Abstract
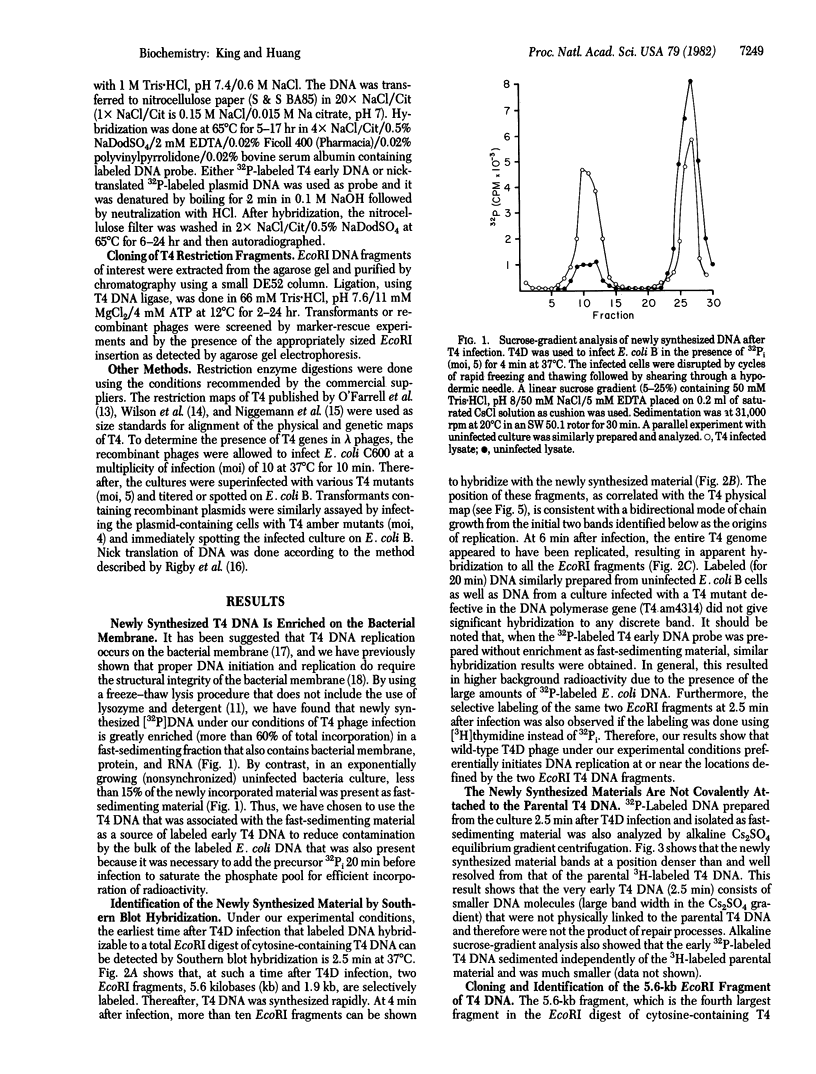
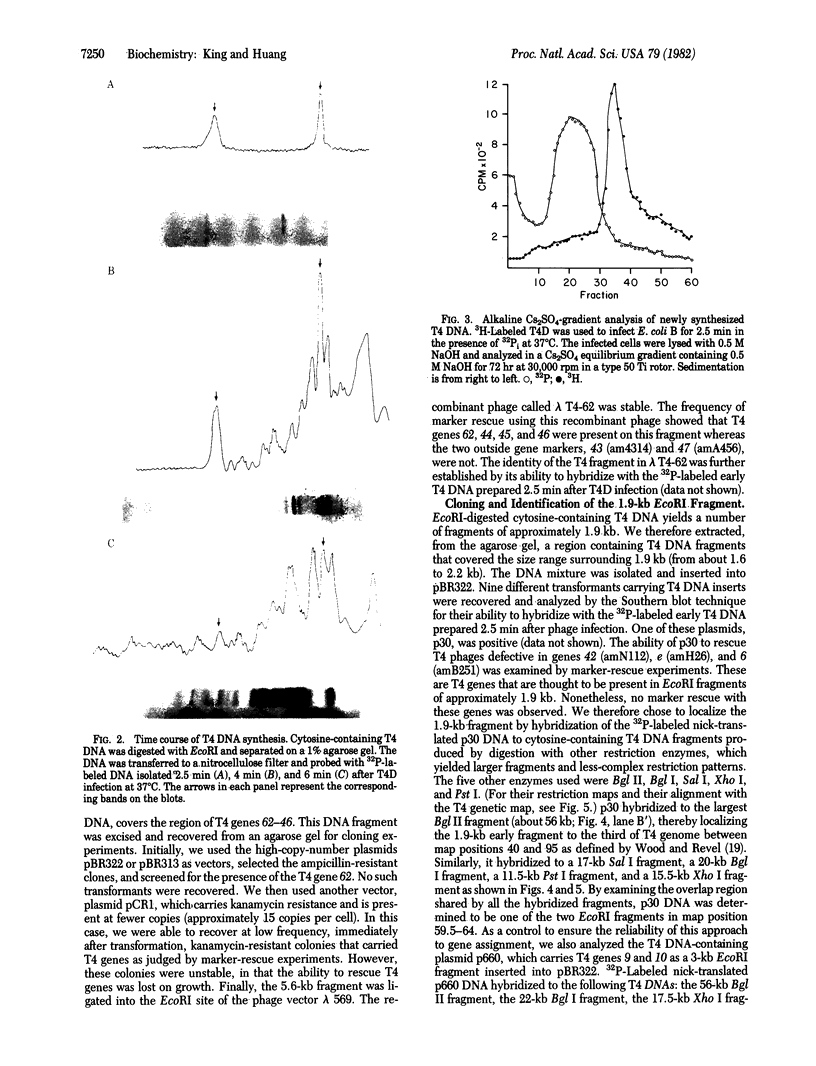
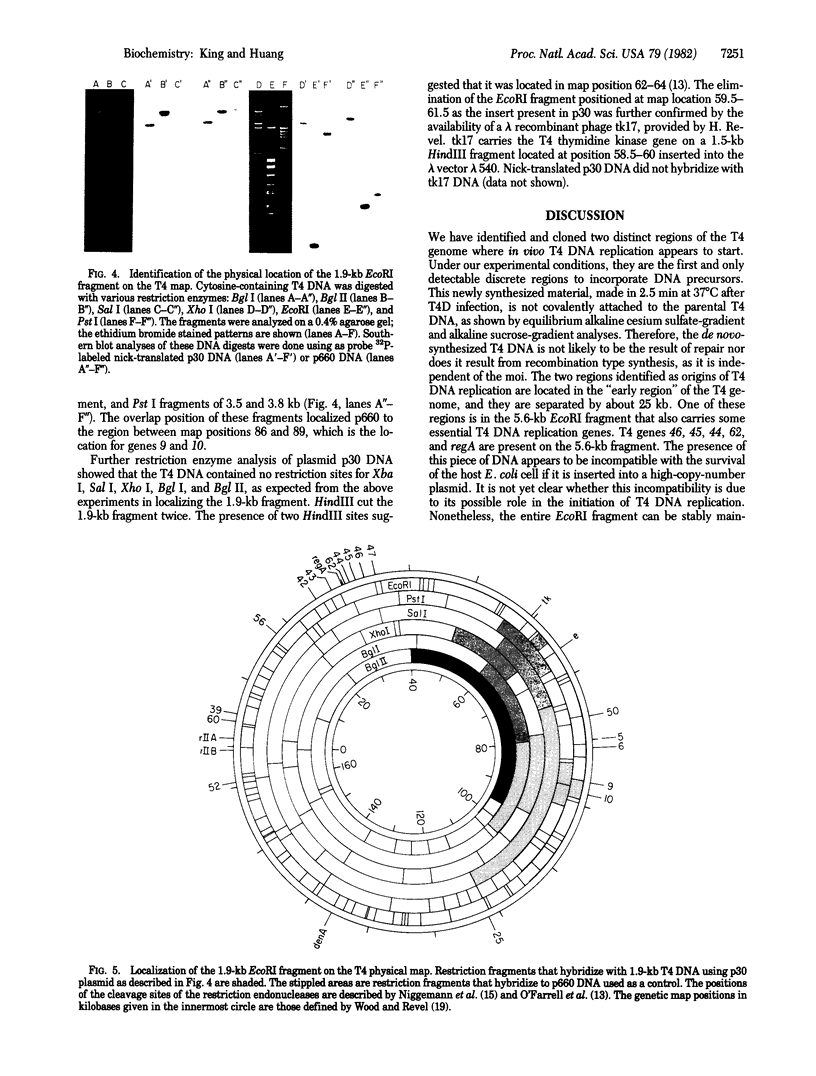
Two physical origins of T4 DNA replication were determined by hybridization of viral DNA prepared 2.5 min after infection to a display of total T4 DNA. This is the earliest time after T4 infection of Escherichia coli at 37 degrees C that labeled and hybridizable DNA can be detected. The two origins, separated by about 25 kilobases, were identified and localized in the early region of the T4 map. One of them is located in a 5.6-kilobase EcoRI fragment containing genes 62-46. The other is located between genes rI and e in a 1.9-kilobase EcoRI fragment. Both of these T4 fragments have been cloned and their interactions with the host cell are discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Craine B. L., Rupert C. S. Identification of a biochemically unique DNA-membrane interaction involving the Escherichia coli origin of replication. J Bacteriol. 1978 Apr;134(1):193–199. doi: 10.1128/jb.134.1.193-199.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delius H., Howe C., Kozinski A. W. Structure of the replicating DNA from bacteriophage T4. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3049–3053. doi: 10.1073/pnas.68.12.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furth M. E., Dove W. F., Meyer B. J. Specificity determinants for bacteriophage lambda DNA replication. III. Activation of replication in lambda ric mutants by transcription outside of ori. J Mol Biol. 1982 Jan 5;154(1):65–83. doi: 10.1016/0022-2836(82)90417-x. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C. P., Hohn B. Identification of a host protein necessary for bacteriophage morphogenesis (the groE gene product). Proc Natl Acad Sci U S A. 1978 Jan;75(1):131–135. doi: 10.1073/pnas.75.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern M. E., Mattson T., Kozinski A. W. Origins of phage T4 DNA replication as revealed by hybridization to cloned genes. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6137–6141. doi: 10.1073/pnas.76.12.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe C. C., Buckley P. J., Carlson K. M., Kozinski A. W. Multiple and specific initiation of T4 DNA replication. J Virol. 1973 Jul;12(1):130–148. doi: 10.1128/jvi.12.1.130-148.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W. M., Buchanan J. M. Synergistic interactions of T4 early proteins concerned with their binding to DNA. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2226–2230. doi: 10.1073/pnas.71.6.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W. M. Inhibition of initiation of bacteriophage T4 DNA replication by perturbation of Escherichia coli host membrane composition. J Virol. 1979 Dec;32(3):917–924. doi: 10.1128/jvi.32.3.917-924.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W. M. Positive regulation of T-even-phage DNA replication by the DNA-delay protein of gene 39. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):495–499. doi: 10.1101/sqb.1979.043.01.055. [DOI] [PubMed] [Google Scholar]
- Liu C. C., Burke R. L., Hibner U., Barry J., Alberts B. Probing DNA replication mechanisms with the T4 bacteriophage in vitro system. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):469–487. doi: 10.1101/sqb.1979.043.01.053. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Liu C. C., Alberts B. M. T4 DNA topoisomerase: a new ATP-dependent enzyme essential for initiation of T4 bacteriophage DNA replication. Nature. 1979 Oct 11;281(5731):456–461. doi: 10.1038/281456a0. [DOI] [PubMed] [Google Scholar]
- Luder A., Mosig G. Two alternative mechanisms for initiation of DNA replication forks in bacteriophage T4: priming by RNA polymerase and by recombination. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1101–1105. doi: 10.1073/pnas.79.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh R. C., Breschkin A. M., Mosig G. Origin and direction of bacteriophage T4 DNA replication. II. A gradient of marker frequencies in partially replicated T4 DNA as assayed by transformation. J Mol Biol. 1971 Sep 14;60(2):213–233. doi: 10.1016/0022-2836(71)90289-0. [DOI] [PubMed] [Google Scholar]
- McCarthy D., Minner C., Bernstein H., Bernstein C. DNA elongation rates and growing point distributions of wild-type phage T4 and a DNA-delay amber mutant. J Mol Biol. 1976 Oct 5;106(4):963–981. doi: 10.1016/0022-2836(76)90346-6. [DOI] [PubMed] [Google Scholar]
- Niggemann E., Green I., Meyer H. P., Rüger W. Physical mapping of bacteriophage T4. Mol Gen Genet. 1981;184(2):289–299. doi: 10.1007/BF00272920. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H., Kutter E., Nakanishi M. A restriction map of the bacteriophage T4 genome. Mol Gen Genet. 1980;179(2):421–435. doi: 10.1007/BF00425473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Saito H., Tabor S., Tamanoi F., Richardson C. C. Nucleotide sequence of the primary origin of bacteriophage T7 DNA replication: relationship to adjacent genes and regulatory elements. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3917–3921. doi: 10.1073/pnas.77.7.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel P. J., Schaechter M. The role of the host cell membrane in the replication and morphogenesis of bacteriophages. Annu Rev Microbiol. 1973;27:261–282. doi: 10.1146/annurev.mi.27.100173.001401. [DOI] [PubMed] [Google Scholar]
- Snyder L., Gold L., Kutter E. A gene of bacteriophage T4 whose product prevents true late transcription on cytosine-containing T4 DNA. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3098–3102. doi: 10.1073/pnas.73.9.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stetler G. L., King G. J., Huang W. M. T4 DNA-delay proteins, required for specific DNA replication, form a complex that has ATP-dependent DNA topoisomerase activity. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3737–3741. doi: 10.1073/pnas.76.8.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G. G., Tanyashin V. I., Murray N. E. Molecular cloning of fragments of bacteriophage T4 DNA. Mol Gen Genet. 1977 Nov 14;156(2):203–214. doi: 10.1007/BF00283493. [DOI] [PubMed] [Google Scholar]
- Wood W. B., Revel H. R. The genome of bacteriophage T4. Bacteriol Rev. 1976 Dec;40(4):847–868. doi: 10.1128/br.40.4.847-868.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zyskind J. W., Smith D. W. Nucleotide sequence of the Salmonella typhimurium origin of DNA replication. Proc Natl Acad Sci U S A. 1980 May;77(5):2460–2464. doi: 10.1073/pnas.77.5.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]