Abstract
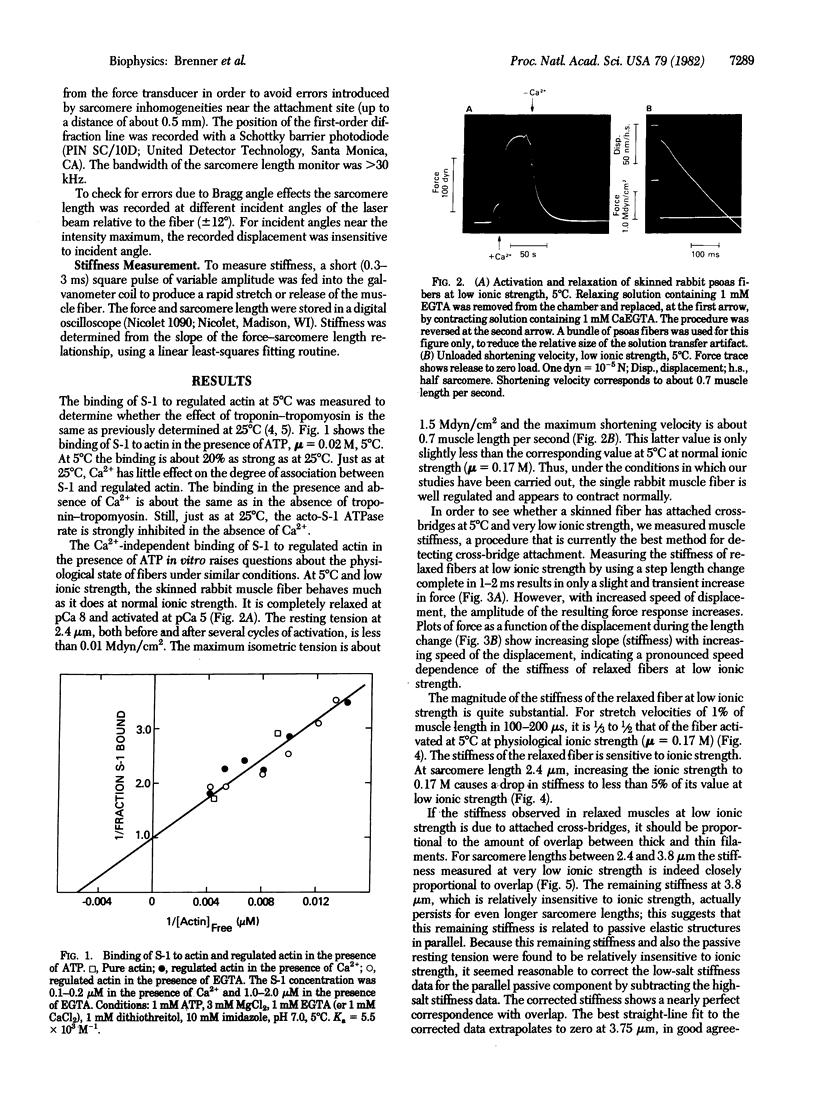
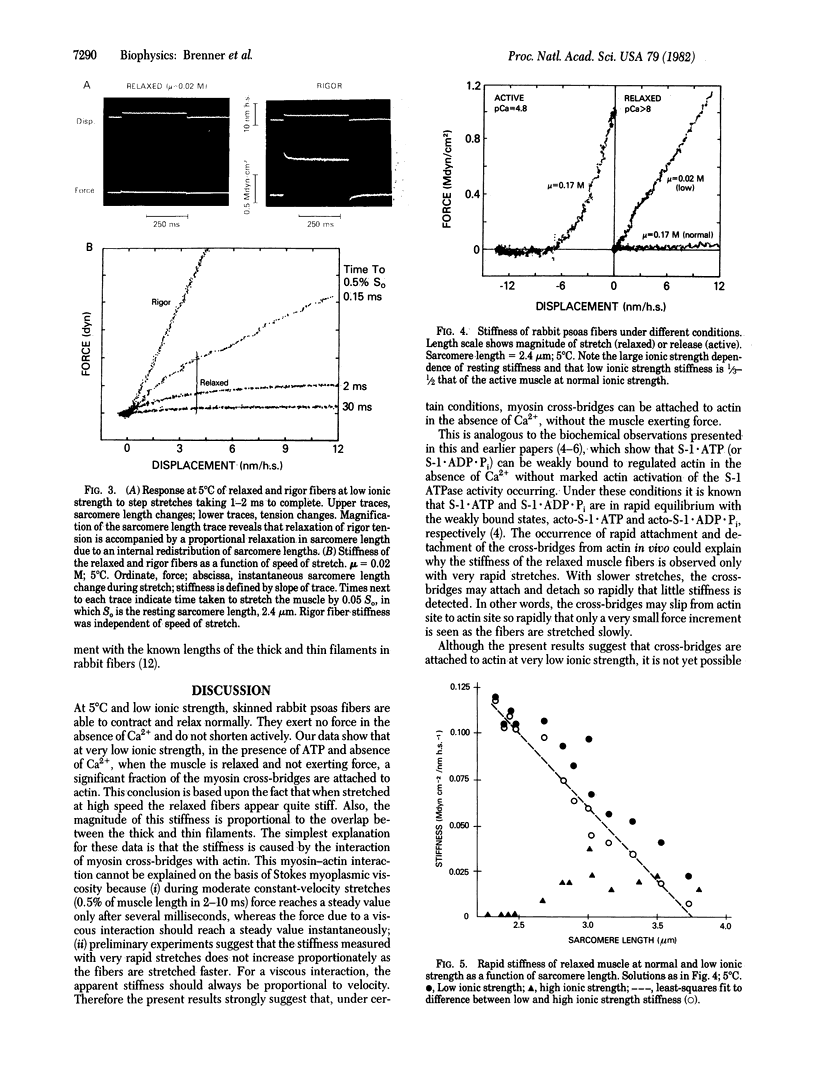
In vitro at low ionic strength (mu = 0.02 M) and 5 degrees C, myosin subfragment-1 shows significant binding to regulated actin in the presence of ATP, independent of the concentration of free Ca2+. Under the same conditions, single skinned rabbit psoas muscle fibers develop force only in the presence of Ca2+ and are relaxed in its absence. However, the stiffness, measured with very rapid stretches (0.5% of muscle length in 0.1 ms), is high even when the fibers are relaxed. This "rapid stiffness" of the resting muscle is sensitive to ionic strength, becoming small at normal ionic strength (mu = 0.17 M). At low ionic strength, the rapid stiffness is approximately proportional to the overlap between the actin and myosin filaments. At zero overlap (sarcomere length = 3.8 microns), the stiffness is less than 20% of the value measured at full overlap. This remaining 20% is relatively insensitive to ionic strength, like the passive resting tension, and it may in fact be due to the structures responsible for the resting tension. Thus, both in vitro binding and the effect of overlap on rapid stiffness measurements in fibers suggest that cross-bridges are attached to actin in relaxed muscle at low ionic strength.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chalovich J. M., Chock P. B., Eisenberg E. Mechanism of action of troponin . tropomyosin. Inhibition of actomyosin ATPase activity without inhibition of myosin binding to actin. J Biol Chem. 1981 Jan 25;256(2):575–578. [PMC free article] [PubMed] [Google Scholar]
- Chalovich J. M., Eisenberg E. Inhibition of actomyosin ATPase activity by troponin-tropomyosin without blocking the binding of myosin to actin. J Biol Chem. 1982 Mar 10;257(5):2432–2437. [PMC free article] [PubMed] [Google Scholar]
- Eastwood A. B., Wood D. S., Bock K. L., Sorenson M. M. Chemically skinned mammalian skeletal muscle. I. The structure of skinned rabbit psoas. Tissue Cell. 1979;11(3):553–566. doi: 10.1016/0040-8166(79)90062-4. [DOI] [PubMed] [Google Scholar]
- Eisenberg E., Kielley W. W. Native tropomyosin: effect on the interaction of actin with heavy meromyosin and subfragment-1. Biochem Biophys Res Commun. 1970 Jul 13;40(1):50–56. doi: 10.1016/0006-291x(70)91044-2. [DOI] [PubMed] [Google Scholar]
- Huxley H. E. Structural difference between resting and rigor muscle; evidence from intensity changes in the lowangle equatorial x-ray diagram. J Mol Biol. 1968 Nov 14;37(3):507–520. doi: 10.1016/0022-2836(68)90118-6. [DOI] [PubMed] [Google Scholar]
- Inoue A., Tonomura Y. Regulation of binding of myosin subfragments with regulated actin by calcium ions in the presence of magnesium ATP. J Biochem. 1982 Apr;91(4):1231–1239. doi: 10.1093/oxfordjournals.jbchem.a133807. [DOI] [PubMed] [Google Scholar]
- Parry D. A., Squire J. M. Structural role of tropomyosin in muscle regulation: analysis of the x-ray diffraction patterns from relaxed and contracting muscles. J Mol Biol. 1973 Mar 25;75(1):33–55. doi: 10.1016/0022-2836(73)90527-5. [DOI] [PubMed] [Google Scholar]
- Stein L. A., Schwarz R. P., Jr, Chock P. B., Eisenberg E. Mechanism of actomyosin adenosine triphosphatase. Evidence that adenosine 5'-triphosphate hydrolysis can occur without dissociation of the actomyosin complex. Biochemistry. 1979 Sep 4;18(18):3895–3909. doi: 10.1021/bi00585a009. [DOI] [PubMed] [Google Scholar]
- Thomas D. D., Cooke R. Orientation of spin-labeled myosin heads in glycerinated muscle fibers. Biophys J. 1980 Dec;32(3):891–906. doi: 10.1016/S0006-3495(80)85024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Ishiwata S., Seidel J. C., Gergely J. Submillisecond rotational dynamics of spin-labeled myosin heads in myofibrils. Biophys J. 1980 Dec;32(3):873–889. doi: 10.1016/S0006-3495(80)85023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner P. D., Giniger E. Calcium-sensitive binding of heavy meromyosin to regulated actin in the presence of ATP. J Biol Chem. 1981 Dec 25;256(24):12647–12650. [PubMed] [Google Scholar]




