-carboxymethylated protein. The determination of the amino acid sequence of the largest cyanogen bromide fragment (154 residues) required additional analysis of subfragments obtained by tryptic cleavage at arginyl residues and by mild acid cleavage of an Asp-Pro peptide bond. Alignment of the cyanogen bromide fragments was accomplished by analysis of a product of limited proteolysis by an endogenous protease and by characterization of the tryptic peptides isolated from S-[14C]carboxymethylated fructose-1,6-bisphosphatase. This sequence information has permitted the identification of several reactive sites of functional and structural significance in pig kidney fructose-1,6-bisphosphatase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benkovic S. J., deMaine M. M. Mechanism of action of fructose 1,6-bisphosphatase. Adv Enzymol Relat Areas Mol Biol. 1982;53:45–82. doi: 10.1002/9780470122983.ch2. [DOI] [PubMed] [Google Scholar]
- Botelho L. H., El-Dorry H. A., Crivellaro O., Chu D. K., Pontremoli S., Horecker B. L. Digestion of rabbit liver fructose 1,6-bisphosphatase with subtilisin: sites of cleavage and activity of the modified enzyme. Arch Biochem Biophys. 1977 Dec;184(2):535–545. doi: 10.1016/0003-9861(77)90463-5. [DOI] [PubMed] [Google Scholar]
- Colombo G., Hubert E., Marcus F. Selective alteration of the regulatory properties of fructose 1,6-diphosphatase by modification with pyridoxal 5'-phosphate. Biochemistry. 1972 May 9;11(10):1798–1803. doi: 10.1021/bi00760a010. [DOI] [PubMed] [Google Scholar]
- Colombo G., Marcus F. Modification of fructose-1,6-diphosphatase with pyridoxal 5'-phosphate. Evidence for the participation of lysyl residues at the active site. Biochemistry. 1974 Jul 16;13(15):3085–3091. doi: 10.1021/bi00712a014. [DOI] [PubMed] [Google Scholar]
- Cunningham B. A., Raushel F. M., Villafranca J. J., Benkovic S. J. Distances between structural metal ion, substrates, and allosteric modifier of fructose bisphosphatase. Biochemistry. 1981 Jan 20;20(2):359–362. doi: 10.1021/bi00505a020. [DOI] [PubMed] [Google Scholar]
- El-Dorry H. A., Chu D. K., Dzugaj A., Botelho L. H., Pontremoli S., Horecker B. L. Primary structure of the S-peptide formed by digestion of rabbit liver fructose 1,6-biphosphatase with subtilisin. Arch Biochem Biophys. 1977 Aug;182(2):763–773. doi: 10.1016/0003-9861(77)90558-6. [DOI] [PubMed] [Google Scholar]
- Fisher W. K., Thompson E. O. Amino acid sequence studies on sheep liver fructose-bisphosphatase. I. The S-peptide. Aust J Biol Sci. 1980 Dec;33(6):665–674. doi: 10.1071/bi9800665. [DOI] [PubMed] [Google Scholar]
- Horecker B. L., Melloni E., Pontremoli S. Fructose 1,6-bisphosphatase: properties of the neutral enzyme and its modification by proteolytic enzymes. Adv Enzymol Relat Areas Mol Biol. 1975;42:193–226. doi: 10.1002/9780470122877.ch4. [DOI] [PubMed] [Google Scholar]
- Humble E., Dahlqvist-Edberg U., Ekman P., Netzel E., Ragnarsson U., Engström L. Amino acid sequence at the phosphorylated site of rat liver fructose-1,6-diphosphatase and phosphorylation of a corresponding synthetic peptide. Biochem Biophys Res Commun. 1979 Oct 12;90(3):1064–1072. doi: 10.1016/0006-291x(79)91934-x. [DOI] [PubMed] [Google Scholar]
- Marcus F., Edelstein I., Saidel L. J., Keim P. S., Heinrikson R. L. The covalent structure of pig kidney fructose 1,6-bisphosphatase: sequence of the 60-residue NH2-terminal peptide produced by digestion with subtilisin. Arch Biochem Biophys. 1981 Jul;209(2):687–696. doi: 10.1016/0003-9861(81)90330-1. [DOI] [PubMed] [Google Scholar]
- Marcus F., Hosey M. M., Keim P. S., Heinrikson R. L. The covalent structure of pig kidney fructose-1,6-bisphosphatase. Sequence of a 63-residue cyanogen bromide peptide containing a phosphorylatable serine. J Biol Chem. 1981 Dec 10;256(23):12208–12212. [PubMed] [Google Scholar]
- Marcus F. Interaction of salicylate at the AMP site of fructose 1,6-bisphosphatase. FEBS Lett. 1976 Nov;70(1):159–162. doi: 10.1016/0014-5793(76)80748-x. [DOI] [PubMed] [Google Scholar]
- Nakai N., Lai C. Y., Horecker B. L. Use of fluorescamine in the chromatographic analysis of peptides from proteins. Anal Biochem. 1974 Apr;58(2):563–570. doi: 10.1016/0003-2697(74)90225-5. [DOI] [PubMed] [Google Scholar]
- Pilkis S. J., El-Maghrabi M. R., Coven B., Claus T. H., Tager H. S., Steiner D. F., Keim P. S., Heinrikson R. L. Phosphorylation of rat hepatic fructose-1,6-bisphosphatase and pyruvate kinase. J Biol Chem. 1980 Apr 10;255(7):2770–2775. [PubMed] [Google Scholar]
- Pisano J. J., Bronzert T. J. Analysis of amino acid phenylthiohydantoins by gas chromatography. J Biol Chem. 1969 Oct 25;244(20):5597–5607. [PubMed] [Google Scholar]
- Rose S. M., Schwartz B. D. Automated isocratic separation of phenylthiohydantoin-amino acids by tandem reverse phase high-pressure liquid chromatography columns. Anal Biochem. 1980 Sep 1;107(1):206–213. doi: 10.1016/0003-2697(80)90513-8. [DOI] [PubMed] [Google Scholar]
- Smithies O., Gibson D., Fanning E. M., Goodfliesh R. M., Gilman J. G., Ballantyne D. L. Quantitative procedures for use with the Edman-Begg sequenator. Partial sequences of two unusual immunoglobulin light chains, Rzf and Sac. Biochemistry. 1971 Dec 21;10(26):4912–4921. doi: 10.1021/bi00802a013. [DOI] [PubMed] [Google Scholar]
- Suda H., Xu G. J., Kutny R. M., Natalini P., Pontremoli S., Horecker B. L. Location of lysyl residues at the allosteric site of fructose 1,6-bisphosphatase. Arch Biochem Biophys. 1982 Aug;217(1):10–14. doi: 10.1016/0003-9861(82)90473-8. [DOI] [PubMed] [Google Scholar]
- Xu G. J., Datta A. G., Singh V. N., Suda H., Pontremoli S., Horecker B. L. Rabbit liver fructose 1,6-bisphosphatase: labeling of the active and allosteric sites with pyridoxal 5-phosphate and sequence of a nonapeptide from the active site. Arch Biochem Biophys. 1981 Aug;210(1):98–103. doi: 10.1016/0003-9861(81)90168-5. [DOI] [PubMed] [Google Scholar]
- Xu G. J., Natalini P., Suda H., Tsolas O., Dzugaj A., Sun S. C., Pontremoli S., Horecker B. L. Rabbit liver fructose-1,6-bisphosphatase: location of an active site lysyl residue in the COOH-terminal fragment generated by a lysosomal proteinase. Arch Biochem Biophys. 1982 Apr 1;214(2):688–694. doi: 10.1016/0003-9861(82)90075-3. [DOI] [PubMed] [Google Scholar]


