Abstract
Anxiolytic benefit following chronic treatment with the glutamate modulating agent riluzole in patients with generalized anxiety disorder (GAD) was previously associated with differential changes in hippocampal NAA concentrations. Here, we investigated the association between hippocampal volume and hippocampal NAA in the context of riluzole response in GAD. Eighteen medication-free adult patients with GAD received 8-week of open-label riluzole. Ten healthy subjects served as a comparison group. Participants underwent magnetic resonance imaging and spectroscopy at baseline and at the end of Week 8. GAD patients who completed all interventions were classified as remitters (n = 7) or non-remitters (n = 6), based on final Hamilton Anxiety Rating Scale (HAM-A) scores ≤ 7. At baseline, GAD patients had a significant reduction in total hippocampal volume compared to healthy subjects (F(1,21) = 6.55, p = 0.02). This reduction was most pronounced in the remitters, compared to non-remitters and healthy subjects. Delta (final – baseline) hippocampal volume was positively correlated with delta NAA in GAD. This positive association was highly significant in the right hippocampus in GAD [r = 0.81, p = 0.002], with no significant association in healthy subjects [Fisher r-to-z p = 0.017]. Across all GAD patients, delta hippocampal volume was positively associated with improvement in HAM-A (rspearman = 0.62, p = 0.03). These preliminary findings support hippocampal NAA and volume as neural biomarkers substantially associated with therapeutic response to a glutamatergic drug.
Keywords: Riluzole, generalized anxiety disorder, biomarkers, glutamate, N-Acetylaspartate, hippocampal volume, magnetic resonance spectroscopy
1. Introduction
Over the last two decades, convergent lines of research have demonstrated aberrant glutamatergic function in mood, anxiety and psychotic disorders. These neurobiological findings have been underscored by preliminary trials showing promising results for novel drugs with glutamate-based mechanisms (Sanacora et al., 2008). One such medication is riluzole, an agent possessing neuroprotective properties (Liu et al., 2011a) approved by the US Food and Drug Administration for treatment of Amyotrophic Lateral Sclerosis. Riluzole is believed to exert its pharmacological effects primarily by reducing pre-synaptic glutamate release and potentiating glutamate reuptake (Pittenger et al., 2008). In open-label trials, riluzole showed significant anxiolytic properties in patients with major depression, bipolar depression, and obsessive-compulsive disorder (for review see (Pittenger et al., 2008)). Notably high remission rates were found among responders to riluzole (65% in (Zarate et al., 2004), 75% in (Sanacora et al., 2007), and 100% in (Zarate et al., 2005)), suggesting a subgroup of patients with robust riluzole responsivity. An improved understanding of this riluzole-responsive subgroup may provide important information to guide the development of rational therapies.
In a pilot 8-week open-label trial, we previously found that riluzole had significant anxiolytic effects in patients with generalized anxiety disorder (GAD) with mild-to-moderate depressive symptoms (Mathew et al., 2008). We also reported that N-Acetylaspartate (NAA), a marker of neuronal integrity, showed utility as a neuroimaging biomarker to assess response to riluzole. More specifically, the proton magnetic resonance spectroscopy (1H-MRS) study revealed a response-by-time interaction, with riluzole responders showing an increase in hippocampal NAA following 8 weeks of treatment. Conversely, non-responders exhibited a relative reduction in NAA levels at the same time point. Hippocampal NAA changes across all GAD patients over 8 weeks of riluzole treatment were positively correlated with improvements in anxiety scores. These findings are consistent with a recent report of increased NAA levels in the anterior cingulate cortex following 6-week riluzole treatment in patients with bipolar depression (Brennan et al., 2010). We hypothesized that changes in NAA levels following riluzole treatment represents alterations in neuroplasticity. As an index of neuroplasticity we measured hippocampal volumetrics as reflected on MRI. Therefore, this study investigates whether hippocampal volume differences paralleled our previously reported NAA changes (Mathew et al., 2008). Accordingly, we used high-resolution magnetic resonance images (MRI) acquired concurrently with the spectroscopy (1H-MRS) scans at baseline and after 8 weeks of riluzole treatment.
Of particular relevance to the current study is convergent evidence of neurotoxicity in mood and anxiety disorders. This neurotoxicity has been hypothesized to be the result of glutamatergic excitotoxicity (Sanacora et al., 2011). A detailed review of the “neuroplasticity hypothesis” is beyond the scope of the current report. However, a brief description of this hypothesis is necessary to delineate the mechanisms underlying hippocampal volumetric changes and why riluzole may favorably impact GAD subjects with reduced hippocampal volume. The “neuroplasticity hypothesis” is primarily based on preclinical findings of increased synaptic and extra-synaptic glutamate in response to stress and glucocorticoids (Krugers et al., 2010; Musazzi et al., 2010; Yuen et al., 2011). This glutamatergic hyperactivity has been shown in preclinical studies to induce excitotoxicity and disrupted neuroplasticity (Hardingham and Bading, 2010). In line with this hypothesis, a replicated human data has shown altered glutamate levels (Kondo et al., 2011; Yuksel and Ongur, 2010), along with cytoarchitectural (Rajkowska and Miguel-Hidalgo, 2007; Schroeter et al., 2010), microstructural (Sexton et al., 2009; Thomason and Thompson, 2011; White et al., 2008) and morphometric brain regional abnormalities in mood and anxiety disorders (Kempton et al., 2011; Radua et al., 2010). Taken together, these findings suggest that patients with chronic GAD may exhibit increased excitotoxicity and hippocampal volume reduction. In addition, this data raises the question if riluzole, a glutamate-modulating agent, would preferentially impact patients with baseline hyperglutamatergic-mediated excitotoxicity, as reflected by reduced hippocampal volume.
Considering these previous reports, we investigated four questions:
Are hippocampal volumetric deficits found in GAD patients compared to healthy subject, and is there a relationship between hippocampal volume and outcome following riluzole treatment?
Are there hippocampal volume changes following 8 weeks of treatment with riluzole in GAD?
Do NAA changes (final – baseline) correlate with hippocampal volume changes (final – baseline)?
Do hippocampal volume changes (final – baseline) correlate with improvement in clinical measures of anxiety in GAD?
2. Experimental Procedures
2.1 Subjects
Sample characteristics were previously described (Mathew et al., 2008). Briefly, 18 patients with GAD and 10 healthy subjects entered the study. Of these participants, 14 medication-free GAD patients (8 women, mean (±SEM) age 33.9 ± 2.7 years) and 10 healthy subjects (6 women, mean (±SEM) age 30.3 ± 2.4 years) successfully completed baseline high-resolution MRI scans (see Fig. 1). Thirteen GAD and 8 healthy subjects completed an additional MRI at Week 8. 11 GAD and 7 healthy subjects had viable MRI and 1H-MRS data at both assessments. All available data were used in statistical analyses (for more details please see online supplemental materials and Table S.1). GAD patients were medication-free for at least 2 weeks prior to the initial scan. Immediately following baseline scans, patients were treated with open-label riluzole (50mg twice per day) monotherapy for 8 weeks (Mathew et al., 2005). All patients met DSM-IV-TR criteria for GAD according to the Structured Clinical Interview for DSM-IV (SCID) (First et al., 1995). GAD patients had a chronic course of illness with moderate severity of illness.
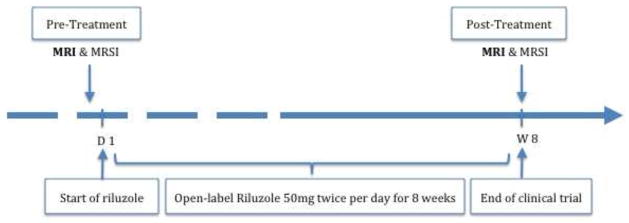
Figure 1. Study Design.
Medication-free patients with primary diagnosis of generalized anxiety disorder (GAD) were treated with riluzole 50mg twice a day for 8 weeks. Healthy controls and GAD participants received magnetic resonance imaging and spectroscopy at baseline and at the end of week 8.
Exclusion criteria for GAD patients included the following: major depressive episode or substance abuse/dependence within 6 months of study entry; lifetime histories of psychosis, bipolar disorder, obsessive-compulsive disorder (OCD), eating disorder, or posttraumatic stress disorder (PTSD); or significant medical or neurologic conditions requiring daily medication treatment. Healthy subjects had no lifetime history of Axis I psychiatric disorders, as established by the SCID-NP (Spitzer et al., 1996). An Institutional Review Board approved the study and all participants provided written informed consent prior to research procedures.
2.2. MRI acquisition and image processing
The MRI and MRS acquisition was performed using a 1.5T GE Genesis Horizon 5.x Signa MR system. Spectroscopy acquisition and processing were previously reported (Mathew et al., 2008). Briefly, the 1H MRS imaging (MRSI) scans were performed using the method of Duyn et al. (Duyn et al., 1993), with TE/TR 280/2300 msec, field of view 240 mm, 32 × 32 circularly sampled k-space phase-encoding steps with one excitation per phase-encoding step, and 256 time-domain points. The raw MRSI data were processed and analyzed voxel by voxel offline on a Sun Microsystems (Mountain View, CA) workstation, using Interactive Data Language (IDL, ITT Visual Information Solutions, Boulder, CO) software package developed in-house by two of the investigators (XM, DCS). From the integral of the point-spread function following spatial filtering and Fourier transformation, we estimated the actual size of each MRSI voxel to be 1.13 cm3 or 40% larger than the nominal voxel size (0.75 × 0.75 × 1.5 cm or 0.83 cm3) that would be derived on the basis of spatial data parameters (Murrough et al., 2010). Voxels that best covered the right and left hippocampus in each subject were selected on the basis of their location on the matching high-resolution MR images. Peak areas derived from spectral fitting were converted to “absolute” (i.e., molar) metabolite concentrations using phantom replacement methodology (Soher et al., 1996). MRI images were acquired using a Sagittal T1-gradient echo volumetric protocol (TR= 34 ms, TE= 5 ms, FOV=240 mm, 1.5-mm thickness with no gaps, totalizing 256 slices per slab, matrix size 256 × 256, NEX=1, voxel size = 0.9375 × 0.9375 × 1.5 mm, FreeSurfer resolution is 1 mm3). Images were converted to ANALYZE format, using MRIcro. Cortical surface reconstruction and volumetric segmentation was performed with the Freesurfer image analysis package (http://surfer.nmr.mgh.harvard.edu/). This processing includes imaging intensity normalization, removal of non-brain tissue, segmentation of the gray/white matter and subcortical volumetric structures (including hippocampus), tessellation of the gray matter white matter boundary, automated topology correction, spherical surface-based intersubject registration based on curvature (sulcus and gyri) and automated panellation of cortical regions. Freesurfer results were visually inspected, however, no manual corrections were necessary. Additionally, FreeSurfer results were confirmed using FSL hippocampal segmentation (ICC = 0.87). The investigators processing the neuroimaging data and generating the estimated hippocampal volume were blinded to gender, age, and treatment status. Although this pipeline provides the volumes of several brain regions, since this is a hypothesis-based study, only the estimated hippocampal (at each hemisphere) and intracranial volumes were used in further analysis (Fischl and Dale, 2000).
2.3. Statistical analyses
A t-test or chi square were conducted to assess demographic and clinical differences between GAD and healthy subjects. As previously reported, age, gender, body mass index (BMI), IQ, and education level did not differ between GAD and healthy subjects (Mathew et al., 2008). GAD patients were subgrouped according to a median split cutoff point of Hamilton Anxiety Rating Scale (HAM-A) scores at the end of week 8 of HAM-A ≤ 7 (HAM-A scores ranged from 1-to-7 for remitters and 9-to-20 for non-remitters). A HAM-A score ≤ 7 represents a standard definition of remission in psychopharmacology trials of GAD (Mathew and Hoffman, 2008). The Penn State Worry Questionnaire (PSWQ), a self-reported anxiety rating scale, was used as a secondary clinical outcome measure. One-way analysis of variance (ANOVA) was performed to examine baseline characteristics among response groups (remitters, non-remitters, and healthy subjects). Age, gender, BMI, IQ, and intracranial volume (ICV) were examined as covariates for right and left hippocampal volume using Pearson’s correlation. However, only ICV had a significant effect on right (r = 0.70, p < 0.001) and left (r = 0.67, p < 0.001) hippocampal volume. As a consequence, ICV was retained as a covariate in hippocampal volume analyses. Providing there were no significant interactions between brain side (left or right) and group, right and left hippocampal volumes were combined.
To address our first hypothesis, a general linear model (GLM) was conducted to study hippocampal volumetric differences among groups while controlling for ICV. Post-hoc analyses of pairwise comparisons of estimated marginal means used Bonferroni adjustment for multiple comparisons. Total hippocampal volume, a measure of combined right and left hippocampi, was used as the primary ROI representative, with post-hoc analyses exploring hemispheric effects. To address the second hypothesis, GLM with repeated measures (mixed effects) was conducted on hippocampal volume as the dependent variable, with time of scan (baseline & post-treatment) as the within-subject factor (repeated measure), group (remitters vs. non-remitters vs. healthy subjects) as the between-subjects factor, and ICV as covariate. To address hypothesis three, delta hippocampal volume and NAA changes over the treatment period were each computed by subtracting the baseline from Week 8 value (final minus baseline). Because delta total hippocampal volume and delta left hippocampal volume were not normally distributed, Spearman’s Rank Order correlation coefficient was used for these variables. ICV had no effect on delta variables, thus partial correlations were not computed for these variables. To address hypothesis four, the correlations between delta hippocampal volume and clinical measures were examined. All tests were two-tailed, with significance level set at p ≤ 0.05.
3. Results
Clinical trial outcomes were previously reported (Mathew et al., 2008). In summary, there was a 64% decline in HAM-A scores over the treatment period of 8 weeks (mean ±SE, from 20 ±0.95 to 7.2 ±1.44, paired t(13) = 8.71, p < 0.001). Of the 13 patients who completed both MRI scans, 7 (54%) met remission criteria (HAM-A ≤ 7) and 6 (46%) were non-remitters. No clinical or demographic differences were found between remitters and non-remitters (see Table 1).
Table 1.
Baseline Characteristics of Remitters, Non-remitters and Healthy Subjects.
| Remitters (n = 7) | Non-remitters (n = 6) | Healthy Subjects (n = 10) | Sig. a | |
|---|---|---|---|---|
|
| ||||
| Mean ± SEM | Mean ± SEM | Mean ± SEM | ||
| Age | 31.9 (5.0) | 31.2 (1.9) | 30.3 (2.4) | .94 |
| Female (N; %) | 4 (57%) | 3 (50%) | 6 (60%) | .93 |
| BMI | 22.81 (1.81) | 22.82 (1.78) | 21.94 (0.61) | .90 |
| IQ | 119.3 (3.2) | 124.8 (1.0) | 118.3 (4.6) | .53 |
| Age of Onset | 17.3 (4.6) | 15.0 (2.0) | N/A | .68 |
| Duration of Illness | 14.6 (4.8) | 16.2 (2.3) | N/A | .78 |
| Psychotropic-naïve (N; %) | 3 (43%) | 2 (33%) | N/A | .72 |
| HAM-A Baseline | 18.3 (0.6) | 21.2 (1.7) | 1.6 (0.5) | < .001b |
| PSWQ Baseline | 65.9 (3.4) | 63.2 (3.6) | 33.7 (3.1) | < .001c |
ANOVA, Independent t-test or Chi square test (significance set at p ≤ .05);
Post-Hoc analysis showed no significant HAM-A difference between Remitters and Non-remitters (p = .14);
Post-Hoc analysis showed no significant PSWQ difference between Remitters and Non-remitters (p = 1.0);
Abbreviations: BMI: Body Mass Index; IQ: was determined with the Wechsler Abbreviated Scale of Intelligence; HAM-A, Hamilton Anxiety Rating Scale; PSWQ, Penn State Worry Questionnaire.
1- Are hippocampal volumetric deficits found in GAD patients compared to healthy subject, and is there a relationship between pre-treatment hippocampal volume and outcome following riluzole treatment?
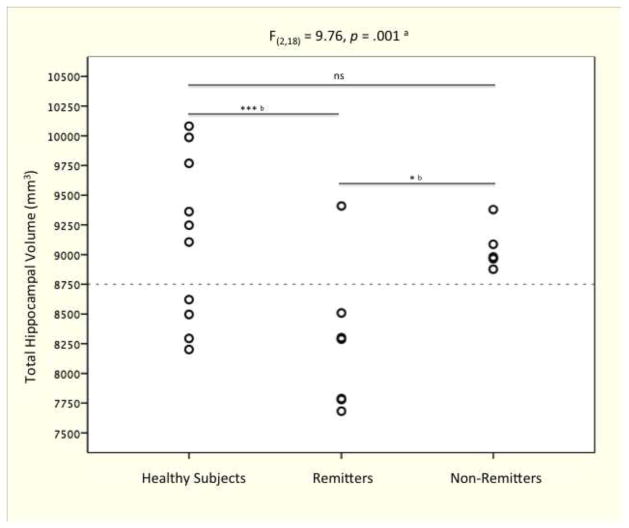
Prior to riluzole treatment, the GAD group had 5% smaller hippocampal volume as compared to healthy subjects (F(1,21) = 6.55, n = 24 [14 GAD and 10 healthy control], p = 0.02). In addition, there was a main effect of treatment response (F(2,18) = 9.76, n = 22 [12 GAD with known response status and 10 healthy control], p = 0.001), with remitters having reduced total hippocampal volume compared to non-remitters (7% smaller, p = 0.02) and healthy subjects (9% smaller, p = 0.001) (see Fig. 2). Post hoc analyses showed similar findings for both hemispheres (right and left) without hemispheric effects.
Figure 2. Baseline Total Hippocampal Volume Differences Among Response Groups.
Post-hoc analyses showed a significantly smaller hippocampus in remitters (HAM-A ≤ 7) as compared to non-remitters and healthy subjects. However there were no significant hippocampal differences between non-remitters and healthy subjects. a. General Linear Model univariate analysis controlling for intracranial volume; b. post-hoc pairwise comparison of estimated marginal means with Bonferroni adjustment for multiple comparisons; * p ≤ .05; *** p ≤ .001; ns: non-significant.
2- Are there hippocampal volume changes following 8 weeks of treatment with riluzole in GAD?
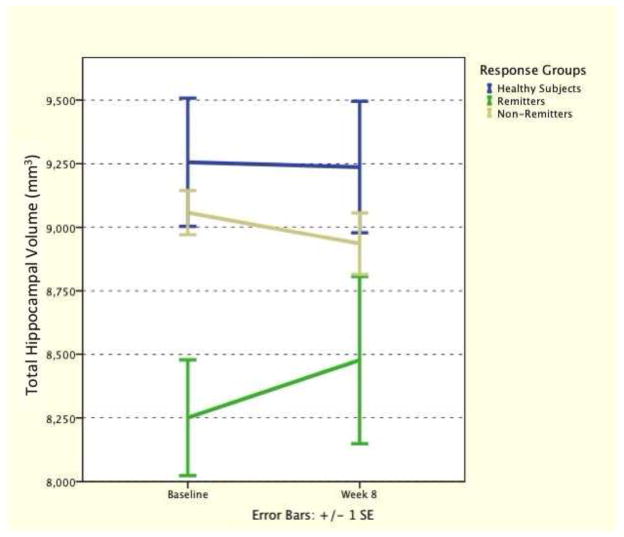
Controlling for ICV, GLM repeated-measures revealed main effect of response group (F(2,16) = 6.70, p = 0.007) but no effect of time (p > 0.5). Time-by-responder group interaction showed a numerical increase in hippocampal volume in remitters, but did not reach statistical significance (F(2,16) = 2.35, p = 0.13) (see Fig. 3).
Figure 3. Hippocampal Volume Changes from Baseline to the End of Week 8.
Riluzole had no statistically significant effect on hippocampal volume changes over the study period.
3- Do NAA changes (final – baseline) correlate with hippocampal volume changes (final – baseline)?
At baseline, there was no correlation between total hippocampal volume and NAA concentration across all study participants (rpartial = 0.16, n = 22, p = 0.48). However, at Week 8, there was a positive correlation between total hippocampal volume and NAA concentration (rpartial = 0.48, n = 19, p = 0.045). This association was evident in the GAD group (rpartial = 0.61, n = 12, p = 0.047), but was not significant in healthy subjects (rpartial = − 0.06, n = 7, p = 0.91).
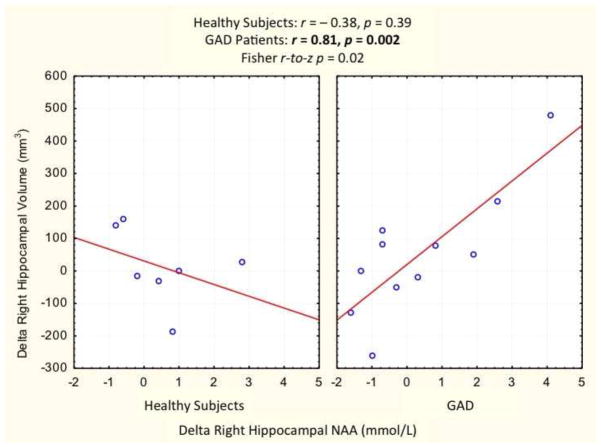
Delta total hippocampal volume (final – baseline) correlated with delta hippocampal NAA in GAD group (rspearman = 0.62, n = 11, p = 0.04). In the GAD group, the positive correlation between delta hippocampal volume and delta hippocampal NAA was especially strong in the right hippocampus (r = 0.81, p = 0.002), but was not significant in healthy subjects (r = − 0.38, p = 0.39; Fisher r-to-z p = 0.02) (see Fig. 4). The corresponding correlations were not significant in the left hippocampus (rspearman = 0.62, p = 0.3).
Figure 4. Association Between Delta Right Hippocampal Volume and N-Acetylaspartate (NAA).
Delta right hippocampal volume highly correlated with delta right hippocampal NAA in GAD patients receiving 8 weeks of riluzole treatment. This correlation was not evident in a sex- and age-matched healthy control group who did not receive the study drug.
4- Do hippocampal volume changes (final – baseline) correlate with improvement in clinical measures of anxiety in GAD?
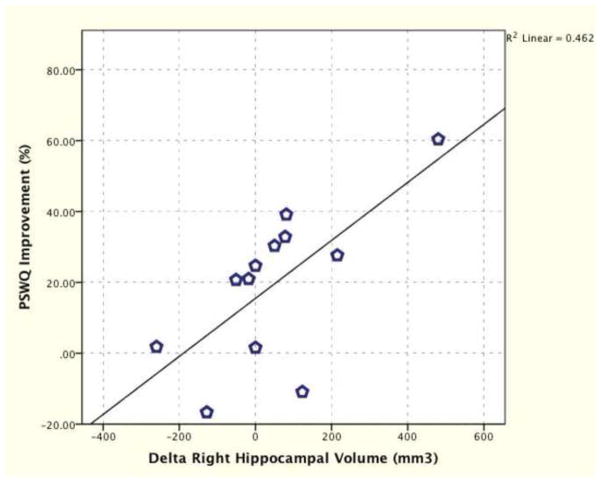
Delta total hippocampal volume (final – baseline) positively correlated with improvement in HAM-A (rspearman = 0.62, n = 12, p = 0.03; Fig. S.1), however, the correlation between delta total hippocampal volume and improvement in PSWQ (p = 0.20) was not significant. Delta right hippocampal volume positively correlated with improvement in HAM-A (r = 0.64, n = 12, p = 0.03; Fig. S.2) and PSWQ (r = 0.68, n = 12, p = 0.01; Fig. 5). Delta left hippocampal volume positively correlated with HAM-A (rspearman = 0.58, n = 12, p = 0.049; Fig. S.3), but did not reach significance with improvement in PSWQ (p = 0.31).
Figure 5. Association Between Delta Right Hippocampal Volume and Penn State Worry Questionnaire (PSWQ).
Delta right hippocampal volume positively correlated with delta PSWQ in GAD patients receiving 8 weeks of riluzole treatment (r = 0.68, n = 12, p = 0.01).
Exploratory analyses were performed relating hippocampal volume to clinical measures. At baseline in the GAD group, there was a positive correlation between left hippocampal volume and HAM-A scores (rpartial = 0.64, n = 12, p = 0.03). In addition, baseline right hippocampal volume was negatively correlated with improvement in PSWQ (rpartial = − 0.72, n = 12, p = 0.01). No additional significant correlations were evident for hippocampal volume measures at baseline or post-treatment with HAM-A or PSWQ.
4. Discussion
In this report, we tested several hypotheses concerning the relationship between hippocampal volume (assessed with MRI), hippocampal NAA (assessed with 1H MRS), and clinical outcomes in GAD patients treated for 8 weeks with the glutamate modulating agent riluzole. First, we confirmed our hypothesis that patients with GAD, compared to age and sex matched healthy volunteers, would show a significant reduction in hippocampal volumes. This reduction was most pronounced in the remitter subgroup, compared to non-remitters and healthy subjects (see Fig. 2). Our second hypothesis, that riluzole treatment would significantly increase hippocampal volume in remitters, was not confirmed. However, it is plausible that this pilot study is underpowered to detect a statistically significant interaction, given the minimal effect size (η2 = 0.23) (see Fig. 3). In this context, the low p value (p = 0.13) combined with correlation between delta volume, NAA, and clinical improvement suggests that riluzole may have increased hippocampal volume in remitters. Concerning the third hypothesis, we found a positive association between changes in hippocampal volume and change of hippocampal NAA in riluzole treated GAD patients. This association was particularly robust in the right hippocampus (r = 0.81)(see Fig. 4). Finally, the fourth hypothesis was supported, as hippocampal volume changes over the treatment period were positively associated with improvement on the HAM-A. Overall, these preliminary findings support the potential utility of hippocampal volumes and NAA as neuroplasticity biomarkers to assess response to this agent.
To our knowledge, our study is the first to examine longitudinal hippocampal volumetric changes and their correlation with hippocampal NAA levels, particularly in the context of treatment with a glutamate-based drug. Reduced cerebral NAA levels have been widely used as a marker of neuronal loss in several neurological and neuropsychiatric disorders. Nonetheless, the use of NAA as a marker of neuronal loss was challenged by later evidence suggesting that NAA levels could also reflect neuronal dysfunction or reversible metabolic impairment (for review see (Moffett et al., 2007)). In addition, NAA has been found in cells other than neurons (Urenjak et al., 1992). These challenges can be partially mitigated by utilizing a multi-modal MR imaging approach. In that regard, our study demonstrates the utility and feasibility of combined MRI-MRS acquisition. This method provided added insight into the neurobiological substrate underlying GAD and its response to riluzole. The study design is enhanced by the contemporaneous acquisition of MRI and MRS data.
Of particular relevance to the current report is the hypothesized role of glia in the pathophysiology of stress related disorders (Valentine and Sanacora, 2009). Glial cells are responsible for clearing glutamate from the intra and extra-synaptic cleft and for converting glutamate to glutamine (Halassa and Haydon, 2010). Thus, glial impairment has been hypothesized to induce heightened glutamatergic-mediated excitotoxicity. Consistent with these findings, animal models of stress demonstrated reduced glial cell density and impaired glutamate transporters (Banasr and Duman, 2008; Fuchs, 2005; Liu et al., 2011b; Zink et al., 2010), suggesting the presence of a stress induced hyperglutamatergic state. Interestingly, riluzole was shown to oppose the effect of glial impairment in rats exposed to stress (Banasr et al., 2010). Furthermore, riluzole is known to exert neuroprotective properties of increasing brain-derived neurotrophic factor (BDNF), glial cell line-derived neurotrophic factor (GDNF) and neurogenesis (Gourley et al., 2011; Katoh-Semba et al., 2002; Mizuta et al., 2001; Tsuchioka et al., 2011). Taken together, these findings strongly implicate riluzole in modulating stress-related glutamatergic dysregulation and neurotoxicity.
These results are potentially consistent with the view that, pre-treatment glutamatergic hyperactivity in hippocampus in high responder patients engendered excitotoxicity and maladaptive neuroplasticity as evident by smaller hippocampi. A hyperglutamatergic state may render a subgroup of GAD patients more responsive to treatment with riluzole – a drug known to decrease glutamate release and facilitate cellular uptake. In line with this hypothesis, Cavus et al. demonstrated a strong correlation between reduced hippocampal volume and elevated extracellular glutamate in the hippocampus of patients with seizure disorder (Cavus et al., 2008). Interestingly, in studies of monoaminergic antidepressants, smaller hippocampal volumes have been associated with poor response in depressed patients (Frodl et al., 2008; Frodl et al., 2004; Hsieh et al., 2002; Kronmuller et al., 2008; MacQueen et al., 2008; Vakili et al., 2000). In contrast, riluzole produced greater effect in patients with smaller hippocampi.
Strength and Limitations
Our study identified a subgroup of GAD patients distinctively responsive to treatment with riluzole. However, it is important to consider the limitations of this pilot, exploratory study, which include the modest sample size, lack of placebo control, and short-term nature of the treatment intervention. In addition, the spectroscopy methods did not permit the quantification of glutamate. Our MRS imaging method used a TE of 288 ms which, while optimal for quantification of NAA by eliminating short T2 compounds and flattening the spectra baseline, is too long for detection of glx (glutamate + glutamine) which requires use of short TE (~30 ms or less). Determining glutamate levels, specifically glutamate/glutamine cycling, would be especially important to further test our hypothesis. Future studies should use recently developed spectroscopy methods using 13C labeled isotopes (13C-MRS) to directly and dynamically monitor glutamate/glutamine cycling. Interestingly, in patients with bipolar depression there was a significant increase in glutamine/glutamate ratio by day 2 of riluzole therapy. These glutamine/glutamate changes were positively associated – at a trend level – with NAA changes over the 6-week treatment period (Brennan et al., 2010). This data further emphasize the need to investigate, using 13C-MRS, the effect of riluzole on glutamate/glutamine cycling and its correlation with clinical and biological measures. Future studies should also attempt to integrate peripheral markers of neuroplasticity (i.e. BDNF) with imaging.
Finally, the strengths of the current study include a well-matched healthy control group, a medication free cohort, and combined structural and spectroscopic acquisition. In addition, the low test-retest variability for hippocampal volume (CV 2.4%) and NAA (CV 4.1%) in healthy subjects confers increased confidence in the validity of the repeated measures.
Conclusions
In contrast to monoaminergic antidepressants, where smaller hippocampal volume has been associated with poor response in depressed patients, the current study is the first to show that riluzole – a glutamate-modulating agent – produces a greater effect in patients with smaller hippocampi. In addition, this study highlights multi-modal neuroimaging, particularly concurrent MR structural and spectroscopy acquisition, in developing mechanistic hypotheses of drug action. The strong correlations between delta hippocampal volume and delta NAA, in the context of differing response to riluzole supports NAA as a neuroplasticity biomarker to assess response to glutamatergic drugs. Moreover, our study supports the hypothesis that disrupted baseline glutamatergic state – as reflected by reductions in hippocampal volume – moderates response to riluzole, and suggests volumetric MRI may have biomarker utility in predicting riluzole response. Finally, the preliminary evidence for hippocampal volume and NAA levels as response biomarkers is an exciting finding that warrants replication in an expanded sample. This line of research is particularly important as the field anticipates novel psychotropic medications, with several glutamate-based drugs currently being tested as treatment for neuropsychiatric disorders (Pankevich et al., 2011).
Supplementary Material
Acknowledgments
This work was supported by Brain and Behavior Research Foundation, Sackler Institute of Columbia University, National Institute of Mental Health (K23-MH-069656), and National Institute on Drug Abuse (T32-DA022975).
Role of the funding source:
The funding sources have no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Contributors
C.G.A. and J.D.C. conceptualized the MRI study, managed the literature searches, undertook the statistical analysis, contributed to the interpretation of the data and wrote the manuscript. A.J. and J.R.S. performed the MRI image processing, segmentations, quality control and analyses. D.C.S. and X.M. performed the MRS and MRI studies, and MRS image processing and analysis. S.J.M. conceived the study, wrote the protocol, performed the study, and contributed to the interpretation of the data. All authors contributed to and have approved the final manuscript.
Conflict of interest
JDC receives grant support from NIMH, NYSTEM, GlaxoSmithKline, Pfizer, and Alexza Pharmaceuticals. He is on the Pfizer advisory board and gives talks for BMS, AstraZeneca, GSK, and Pfizer. SJM: Grant or Research Support: NIMH, NARSAD, Dept. of Veterans Affairs. Consultant (last 24 months): Cephalon, Roche, Noven, AstraZeneca. No biomedical financial interests or potential conflicts of interest are reported for CGA, AJ, JRS, XM, and DCS.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banasr M, Chowdhury GM, Terwilliger R, Newton SS, Duman RS, Behar KL, Sanacora G. Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Molecular Psychiatry. 2010;15:501–511. doi: 10.1038/mp.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasr M, Duman RS. Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biological Psychiatry. 2008;64:863–870. doi: 10.1016/j.biopsych.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan BP, Hudson JI, Jensen JE, McCarthy J, Roberts JL, Prescot AP, Cohen BM, Pope HG, Jr, Renshaw PF, Ongur D. Rapid enhancement of glutamatergic neurotransmission in bipolar depression following treatment with riluzole. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2010;35:834–846. doi: 10.1038/npp.2009.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavus I, Pan JW, Hetherington HP, Abi-Saab W, Zaveri HP, Vives KP, Krystal JH, Spencer SS, Spencer DD. Decreased hippocampal volume on MRI is associated with increased extracellular glutamate in epilepsy patients. Epilepsia. 2008;49:1358–1366. doi: 10.1111/j.1528-1167.2008.01603.x. [DOI] [PubMed] [Google Scholar]
- Duyn JH, Gillen J, Sobering G, van Zijl PC, Moonen CT. Multisection proton MR spectroscopic imaging of the brain. Radiology. 1993;188:277–282. doi: 10.1148/radiology.188.1.8511313. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders: Patient Edition (SCIDI/P. Version 2.0. Biometric Research, New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Jager M, Smajstrlova I, Born C, Bottlender R, Palladino T, Reiser M, Moller HJ, Meisenzahl EM. Effect of hippocampal and amygdala volumes on clinical outcomes in major depression: a 3-year prospective magnetic resonance imaging study. J Psychiatry Neurosci. 2008;33:423–430. [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Meisenzahl EM, Zetzsche T, Hohne T, Banac S, Schorr C, Jager M, Leinsinger G, Bottlender R, Reiser M, Moller HJ. Hippocampal and amygdala changes in patients with major depressive disorder and healthy controls during a 1-year follow-up. The Journal of clinical psychiatry. 2004;65:492–499. doi: 10.4088/jcp.v65n0407. [DOI] [PubMed] [Google Scholar]
- Fuchs E. Social stress in tree shrews as an animal model of depression: an example of a behavioral model of a CNS disorder. CNS Spectrums. 2005;10:182–190. doi: 10.1017/s1092852900010038. [DOI] [PubMed] [Google Scholar]
- Gourley SL, Espitia JW, Sanacora G, Taylor JR. Antidepressant-like properties of oral riluzole and utility of incentive disengagement models of depression in mice. Psychopharmacology. 2011 doi: 10.1007/s00213-011-2403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Haydon PG. Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu Rev Physiol. 2010;72:335–355. doi: 10.1146/annurev-physiol-021909-135843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci. 2010;11:682–696. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh MH, McQuoid DR, Levy RM, Payne ME, MacFall JR, Steffens DC. Hippocampal volume and antidepressant response in geriatric depression. Int J Geriatr Psychiatry. 2002;17:519–525. doi: 10.1002/gps.611. [DOI] [PubMed] [Google Scholar]
- Katoh-Semba R, Asano T, Ueda H, Morishita R, Takeuchi IK, Inaguma Y, Kato K. Riluzole enhances expression of brain-derived neurotrophic factor with consequent proliferation of granule precursor cells in the rat hippocampus. The FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2002;16:1328–1330. doi: 10.1096/fj.02-0143fje. [DOI] [PubMed] [Google Scholar]
- Kempton MJ, Salvador Z, Munafo MR, Geddes JR, Simmons A, Frangou S, Williams SC. Structural neuroimaging studies in major depressive disorder. Meta-analysis and comparison with bipolar disorder. Archives of General Psychiatry. 2011;68:675–690. doi: 10.1001/archgenpsychiatry.2011.60. [DOI] [PubMed] [Google Scholar]
- Kondo DG, Hellem TL, Sung YH, Kim N, Jeong EK, Delmastro KK, Shi X, Renshaw PF. Review: magnetic resonance spectroscopy studies of pediatric major depressive disorder. Depress Res Treat. 2011;2011:650450. doi: 10.1155/2011/650450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronmuller KT, Pantel J, Kohler S, Victor D, Giesel F, Magnotta VA, Mundt C, Essig M, Schroder J. Hippocampal volume and 2-year outcome in depression. The British journal of psychiatry: the journal of mental science. 2008;192:472–473. doi: 10.1192/bjp.bp.107.040378. [DOI] [PubMed] [Google Scholar]
- Krugers HJ, Hoogenraad CC, Groc L. Stress hormones and AMPA receptor trafficking in synaptic plasticity and memory. Nature reviews Neuroscience. 2010;11:675–681. doi: 10.1038/nrn2913. [DOI] [PubMed] [Google Scholar]
- Liu AY, Mathur R, Mei N, Langhammer CG, Babiarz B, Firestein BL. Neuroprotective drug riluzole amplifies the heat shock factor 1 (HSF1)- and glutamate transporter 1 (GLT1)-dependent cytoprotective mechanisms for neuronal survival. J Biol Chem. 2011a;286:2785–2794. doi: 10.1074/jbc.M110.158220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Li B, Zhu HY, Wang YQ, Yu J, Wu GC. Glia atrophy in the hippocampus of chronic unpredictable stress-induced depression model rats is reversed by electroacupuncture treatment. Journal of Affective Disorders. 2011b;128:309–313. doi: 10.1016/j.jad.2010.07.007. [DOI] [PubMed] [Google Scholar]
- MacQueen GM, Yucel K, Taylor VH, Macdonald K, Joffe R. Posterior hippocampal volumes are associated with remission rates in patients with major depressive disorder. Biological Psychiatry. 2008;64:880–883. doi: 10.1016/j.biopsych.2008.06.027. [DOI] [PubMed] [Google Scholar]
- Mathew SJ, Amiel JM, Coplan JD, Fitterling HA, Sackeim HA, Gorman JM. Open-label trial of riluzole in generalized anxiety disorder. The American journal of psychiatry. 2005;162:2379–2381. doi: 10.1176/appi.ajp.162.12.2379. [DOI] [PubMed] [Google Scholar]
- Mathew SJ, Hoffman E. Pharmacotherapy for Generalized Anxiety Disorder. Oxford handbook of anxiety and related disorders. 2008;350 [Google Scholar]
- Mathew SJ, Price RB, Mao X, Smith EL, Coplan JD, Charney DS, Shungu DC. Hippocampal N-acetylaspartate concentration and response to riluzole in generalized anxiety disorder. Biol Psychiatry. 2008;63:891–898. doi: 10.1016/j.biopsych.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuta I, Ohta M, Ohta K, Nishimura M, Mizuta E, Kuno S. Riluzole stimulates nerve growth factor, brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor synthesis in cultured mouse astrocytes. Neuroscience Letters. 2001;310:117–120. doi: 10.1016/s0304-3940(01)02098-5. [DOI] [PubMed] [Google Scholar]
- Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol. 2007;81:89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Mao X, Collins KA, Kelly C, Andrade G, Nestadt P, Levine SM, Mathew SJ, Shungu DC. Increased ventricular lactate in chronic fatigue syndrome measured by 1H MRS imaging at 3.0 T. II: comparison with major depressive disorder. NMR Biomed. 2010;23:643–650. doi: 10.1002/nbm.1512. [DOI] [PubMed] [Google Scholar]
- Musazzi L, Milanese M, Farisello P, Zappettini S, Tardito D, Barbiero VS, Bonifacino T, Mallei A, Baldelli P, Racagni G, Raiteri M, Benfenati F, Bonanno G, Popoli M. Acute stress increases depolarization-evoked glutamate release in the rat prefrontal/frontal cortex: the dampening action of antidepressants. PLoS One. 2010;5:e8566. doi: 10.1371/journal.pone.0008566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankevich DE, Davis M, Altevogt BM. Glutamate-Related Biomarkers in Drug Development for Disorders of the Nervous System: Workshop Summary. Washington (DC): 2011. [PubMed] [Google Scholar]
- Pittenger C, Coric V, Banasr M, Bloch M, Krystal JH, Sanacora G. Riluzole in the treatment of mood and anxiety disorders. CNS Drugs. 2008;22:761–786. doi: 10.2165/00023210-200822090-00004. [DOI] [PubMed] [Google Scholar]
- Radua J, van den Heuvel OA, Surguladze S, Mataix-Cols D. Meta-analytical comparison of voxel-based morphometry studies in obsessive-compulsive disorder vs other anxiety disorders. Archives of General Psychiatry. 2010;67:701–711. doi: 10.1001/archgenpsychiatry.2010.70. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ. Gliogenesis and glial pathology in depression. CNS & neurological disorders drug targets. 2007;6:219–233. doi: 10.2174/187152707780619326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Kendell SF, Levin Y, Simen AA, Fenton LR, Coric V, Krystal JH. Preliminary evidence of riluzole efficacy in antidepressant-treated patients with residual depressive symptoms. Biological Psychiatry. 2007;61:822–825. doi: 10.1016/j.biopsych.2006.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression An emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Zarate CA, Krystal JH, Manji HK. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov. 2008;7:426–437. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter ML, Abdul-Khaliq H, Sacher J, Steiner J, Blasig IE, Mueller K. Mood disorders are glial disorders: evidence from in vivo studies. Cardiovasc Psychiatry Neurol. 2010;2010:780645. doi: 10.1155/2010/780645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton CE, Mackay CE, Ebmeier KP. A systematic review of diffusion tensor imaging studies in affective disorders. Biological Psychiatry. 2009;66:814–823. doi: 10.1016/j.biopsych.2009.05.024. [DOI] [PubMed] [Google Scholar]
- Soher BJ, van Zijl PC, Duyn JH, Barker PB. Quantitative proton MR spectroscopic imaging of the human brain. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 1996;35:356–363. doi: 10.1002/mrm.1910350313. [DOI] [PubMed] [Google Scholar]
- Spitzer R, Williams J, Gibbon M. Structured clinical interview for DSM-IV: non-patient edition (SCID-NP) New York: Biometrics Research Department. New York State Psychiatric Institute; 1996. [Google Scholar]
- Thomason ME, Thompson PM. Diffusion imaging, white matter, and psychopathology. Annu Rev Clin Psychol. 2011;7:63–85. doi: 10.1146/annurev-clinpsy-032210-104507. [DOI] [PubMed] [Google Scholar]
- Tsuchioka M, Hisaoka K, Yano R, Shibasaki C, Kajiatani N, Takebayashi M. Riluzole-induced glial cell line-derived neurotrophic factor production is regulated through fibroblast growth factor receptor signaling in rat C6 glioma cells. Brain Research. 2011;1384:1–8. doi: 10.1016/j.brainres.2011.01.100. [DOI] [PubMed] [Google Scholar]
- Urenjak J, Williams SR, Gadian DG, Noble M. Specific expression of N-acetylaspartate in neurons, oligodendrocyte-type-2 astrocyte progenitors, and immature oligodendrocytes in vitro. Journal of neurochemistry. 1992;59:55–61. doi: 10.1111/j.1471-4159.1992.tb08875.x. [DOI] [PubMed] [Google Scholar]
- Vakili K, Pillay SS, Lafer B, Fava M, Renshaw PF, Bonello-Cintron CM, Yurgelun-Todd DA. Hippocampal volume in primary unipolar major depression: a magnetic resonance imaging study. Biological Psychiatry. 2000;47:1087–1090. doi: 10.1016/s0006-3223(99)00296-6. [DOI] [PubMed] [Google Scholar]
- Valentine GW, Sanacora G. Targeting glial physiology and glutamate cycling in the treatment of depression. Biochem Pharmacol. 2009;78:431–439. doi: 10.1016/j.bcp.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T, Nelson M, Lim KO. Diffusion tensor imaging in psychiatric disorders. Topics in Magnetic Resonance Imaging: TMRI. 2008;19:97–109. doi: 10.1097/RMR.0b013e3181809f1e. [DOI] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Ren Y, Feng J, McEwen BS, Yan Z. Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory. Molecular Psychiatry. 2011;16:156–170. doi: 10.1038/mp.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuksel C, Ongur D. Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biol Psychiatry. 2010;68:785–794. doi: 10.1016/j.biopsych.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Payne JL, Quiroz J, Sporn J, Denicoff KK, Luckenbaugh D, Charney DS, Manji HK. An open-label trial of riluzole in patients with treatment-resistant major depression. The American journal of psychiatry. 2004;161:171–174. doi: 10.1176/appi.ajp.161.1.171. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Quiroz JA, Singh JB, Denicoff KD, De Jesus G, Luckenbaugh DA, Charney DS, Manji HK. An open-label trial of the glutamate-modulating agent riluzole in combination with lithium for the treatment of bipolar depression. Biological Psychiatry. 2005;57:430–432. doi: 10.1016/j.biopsych.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Zink M, Vollmayr B, Gebicke-Haerter PJ, Henn FA. Reduced expression of glutamate transporters vGluT1, EAAT2 and EAAT4 in learned helpless rats, an animal model of depression. Neuropharmacology. 2010;58:465–473. doi: 10.1016/j.neuropharm.2009.09.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.