Abstract
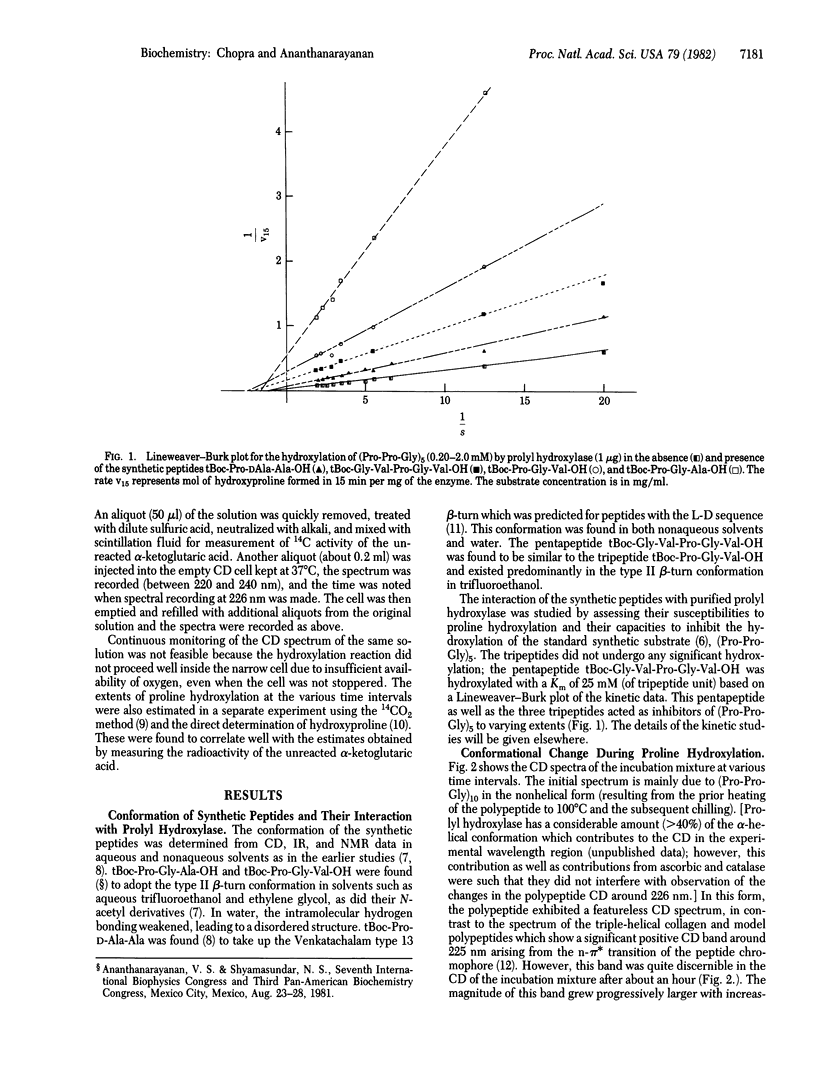
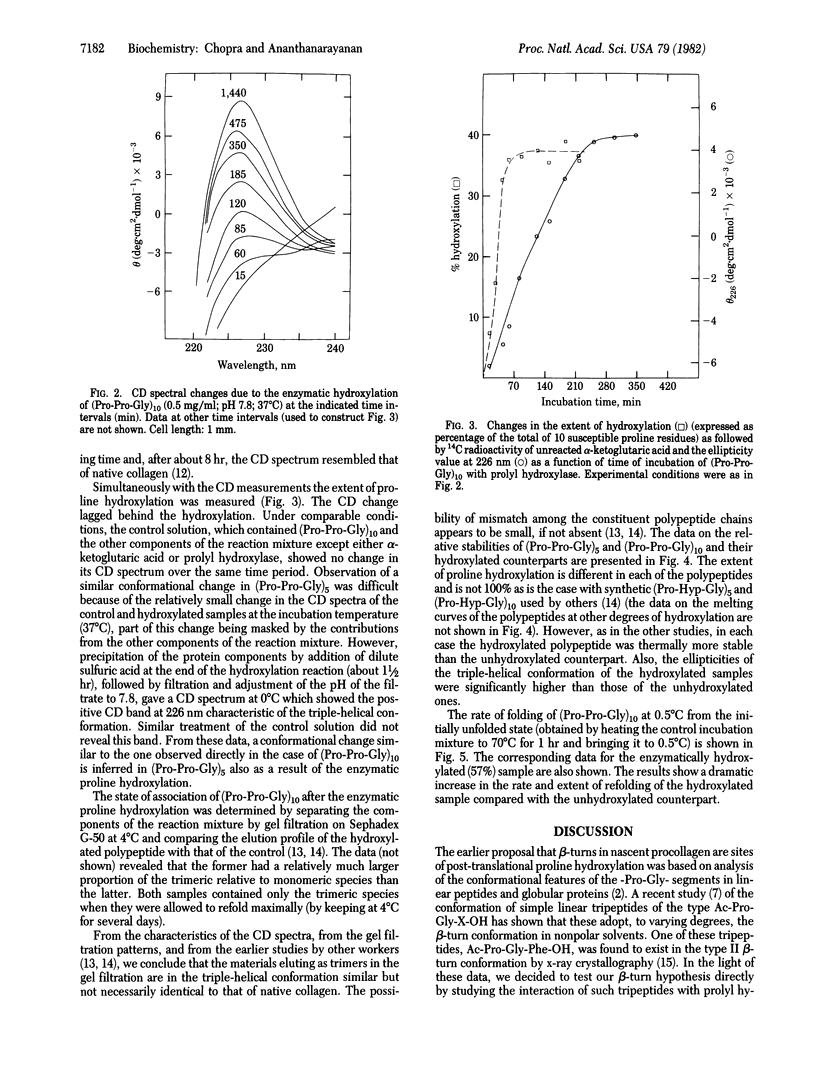
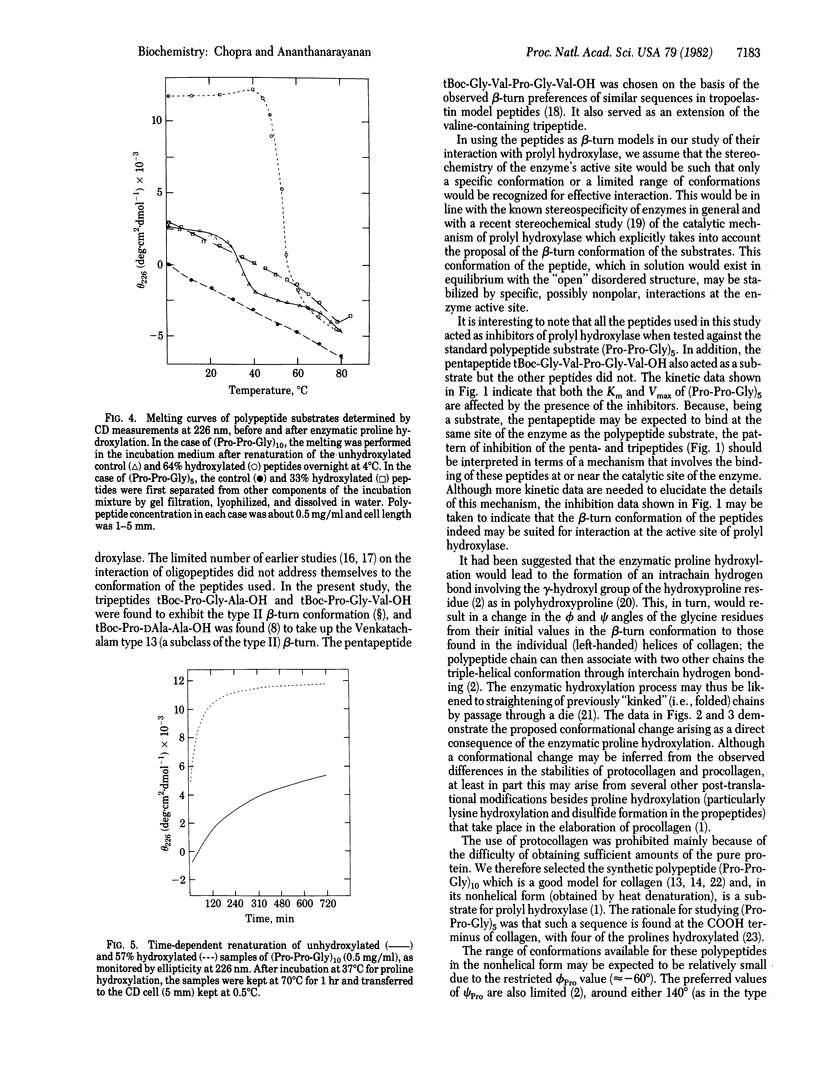
In 1979 it was proposed that prolyl hydroxylase (prolyl-glycyl-peptide,2-oxoglutarate:oxygen oxidoreductase, EC 1.14.11.2) recognizes the beta-turn conformation in nascent procollagen chains and that the hydroxylation process involves a conformational change resulting in "straightening" of the beta-turn segments into the linear triple-helical conformation of native collagen. We present experimental data that verify both these postulates. The following peptides were synthesized and studied for their conformation and interaction with prolyl hydroxylase: tBoc-Pro-Gly-Ala-OH, tBoc-Pro-Gly-Val-OH, tBoc-Gly-Val-Pro-Gly-Val-OH, and tBoc-Pro-DAla-Ala-OH. Spectral data showed that these peptides preferred a beta-turn conformation. All of them acted as inhibitors of the enzyme; the pentapeptide also acted as a substrate. To mimic the biosynthetic event, a collagen model polypeptide, (Pro-Pro-Gly)10, was incubated at 37 degrees C with purified prolyl hydroxylase and the necessary cosubstrates and cofactors at pH 7.8. A progressive change from the initially nonhelical to the triple-helical conformation, as monitored by CD spectra and gel filtration, occurred during the course of proline hydroxylation. In addition to leading to increased thermal stability of the triple-helical conformation in (Pro-Pro-Gly)10 and (Pro-Pro-Gly)5, the enzymatic incorporation of the hydroxyproline residues was found to enable these polypeptides to fold into this conformation faster than the unhydroxylated counterparts. These conformational implications of proline hydroxylation in collagen may also be of use in the study of the complement subcomponent Clq and of acetylcholine esterase which contain collagen-like regions in them.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ananthanarayanan V. S., Shyamasundar N. Circular dichroism of type 13 beta -turn in linear tripeptides containing L-proline and D-alanine. Biochem Biophys Res Commun. 1981 Sep 16;102(1):295–301. doi: 10.1016/0006-291x(81)91520-5. [DOI] [PubMed] [Google Scholar]
- Berg R. A., Prockop D. J. The thermal transition of a non-hydroxylated form of collagen. Evidence for a role for hydroxyproline in stabilizing the triple-helix of collagen. Biochem Biophys Res Commun. 1973 May 1;52(1):115–120. doi: 10.1016/0006-291x(73)90961-3. [DOI] [PubMed] [Google Scholar]
- Brahmachari S. K., Ananthanarayanan V. S. Beta-turns in nascent procollagen are sites of posttranslational enzymatic hydroxylation of proline. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5119–5123. doi: 10.1073/pnas.76.10.5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown F. R., 3rd, Di Corato A., Lorenzi G. P., Blout E. R. Synthesis and structural studies of two collagen analogues: poly (L-prolyl-L-seryl-glycyl) and poly (L-prolyl-L-alanyl-glycyl). J Mol Biol. 1972 Jan 14;63(1):85–99. doi: 10.1016/0022-2836(72)90523-2. [DOI] [PubMed] [Google Scholar]
- Bächinger H. P., Bruckner P., Timpl R., Prockop D. J., Engel J. Folding mechanism of the triple helix in type-III collagen and type-III pN-collagen. Role of disulfide bridges and peptide bond isomerization. Eur J Biochem. 1980 May;106(2):619–632. doi: 10.1111/j.1432-1033.1980.tb04610.x. [DOI] [PubMed] [Google Scholar]
- Bächinger H. P., Fessler L. I., Timpl R., Fessler J. H. Chain assembly intermediate in the biosynthesis of type III procollagen in chick embryo blood vessels. J Biol Chem. 1981 Dec 25;256(24):13193–13199. [PubMed] [Google Scholar]
- Chandrasekaran R., Lakshminarayanan A. V., Pandya U. V., Ramachandran G. N. Conformation of the LL and LD hairpin bends with internal hydrogen bonds in proteins and peptides. Biochim Biophys Acta. 1973 Mar 23;303(1):14–27. doi: 10.1016/0005-2795(73)90143-8. [DOI] [PubMed] [Google Scholar]
- Edwards C. A., O'Brien W. D., Jr Modified assay for determination of hydroxyproline in a tissue hydrolyzate. Clin Chim Acta. 1980 Jun 10;104(2):161–167. doi: 10.1016/0009-8981(80)90192-8. [DOI] [PubMed] [Google Scholar]
- Engel J., Chen H. T., Prockop D. J., Klump H. The triple helix in equilibrium with coil conversion of collagen-like polytripeptides in aqueous and nonaqueous solvents. Comparison of the thermodynamic parameters and the binding of water to (L-Pro-L-Pro-Gly)n and (L-Pro-L-Hyp-Gly)n. Biopolymers. 1977 Mar;16(3):601–622. doi: 10.1002/bip.1977.360160310. [DOI] [PubMed] [Google Scholar]
- Hanauske-Abel H. M., Günzler V. A stereochemical concept for the catalytic mechanism of prolylhydroxylase: applicability to classification and design of inhibitors. J Theor Biol. 1982 Jan 21;94(2):421–455. doi: 10.1016/0022-5193(82)90320-4. [DOI] [PubMed] [Google Scholar]
- Kedersha N. L., Berg R. A. An improved method for the purification of vertebrate prolyl hydroxylase by affinity chromatography. Coll Relat Res. 1981 Jul;1(4):345–353. doi: 10.1016/s0174-173x(81)80011-8. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y., Fujimoto D., Tamiya N. The enzymic hydroxylation of protocollagen models. Biochem J. 1969 Nov;115(3):569–574. doi: 10.1042/bj1150569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivirikko K. I., Prockop D. J. Hydroxylation of proline in synthetic polypeptides with purified protocollagen hydroxylase. J Biol Chem. 1967 Sep 25;242(18):4007–4012. [PubMed] [Google Scholar]
- Kobayashi Y., Suezaki Y., Inouye K., Tanaka K., Kakiuchi K., Kyogoku Y. Investigation of the formation of triple helices between (Pro-Pro-Gly)n's of different degrees of polymerization using gel filtration. Biopolymers. 1977 Feb;16(2):247–257. doi: 10.1002/bip.1977.360160203. [DOI] [PubMed] [Google Scholar]
- Okuyama K., Okuyama K., Arnott S., Takayanagi M., Kakudo M. Crystal and molecular structure of a collagen-like polypeptide (Pro-Pro-Gly)10. J Mol Biol. 1981 Oct 25;152(2):427–443. doi: 10.1016/0022-2836(81)90252-7. [DOI] [PubMed] [Google Scholar]
- Reid K. B., Lowe D. M., Porter R. R. Isolation and characterization of C1q, a subcomponent of the first component of complement, from human and rabbit sera. Biochem J. 1972 Dec;130(3):749–763. doi: 10.1042/bj1300749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads R. E., Udenfriend S. Purification and properties of collagen proline hydroxylase from newborn rat skin. Arch Biochem Biophys. 1970 Aug;139(2):329–339. doi: 10.1016/0003-9861(70)90485-6. [DOI] [PubMed] [Google Scholar]
- Sakakibara S., Inouye K., Shudo K., Kishida Y., Kobayashi Y., Prockop D. J. Synthesis of (Pro-Hyp-Gly) n of defined molecular weights. Evidence for the stabilization of collagen triple helix by hydroxypyroline. Biochim Biophys Acta. 1973 Mar 23;303(1):198–202. doi: 10.1016/0005-2795(73)90164-5. [DOI] [PubMed] [Google Scholar]
- Tuderman L., Kuutti E. R., Kivirikko K. I. An affinity-column procedure using poly(L-proline) for the purification of prolyl hydroxylase. Purification of the enzyme from chick embryos. Eur J Biochem. 1975 Mar 3;52(1):9–16. doi: 10.1111/j.1432-1033.1975.tb03967.x. [DOI] [PubMed] [Google Scholar]
- Urry D. W., Long M. M. Conformations of the repeat peptides of elastin in solution: an application of proton and carbon-13 magnetic resonance to the determination of polypeptide secondary structure. CRC Crit Rev Biochem. 1976 Jun;4(1):1–45. doi: 10.3109/10409237609102557. [DOI] [PubMed] [Google Scholar]
- Venkatachalam C. M. Stereochemical criteria for polypeptides and proteins. V. Conformation of a system of three linked peptide units. Biopolymers. 1968 Oct;6(10):1425–1436. doi: 10.1002/bip.1968.360061006. [DOI] [PubMed] [Google Scholar]
- Watkins M. S., Hitt A. S., Bulger J. E. The binding of 18S acetylcholinesterase to sphingomyelin and the role of the collagen-like tail. Biochem Biophys Res Commun. 1977 Dec 7;79(3):640–647. doi: 10.1016/0006-291x(77)91160-3. [DOI] [PubMed] [Google Scholar]


