Abstract
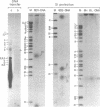
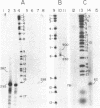
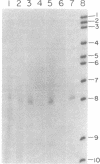
The initiation sites for heavy (H) and light (L) strand transcription in HeLa cell mitochondrial DNA have been investigated by mapping experiments utilizing in vitro "capped" mitochondrial RNA molecules or nascent RNA chains. Mitochondrial poly(A)-containing RNA molecules were labeled at their 5' ends with [alpha-32P]GTP and guanylyltransferase ("capping" enzyme) and mapped on the mitochondrial genome by DNA transfer hybridization and S1 nuclease protection experiments. A mapping site for the capped 5' ends was found on the H strand very near to the 5' terminus of the 12S rRNA gene, and another site was found on the L strand very near to the 5' terminus of the 7S RNA coding sequence. In parallel experiments, the 5' ends of the nascent chains isolated from mitochondrial DNA transcription complexes were similarly mapped very near to the 5' termini of the 12S rRNA gene and of the 7S RNA coding sequence. The in vitro capped RNA molecules and the nascent chains thus presumably identify the same transcriptional initiation sites on the H strand and the L strand. The occurrence of a second possible initiation site for H-strand transcription 90-110 nucleotides upstream of that described above--i.e., 20-40 nucleotides upstream of the tRNAPhe gene--had been previously indicated by a mapping analysis of the nascent RNA chains and has been confirmed in the present work. The presence of two initiation sites for H-strand transcription can be correlated with other types of evidence that point to two different transcription events leading to the synthesis of a polycistronic molecule corresponding to the almost entire H strand and to the synthesis of the rRNA species.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloni Y., Attardi G. Expression of the mitochondrial genome in HeLa cells. XI. Isolation and characterization of transcription complexes of mitochondrial DNA. J Mol Biol. 1972 Sep 28;70(2):363–373. doi: 10.1016/0022-2836(72)90545-1. [DOI] [PubMed] [Google Scholar]
- Aloni Y., Attardi G. Symmetrical in vivo transcription of mitochondrial DNA in HeLa cells. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1757–1761. doi: 10.1073/pnas.68.8.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amalric F., Merkel C., Gelfand R., Attardi G. Fractionation of mitochondrial RNA from HeLa cells by high-resolution electrophoresis under strongly denaturing conditions. J Mol Biol. 1978 Jan 5;118(1):1–25. doi: 10.1016/0022-2836(78)90241-3. [DOI] [PubMed] [Google Scholar]
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Cantatore P., Attardi G. Mapping of nascent light and heavy strand transcripts on the physical map of HeLa cell mitochondrial DNA. Nucleic Acids Res. 1980 Jun 25;8(12):2605–2625. doi: 10.1093/nar/8.12.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson T., Edwards J., Levens D., Locker J., Rabinowitz M. Transcriptional initiation and processing of the small ribosomal RNA of yeast mitochondria. J Biol Chem. 1982 Jun 10;257(11):6494–6500. [PubMed] [Google Scholar]
- Crews S., Attardi G. The sequences of the small ribosomal RNA gene and the phenylalanine tRNA gene are joined end to end in human mitochondrial DNA. Cell. 1980 Mar;19(3):775–784. doi: 10.1016/s0092-8674(80)80053-5. [DOI] [PubMed] [Google Scholar]
- Gelfand R., Attardi G. Synthesis and turnover of mitochondrial ribonucleic acid in HeLa cells: the mature ribosomal and messenger ribonucleic acid species are metabolically unstable. Mol Cell Biol. 1981 Jun;1(6):497–511. doi: 10.1128/mcb.1.6.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levens D., Ticho B., Ackerman E., Rabinowitz M. Transcriptional initiation and 5' termini of yeast mitochondrial RNA. J Biol Chem. 1981 May 25;256(10):5226–5232. [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Martin S. A., Paoletti E., Moss B. Purification of mRNA guanylyltransferase and mRNA (guanine-7-) methyltransferase from vaccinia virions. J Biol Chem. 1975 Dec 25;250(24):9322–9329. [PubMed] [Google Scholar]
- Monroy G., Spencer E., Hurwitz J. Characteristics of reactions catalyzed by purified guanylyltransferase from vaccinia virus. J Biol Chem. 1978 Jun 25;253(12):4490–4498. [PubMed] [Google Scholar]
- Montoya J., Ojala D., Attardi G. Distinctive features of the 5'-terminal sequences of the human mitochondrial mRNAs. Nature. 1981 Apr 9;290(5806):465–470. doi: 10.1038/290465a0. [DOI] [PubMed] [Google Scholar]
- Murphy W. I., Attardi B., Tu C., Attardi G. Evidence for complete symmetrical transcription in vivo of mitochondrial DNA in HeLa cells. J Mol Biol. 1975 Dec 25;99(4):809–814. doi: 10.1016/s0022-2836(75)80187-2. [DOI] [PubMed] [Google Scholar]
- Ojala D. K., Montoya J., Attardi G. The putative mRNA for subunit II of human cytochrome c oxidase starts directly at the translation initiator codon. Nature. 1980 Sep 4;287(5777):79–82. doi: 10.1038/287079a0. [DOI] [PubMed] [Google Scholar]
- Ojala D., Attardi G. A detailed physical map of HeLa cell mitochondria DNA and its alignment with the positions of known genetic markers. Plasmid. 1977 Nov;1(1):78–105. doi: 10.1016/0147-619x(77)90010-5. [DOI] [PubMed] [Google Scholar]
- Ojala D., Attardi G. Fine mapping of the ribosomal RNA genes of HeLa cell mitochondrial DNA. J Mol Biol. 1980 Apr;138(2):411–420. doi: 10.1016/0022-2836(80)90296-x. [DOI] [PubMed] [Google Scholar]
- Ojala D., Crews S., Montoya J., Gelfand R., Attardi G. A small polyadenylated RNA (7 S RNA), containing a putative ribosome attachment site, maps near the origin of human mitochondrial DNA replication. J Mol Biol. 1981 Aug 5;150(2):303–314. doi: 10.1016/0022-2836(81)90454-x. [DOI] [PubMed] [Google Scholar]
- Ojala D., Merkel C., Gelfand R., Attardi G. The tRNA genes punctuate the reading of genetic information in human mitochondrial DNA. Cell. 1980 Nov;22(2 Pt 2):393–403. doi: 10.1016/0092-8674(80)90350-5. [DOI] [PubMed] [Google Scholar]
- Ojala D., Montoya J., Attardi G. tRNA punctuation model of RNA processing in human mitochondria. Nature. 1981 Apr 9;290(5806):470–474. doi: 10.1038/290470a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Venkatesan S., Gershowitz A., Moss B. Modification of the 5' end of mRNA. Association of RNA triphosphatase with the RNA guanylyltransferase-RNA (guanine-7-)methyltransferase complex from vaccinia virus. J Biol Chem. 1980 Feb 10;255(3):903–908. [PubMed] [Google Scholar]